Tripartite motif protein 25 is associated with epirubicin resistance in hepatocellular carcinoma cells via regulating PTEN/AKT pathway
Peng Yuan and Aidong Zheng contributed equally to this work.
Abstract
Tripartite motif protein 25 (TRIM25) expression was altered in various human cancers. Herein, we found that the expression of TRIM25 was elevated in hepatocellular carcinoma (HCC) tissues and cell lines. Knockdown of TRIM25 increased the sensitivity of HCC HepG2 cells to epirubicin (EPI), as indicated by reduced cell viability, enhanced cell apoptosis, and downregulated P-glycoprotein (P-gp) and multiple drug-resistance protein 1 (MRP1). Moreover, TRIM25 knockdown strengthened the effects of EPI on phosphatase and tensin homolog (PTEN) and phosphorylated (p)-AKT. However, overexpression of TRIM25 exerted an opposite effect, weakening the sensitivity of Huh7 to EPI, and obviously increasing PTEN and reducing p-AKT. Most important, all the changes induced by TRIM25 overexpression in Huh7 were reversed with additional treatment of LY294002 (an AKT pathway inhibitor). Notably, coimmunoprecipitation experiments confirmed the interaction between TRIM25 and PTEN. Knockdown of TRIM25 resulted in reduced ubiquitination of PTEN protein. Collectively, our data suggested that TRIM25 enhanced EPI resistance via modulating PTEN/AKT pathway, and targeting TRIM25 may enhance the sensitivity of HCC cells toward chemotherapy drugs.
Abbreviations
-
- EPI
-
- epirubicin
-
- FITC
-
- fluorescein isothiocyanate
-
- HCC
-
- hepatocellular carcinoma
-
- MRP1
-
- multiple drug-resistance protein 1
-
- P-gp
-
- P-glycoprotein
-
- PBS
-
- phosphate-buffered saline
-
- PDX
-
- patient-derived xenograft
-
- PI3K
-
- phosphoinositide 3-kinase
-
- PTEN
-
- phosphatase and tensin homolog
-
- SEM
-
- standard error of the mean
-
- TACE
-
- transcatheter arterial chemoembolization
-
- TCGA
-
- The Cancer Genome Atlas
-
- TRIM25
-
- tripartite motif protein 25
Introduction
Hepatocellular carcinoma (HCC) is a pervasive and deadly malignancy with a 5-year survival rate of merely 30–40% (Zhu et al., 2015). Currently, transcatheter arterial chemoembolization (TACE) combined with injecting anticancer drugs into the liver is accepted as a promising therapy for this disease (Bai et al., 2018). Epirubicin (EPI), an anthracycline drug, is a conventional and effective antitumor agent that used as in the first-line for TACE in chemotherapy in HCC (Tacar et al., 2013; Finn, 2014; Bai et al., 2018). Mechanically, EPI works by intercalating DNA strands and inhibiting DNA and RNA synthesis (Tacar et al., 2013). However, during long-course of EPI monotherapy, HCC cells may eventually develop acquired resistance to EPI, rendering the advantage of this drug merely modest, and resulting in HCC recurrence and poor prognosis (Han & Park, 2008). Therefore, identification of novel molecular targets is necessary to develop synergetic anticancer effects and improve EPI-based chemotherapy in HCC.
Members of tripartite motif (TRIM) protein family have conserved architecture (structurally including B-box, coiled coil, and zinc-finger). Many members of TRIM proteins have been identified as cancer-associated E3 ligase, participating in carcinogenesis of various human cancers including HCC (Hatakeyama, 2017). Recent studies have suggested that some members of TRIM family proteins play important roles in chemoresistance (Zhang et al., 2015; Ni et al., 2016; Liu et al., 2017; Tan et al., 2018; Yu et al., 2018; Zhao et al., 2018). The expression of TRIM25, a member of TRIM protein, was increased in gastric (Zhu et al., 2016), lung cancer (Qin et al., 2016), breast cancer (Urano et al., 2002), and prostate cancer (Takayama et al., 2018), but reduced in endometrial cancer (Nakayama et al., 2005). It is reported that TRIM25 promoted breast cancer metastasis (Walsh et al., 2017). Evidence has shown that TRIM25 could enhance the resistance of lung cancer cells to cisplatin and doxorubicin (Qin et al., 2016; Qin et al., 2017). However, whether TRIM25 played a role in EPI resistance in HCC, and whether targeting TRIM25 produced synergized effect in EPI-based chemotherapy has not been elucidated.
