The altered expression of homing factors in CD34+ hematopoietic stem cells following G-CSF injection and its effects on transplantation quality in ALL patients
Abstract
Hematopoietic stem cells (HSCs) transplantation is considered a suitable treatment for malignant or nonmalignant hematological diseases. This study aims to investigate the HSCs homing factors in bone marrow (BM) donors of acute lymphoblastic leukemia (ALL) patients following granulocyte colony-stimulating factor (G-CSF) injection, as well as the G-CSF effects on BM transplantation quality in these patients. To mobilize HSCs into peripheral blood, G-CSF was used for ALL patient's BM donors. For HSCs counting, CD34+ cells were evaluated in analogous and autologous donors using flow cytometry. The expression of stem cell homing factors in CD34+ cells and peripheral blood mononuclear cells (PBMCs) were investigated using a real-time polymerase chain reaction. Finally, hematological factors after BM transplantation in ALL patients were assessed. According to our results, after G-CSF injection, the level of CD34+ HSCs was statistically increased. Besides, autologous donors showed a higher level of CD34+ cells compared to analogous donors before and after G-CSF injection. Additionally, a higher number of CD34+ HSCs was achieved in the autologous samples following G-CSF injection. Furthermore, after G-CSF injection, the expression of matrix metalloproteinase (MMP)-2, MMP-9 was increased; while, stromal cell-derived factor 1, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 expression were decreased. Moreover, the expression of C-X-C chemokine receptor type 4, lymphocyte function-associated antigen 1, and very late antigen-4 in CD34+ cells and PBMCs were decreased. BM transplantation on Day 90 also caused an increased level of white blood cells, red blood cells, and platelets as compared to the first day; however, no statistical differences were observed in hemoglobin level. In conclusion, G-CSF by altering the expression of HSCs homing factors in ALL donors improves BM transplantation quality in ALL patients.
1 INTRODUCTION
Acute lymphoblastic leukemia (ALL) is a malignant disease characterized by a large number of immature lymphocytes. This disease is common in children; however, in adults, it poses a devastating threat (Terwilliger & Abdul-Hay, 2017). In this regard, hematopoietic stem cells (HSCs) transplantation has been shown to be effective in the treatment of cancers, including malignant and nonmalignant hematopoietic disorders (Yaghoubi et al., 2020). HSCs as immature multipotent adult stem cells can differentiate into any mature blood cells (Demirci et al., 2020; Parhizkar et al., 2021). In this therapeutic strategy, HSCs capable to restore normal bone marrow (BM) function, are injected intravenously into patients undergoing chemotherapy and radiation (Shirvaikar et al., 2012). HSCs transplantation exhibits long-term engraftment and the ability to recreate the entire hematopoietic system in the recipient (Gunsilius et al., 2001). The most common sources of HSCs include BM, umbilical cord blood, and peripheral blood HSCs mobilization using granulocyte colony-stimulating factor (G-CSF) (Yaghoubi et al., 2020). Mobilized HSCs collection has almost been substituted with BM harvest for both autologous and a majority of allogeneic transplants because of HSCs easy collection by apheresis, and faster hematopoietic recovery after transplantation (Shirvaikar et al., 2012). In steady-state homeostasis, about 0.06% of BM HSCs continuously circulate in peripheral blood (Körbling & Anderlini, 2001). However, this number can be increased by using chemotherapeutic drugs, cytokines, and growth factors that mobilize HSCs from BM into peripheral blood (Shirvaikar et al., 2012). Among these factors, G-CSF is mostly utilized as mobilizing agent for HSCs transplantation (Gunsilius et al., 2001). It has been reported that adhesion molecules, chemokine, and enzymes could be involved in peripheral blood CD34+ HSCs mobilization (Liesveld et al., 2020). Hence, this study aimed to evaluate the effects of G-CSF on stem cell homing factors expression including MMP-2, MMP-4, stromal cell-derived factor 1 (SDF-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) along with C-X-C chemokine receptor type 4 (CXCR-4), very late antigen-4 (VLA-4), and lymphocyte function-associated antigen 1 in CD34+ HSCs and peripheral blood mononuclear cells (PBMCs) in ALL donors, and transplantation quality in ALL patients.
2 MATERIALS AND METHODS
2.1 Study population
Thirteen ALL patients under chemotherapy or radiation and seven healthy individuals referred to Shahid Ghazi Hospital at Tabriz university of medical science participated in this study and were considered autologous and analogous to BM transplantation donors, respectively. Written informed consent was obtained from each participant before the study began. HSC transplantation donors received 5–10 μg/kg of G-CSF per day for 5–7 days. This study was approved by the ethics committee of Tabriz University of Medical Sciences (Code: IR.TBZMED.REC.1397.343).
2.2 PBMCs isolation
A peripheral blood sample (10 ml) was obtained from HSC transplantation donors in heparinized tubes before and after G-CSF injection. PBMCs isolation from the blood sample was done using Ficoll 1.077 g/ml (lymphosep) (Biosera) with gradient centrifugation (450g, 25 min).
2.3 Flow cytometry analysis
About 2 × 106 of PBMCs were used to count CD34+ HSCs, and the surface markers were investigated by flow cytometry (BD FACS Calibur) using a panel of fluorescein isothiocyanate-conjugated monoclonal antibodies including HSCs CD34+ markers (BD Biosciences).
2.4 Magnetic-activated cell sorting (MACS)
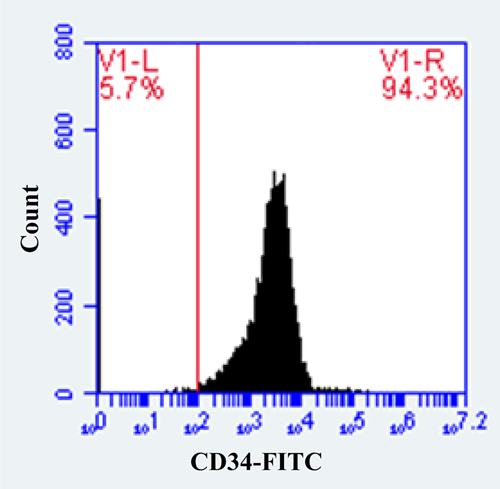
MACS technique was used for CD34+ cells isolation according to selection protocol (Miltenyi Biotec). Isolated CD34+ cells with a purity of over 92% (Figure 1) were washed with phosphate-buffered saline (PBS). Then, 105 cells/ml of each donor were separately cultured in RPMI 1640 containing 100 U/ml of penicillin, 10% fetal bovine serum (FBS), and 200 mM l-glutamine and incubated for 48 h at 5% CO2 and 37°C. Then the number of harvested CD34+ HSCs was evaluated by flow cytometry.
2.5 Real-time polymerase chain reaction (PCR)
TRIzol reagent (Invitrogen) was used for total RNA extraction. Complementary DNA (cDNA) was synthesized with random hexamer primers and oligo (dT) by M-MLV (H-) Revert Aid Reverse Transcriptase kit (Thermo Fisher). Real-time PCR was used for the measurement of CD34+ HSCs homing factors including matrix metalloproteinase (MMP)-2, MMP-9, ICAM-1, VCAM-1, and stromal cell-derived factor 1 (SDF-1) along with C-X-C chemokine receptor type 4 (CXCR-4), VLA-4, and lymphocyte function-associated antigen 1 (LFA-1). In this method, SYBR Green and gene-specific primers were applied in a real-time PCR System (Corbett Research, Bosch Institute). The sequences of gene-specific primers are shown in Table 1. formula was utilized for evaluating the results in the samples.
| Gene | Primer | Sequence (5′ → 3′) |
|---|---|---|
| CXCR4 | Forward | CTCCTCTTTGTCATCACGCTTCC |
| Reverse | GGATGAGGACACTGCTGTAGAG | |
| CD11a (LFA-1) | Forward | CTGCTTTTGCCAGCCTCTCTGT |
| Reverse | GCTCACAGGTATCTGGCTATGG | |
| CD49d (VLA-4) | Forward | GCATACAGGTGTCCAGCAGAGA |
| Reverse | AGGACCAAGGTGGTAAGCAGCT | |
| ICAM-1 | Forward | AGCGGCTGACGTGTGCAGTAAT |
| Reverse | TCTGAGACCTCTGGCTTCGTCA | |
| VCAM-1 | Forward | GATTCTGTGCCCACAGTAAGGC |
| Reverse | TGGTCACAGAGCCACCTTCTTG | |
| MMP-2 | Forward | AGCGAGTGGATGCCGCCTTTAA |
| Reverse | CATTCCAGGCATCTGCGATGAG | |
| MMP-9 | Forward | GCCACTACTGTGCCTTTGAGTC |
| Reverse | CCCTCAGAGAATCGCCAGTACT | |
| SDF-1 | Forward | CTCAACACTCCAAACTGTGCCC |
| Reverse | CTCCAGGTACTCCTGAATCCAC | |
| GAPDH | Forward | GTCTCCTCTGACTTCAACAGCG |
| Reverse | ACCACCCTGTTGCTGTAGCCAA |
- Abbreviations: CXCR-4, C-X-C chemokine receptor type 4; ICAM-1, intercellular adhesion molecule 1; LFA-1, lymphocyte function-associated antigen 1; MMP, matrix metalloproteinase; SDF-1, stromal-derived factor 1; VCAM-1, vascular cell adhesion molecule 1; VLA-4, very late antigen 4.
2.6 Investigation of hematological factors
Complete blood count (CBC) was carried out with Technicon Auto-Analyzer II (SEAL Analytical) for evaluating white blood cells (WBCs), red blood cells (RBCs), platelets, and hemoglobin in samples collected after BM transplantation on the first day and 3 months after BM transplantation on Day 90.
2.7 Statistical analysis
The results are presented as mean ± SD. Statistical analysis was performed using SPSS version 24.0. All graphs illustration was done by Graph Pad Prism version 8.00. Analysis of variance and unpaired t-test were used to compare means between groups. p < .05 was considered as statistically significant.
3 RESULTS
3.1 Frequency evaluation of CD34+ HSCs
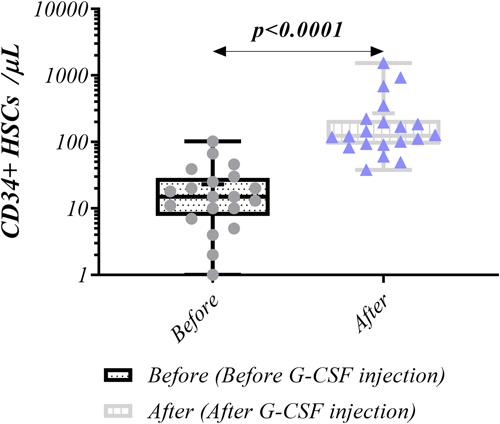
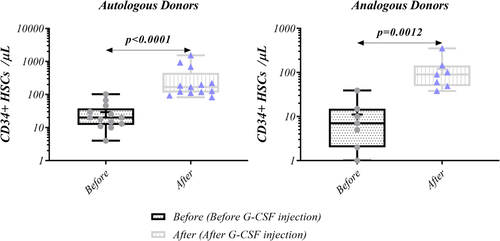
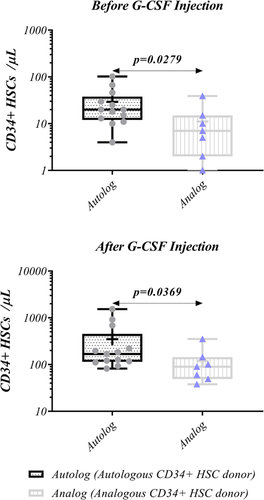
Flow cytometry was used to measure the frequency of CD34+ cells in autologous and analogous ALL donors. After G-CSF injection the level of CD34+ cells substantially elevated (123.5 [38–1532] vs. 15 [0–102], p < 0.0001) (Figure 2). Similar result was also observed for both autologous and analogous donors (168 [82–1532] vs. 20 [4–102], p < 0.0001, and 90 [38–352] vs. 7 [0–39], p = 0.0012, respectively) (Figure 3). Moreover, in both before and after G-CSF injection, CD34+ cells frequency was higher in autologous donors than analogous counterparts (20 vs. 7, p = 0.0279, 168 vs. 90, p = 0.0369, respectively) (Figure 4, Table 2).
| CD34 + stem cell count | |||
|---|---|---|---|
| Variable | Autologous donor | Analogous donor | p Value |
| Median (min–max) (N = 13) | Median (min–max) (N = 7) | ||
| Harvested CD34+ HSCs per kg | 2.75 × 106 (1.04 × 106–7.8 × 106) | 1.1 × 106 (0.85 × 106–4.4 × 106) | 0.0456 |
| CD34+ HSCs per μl (before G-CSF injection) | 20 (4–102) | 7 (0–39) | 0.0279 |
| CD34+ HSCs per μl (after G-CSF injection) | 168 (82–1532) | 90 (38–352) | 0.0369 |
| Before G-CSF injection | After G-CSF injection | ||
|---|---|---|---|
| Variable | Median (min–max) | Median (min–max) | p Value |
| CD34+ HSCs per μl (N = 20) | 15 (0–102) | 123.5 (38–1532) | <0.0001 |
| CD34+ HSCs per μl (autologous donors; N = 13) | 20 (4–102) | 168 (82–1532) | <0.0001 |
| CD34+ HSCs per μl (analogous donors; N = 7) | 7 (0–39) | 90 (38–352) | 0.0012 |
| Relative gene expression (fold change) | |||
|---|---|---|---|
| Before G-CSF injection | After G-CSF injection | ||
| Variable | mean ± SD (N = 20) | mean ± SD (N = 20) | p Value |
| MMP-2 | 1.000 ± 0.06464 | 1.527 ± 0.6628 | 0.0004 |
| MMP-9 | 1.000 ± 0.09755 | 1.679 ± 0.7681 | 0.0012 |
| SDF-1 | 1.000 ± 0.1096 | 0.7650 ± 0.3804 | 0.0317 |
| ICAM-1 | 1.000 ± 0.05629 | 0.6980 ± 0.3810 | 0.0012 |
| VCAM-1 | 1.000 ± 0.06890 | 0.7170 ± 0.4238 | 0.0055 |
- Note: Data are presented as mean ± standard error (SE) or standard division (SD). p < 0.05 was considered as statistically significant.
- Abbreviations: HSC, hematopoietic stem cell; ICAM-1, intercellular adhesion molecule 1; MMP, matrix metalloproteinase; SDF-1, stromal-derived factor 1; VCAM-1, vascular cell adhesion molecule 1.
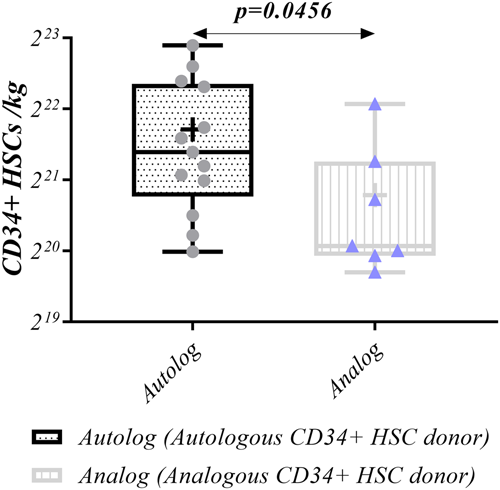
3.2 Harvested isolated CD34 + HSCs
A higher number of harvested CD34+ HSCs (average ≥ 2 × 106 per kg weight) in FBS-containing media was achieved in autologous samples compared to analogous following G-CSF injection (2.75 × 106 [1.04 × 106–7.8 × 106] vs. 1.1 × 106 [0.85 × 106–4.4 × 106], p = 0.0456, respectively) (Figure 5, Table 2).
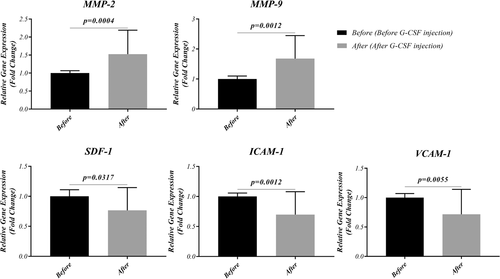
3.3 Expression level of MMP-2, MMP-9, SDF-1, ICAM-1, and VCAM-1 in PBMCs
To study the mRNA expression level of MMP-2, MMP-9, SDF-1, ICAM-1, and VCAM-1 before and after G-CSF injection, real-time PCR was performed. Data revealed an increased expression level of MMP-2 (1.527 ± 0.6628 vs. 1.000 ± 0.06464, p = 0.0004, respectively) and MMP-9 (1.679 ± 0.7681 vs. 1.000 ± 0.09755, p = 0.0012, respectively) following G-CSF injection. While, the expression of SDF-1 (0.7650 ± 0.3804 vs. 1.000 ± 0.1096, p = 0.0317, respectively), ICAM-1 (0.6980 ± 0.3810 vs. 1.000 ± 0.05629, p = 0.0012, respectively) and VCAM-1 (0.7170 ± 0.4238 vs. 1.000 ± 0.06890, p = 0.0055, respectively) was significantly decreased (Figure 6, Table 2).
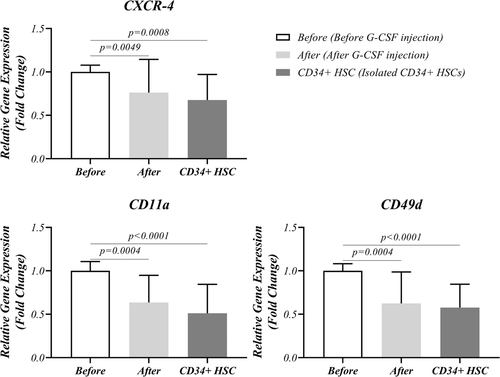
3.4 Expression level of CXCR-4, LFA-1 (CD11a), and VLA-4 (CD49d) in isolated CD34+ HSCs and PBMCs
The expression level of CXCR-4, LFA-1, and VLA-4 in isolated mobilized CD34+ HSCs was also evaluated in PBMCs before and after G-CSF injection. A significant decreased expression of CXCR-4 (0.7615 ± 0.3827 vs. 1.000 ± 0.07780, p = 0.0049), LFA-1 (0.6360 ± 0.3117 vs. 1.000 ± 0.1068, p = 0.0004), and VLA-4 (0.6240 ± 0.3624 vs. 1.000 ± 0.08156, p = 0.0004) was observed after G-CSF injection in PBMCs. Similar results were also observed in CD34+ cells regarding CXCR-4 (0.6750 ± 0.2958 vs. 1.000 ± 0.07780, p = 0.0008), LFA-1 (0.5105 ± 0.3341 vs. 1.000 ± 0.1068, p < 0.0001) and VLA-4 (0.5770 ± 0.2691 vs. 1.000 ± 0.08156, p < 0.0001) after mentioned injection (Figure 7, Table 3).
| Variable | Before (G1) | After (G2) | CD34+ HSC (G3) | p Value | ||
|---|---|---|---|---|---|---|
| Mean ± SD (N = 20) | Mean ± SD (N = 20) | Mean ± SD (N = 20) | G1 vs. G2 | G1 vs. G3 | G2 vs. G3 | |
| CXCR-4 | 1.000 ± 0.07780 | 0.7615 ± 0.3827 | 0.6750 ± 0.2958 | 0.0049 | 0.0008 | NS |
| CD11a (LFA-1) | 1.000 ± 0.1068 | 0.6360 ± 0.3117 | 0.5105 ± 0.3341 | 0.0004 | <0.0001 | NS |
| CD49d (VLA-4) | 1.000 ± 0.08156 | 0.6240 ± 0.3624 | 0.5770 ± 0.2691 | 0.0004 | <0.0001 | NS |
- Note: Data are presented as mean ± standard division. p < 0.05 was considered as statistically significant.
- Abbreviations: CXCR-4, C-X-C chemokine receptor type 4; LFA-1, lymphocyte function-associated antigen 1; VLA-4, very late antigen 4.
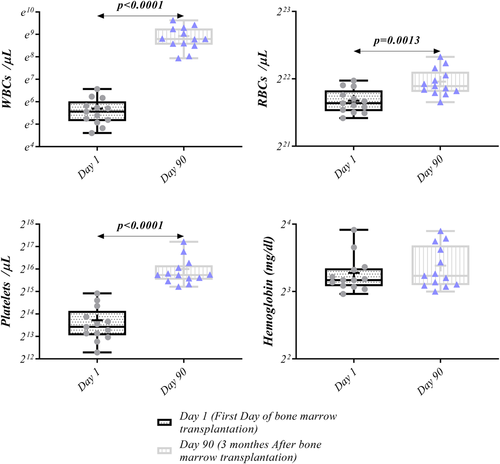
3.5 Hematological factors on Days 1 and 90 after BM transplantation
Hematological factors analysis of collected samples after BM transplantation on Days 1 and 90 demonstrated increased number of WBCs (0.26 × 103 [0.1 × 103–0.71 × 103] vs. 6.6 × 103 [2.5 × 103–15.2 × 103], p < 0.0001), RBCs (3.265 × 106 [2.8 × 106–4.12 × 106] vs. 3.895 × 106 [3.3 × 106–5.26 × 106], p = 0.0013), and platelets (11,000 [5000–31,000] vs. 54,500 [38,000–152,000], p < 0.0001) on Day 90 compared to Day 1. No statistical differences were found in Hemoglobin level between Days 1 and 90 (Figure 8, Table 4).
| Variable | First day (min–max) | Day of 90 (min–max) | p Value |
|---|---|---|---|
| (N = 13) | (N = 13) | ||
| WBCs (µl) | 0.26 × 103 (0.1 × 103–0.71 × 103) | 6.6 × 103 (2.5 × 103–15.2 × 103) | <0.0001 |
| RBCs (µl) | 3.265 × 106 (2.8 × 106–4.12 × 106) | 3.895 × 106 (3.3 × 106–5.26 × 106) | 0.0013 |
| Platelets (µl) | 11,000 (5000–31,000) | 54,500 (38,000–15,2000) | <0.0001 |
| First day Mean ± SD | Day of 120 Mean ± SD | ||
|---|---|---|---|
| Variable | (N = 13) | (N = 13) | p Value |
| Hemoglobin (mg/dl) | 9.7 ± 2.027 | 10.44 ± 2.36 | NS |
- Note: Data are presented as median ± standard error (SE). p < 0.05 was considered as statistically significant.
- Abbreviations: RBC, red blood cell; WBC, white blood cell.
4 DISCUSSION
The continual traffic of HSCs between BM and peripheral blood is required for normal hematopoiesis during homeostasis. Improvement of this physiological process, for instance through forcefully mobilizing HSCs from the BM into the circulation, is critical in clinical transplantation (Gertz, 2010). Regarding the role of stem cell homing factors in CD34+ HSCs mobilization (Liesveld et al., 2020) and their expression in PBMCs (Diaz et al., 2005; Figenschau et al., 2018; Martinesi et al., 2008; Ohashi et al., 1998), in the current study, the effects of G-CSF injection on these factors expression level were evaluated in HSCs and PBMCs in ALL donors in the present study. Moreover, BM transplantation quality in ALL patients was also assessed.
Peripheral blood HSCs are the most commonly used stem cells in allogeneic and autologous transplantations. It is necessary to collect and mobilize sufficient HSCs for successful BM transplantation. According to our findings, following G-CSF injection, the level of CD34+ HSCs was increased. In other words, this cytokine enhanced the HSCs mobilization from BM into peripheral blood (Shirvaikar et al., 2012). Moreover, a higher level of CD34+ HSCs was observed in autologous donors as compared to analogous counterparts both before and after G-CSF injection. Additionally, the minimum dose for harvested CD34+ was successfully achieved in autologous donors, suggesting the higher effectiveness of G-CSF injection in autologous donors' HSCs mobilization.
Our investigations also revealed that G-CSF injection increases the expression level of MMP-2, and MMP-9; while, the expression of SDF-1, ICAM-1, and VCAM-1 is decreased. MMPs are known to facilitate cell migration by breaking the basal membrane barriers made of extracellular matrix proteins. It has been reported that upon G-CSF stimulation in mice, neutrophils release MMP-9, resulting in HSCs mobilization (Pelus et al., 2004). Increased MMP-2 and MMP-9 production in mobilized HSCs, but not in steady-state in BM indicates the participation of these proteases in the migration process from endothelial into the blood circulation (Janowska-Wieczorek et al., 1999; Janowska-Wieczorek et al., 2000). Moreover, it has been shown that MMP inhibitors prevent the mobilization of murine stem cells stimulated by G-CSF (Heissig et al., 2002).
SDF-1 is a member of the CXC chemokine ligand superfamily expressed by BM stromal cells. This factor is a key molecule in anchoring HSCs to BM, activating main adhesion molecules, and regulating stem cell development. Reduced SDF-1 levels in BM may inhibit stem cell retention and facilitate their release (Petit et al., 2002). Several studies on murine models have reported decreased SDF-1 levels in BM after G-CSF injection, and increased HSCs mobilization, suggesting that SDF-1 reduction is a vital step for HSC mobilization (Lévesque et al., 2003; Petit et al., 2002). Cleavage and inactivation of SDF-1 are mediated by MMP-2 and MMP-9 proteases which impair its binding to the CXCR-4 receptor during mobilization (McQuibban et al., 2001). Other molecules involved in maintaining HSCs in BM are adhesion molecules including VCAM-1, and ICAM-1 on stromal cells, which mediate HSCs rolling and tethering processes (Doan & Chute, 2012; Liesveld et al., 2020). In this regard, G-CSF injection results in proteolytic degradation of VCAM-1 leading to a VCAM-1 decrease in mouse BM and HSCs mobilization (Lévesque et al., 2001, 2002). Moreover, Liu Y et al. (2018) indicated that the ICAM-1deficiency in the niche contributes to enhanced HSCs mobilization in mice.
Alongside the proteases and adhesion molecules' significance in HSCs mobilization, CXCR4, LFA-1, and VLA-4 receptors can play a vital role in G-CSF-induced mobilization. With regard to the expression of these molecules on both PBMCs and CD34+ HSCs, we compared the effects of G-CSF on these two kinds of cells. After G-CSF injection, the expression of CXCR4, LFA-1, and VLA-4 receptors significantly decreased in PBMCs and CD34+ SCs. Additionally, the effect of G-CSF on CD34+ HSCs was greater than PBMCs, and caused the more efficient mobilization of CD34+ cells into the peripheral blood. HSCs possess a variety of cell surface molecules including CXCR4, VLA-4, and LFA-1, that are involved in HSCs maintenance in BM (Rettig et al., 2012). In this regard, low CXCR4 expression levels and decreased migration to SDF-1 gradients have been observed in mobilized CD34+ cells (Aiuti et al., 1997; Peled et al., 1999). In addition, G-CSF-treated patients showed a lower level of CXCR4 and SDF-1 in the periphery, which are associated with higher stem cell mobilization rates (Gazitt & Liu, 2001; Voermans et al., 2001). Moreover, several studies have reported decreased expression of LFA-1 and VLA-4 in G-CSF-induced mobilized HSCs which is consistent with our findings (Dercksen et al., 1995; Möhle et al., 1993). Additionally, hematological factors evaluation in our study showed good transplantation quality on Day 90 in all patients undergoing mobilized HSCs transplantation.
5 CONCLUSION
In conclusion, we thoroughly evaluated stem cell homing factors involved in G-CSF-induced mobilization of CD34+ HSCs in ALL donors' BM transplantation. We observed a higher level of CD34+ HSCs in autologous donors as compared to analogous counterparts both before and after G-CSF injection. Interestingly, we showed higher effectiveness of G-CSF injection in autologous donor HSCs mobilization. Moreover, we indicated that during HSCs mobilization, the expression level of proteases was increased, and HSCs homing factors were reduced. Finally, according to our results all patients undergoing G-CSF-induced mobilized HSCs transplantation exhibited good transplantation quality. These data recommend that the administration of G-CSF in ALL donors leads to good quality BM transplantation in ALL patients by affecting CD34+ HSCs homing factors.
AUTHOR CONTRIBUTIONS
Seyede-Leila Shafiei wrote the manuscript and helped with laboratory assays. Seyede Fatemeh Hosseini and Saeedeh Torabi Goudarzi helped in laboratory assays. Mehdi Talebi and Mehdi Edalati helped in sample collection. Mohammad Sadegh Soltani-Zangbar helped with data analysis. Aliakbar Movassaghpour helped in the final edition of the manuscript. Aliakbar Movassaghpour and Mehdi Yousefi helped in the conceptualization and supervised the study.
ACKNOWLEDGMENTS
This study is a part of the MSCs Thesis for Seyede-Lelia Shafiei. The current study was supported by the Hematology Oncology Research Center at Tabriz University of Medical Sciences, Tabriz, Iran (Grant No: 62486).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