Activation of phosphoinositide 3-kinase (PI3K)/AKT tyrosine kinase cascade (also known as AKT or PKB pathway) contributes to anti-apoptosis and cell survival in various cancers (Narlaet al., 2018). Increased AKT activity accounts for acquired resistance to antitumor agents (Cassinelli et al., 2013). Thus, developing AKT inhibitors may be employed as chemotherapeutic adjuvants to modulate drug resistance in combinatorial regimens (Huang & Hung, 2009). Phosphatase and tensin homolog (PTEN) is an inhibitor of PI3K and acts as a tumor suppressor in HCC (Hu et al., 2003; Meng et al., 2007). The regulatory functions of other members of TRIM proteins, such as TRIM59 and TRIM14, on PI3K/AKT have been described (Sun et al., 2017; Xu et al., 2017). However, little is known about the roles of TRIM25 in regulating AKT in response to EPI resistance in HCC cells. At present, we investigated the significance of TRIM25 in EPI resistance of HCC cells. Cell proliferation and apoptosis assays were performed to assess drug sensitivity. We found that knockdown of TRIM25 sensitized HCC cells to EPI via inhibiting PTEN ubiquitination and blocking AKT activation. Our study suggested that TRIM25 may be a novel promising marker of HCC, and an attractive target for chemotherapies in HCC.
Material and methods
Patients and clinical samples
Twenty-five pairs of liver samples of HCCs, as well as the matched adjacent normal tissues were recruited from patients at the Department of Oncology, The People's Hospital of Funing County (Yancheng, China). All clinical samples were obtained with signed consent. Our research was permitted by the Ethics Committee of The People's Hospital of Funing County.
Analysis of TCGA (The Cancer Genome Atlas) data set
TCGA HCC data set was assessed and TRIM25 expression in HCC (n = 371) and normal liver tissues (n = 50) was analyzed through the UALCAN (Chandrashekar et al., 2017).
Immunohistochemical (IHC) staining
IHC staining was performed as previously described (Wang et al., 2019). After deparaffinization and hydration, endogenous peroxidase was blocked and antigen retrieval was performed. The tissue sections were incubated with anti-TRIM25 (1:200, ab167154; Abcam) followed by incubation with a secondary antibody. Histological examination was performed using 3,3′-diaminobenzidine kit (DAB) and the nuclei were counterstained with hematoxylin.
Cells lines and culture
Human hepatocyte L02 cells and five HCC cell lines (SK-HEP-1, Hep3B, SMMC-7721, HepG2, and Huh7) were acquired from the Chinese Academy of Sciences. Cells grew at 37°C under 5% CO2 in a high-glucose Dulbecco's modified Eagle's medium (SH30243.01; HyClone) with 10% (v/v) of fetal bovine serum (FBS) and 1% (v/v) penicillin (P1400-100; Solarbio).
Knockdown and overexpression of TRIM25
Three shRNAs targeting TRIM25 (shTRIM25-1, -2, -3) were designed and the target sequence are listed in Table S1. The shRNA oligonucleotides were annealed and cloned into the PLKO.1 (Addgene).
Human TRIM25 gene was amplified with the designed primers of were 5′- CGGAATTCATGGCAGAGCTGTGCCC-3′ (forward) and 5′- CGGGATCCCTACTTGGGGGAGCAGATG-3′ (reverse), and cloned into the pLVX-Puro (Clontech). Plasmid expressing FLAG-tagged TRIM25 (wild-type, WT) or E3 ligase-defective TRIM25 mutant (ΔRING) was constructed as previously described (Gack et al., 2007). Plasmid expressing TRIM25 shRNAs, control shRNA (shNC), TRIM25, or Vector was transfected into 293T cells combined with psPAX2 and pMD2G (Addgen) by using Lipofectamine 2000 reagent (Invitrogen). At 48–72 h post-transfection, lentiviruses were collected to infect the target cell lines.
Cell treatment
HepG2 cells were infected with lentivirus overexpressing TRIM25 or Vector for 24 h, and then treated with EPI (E122334; Aladdin) (2.5 μg/mL) or Vehicle (DMSO) for 24 h.
Huh7 cells were infected with lentivirus expressing TRIM25 shRNA or shNC for 24 h, and then treated with EPI (E122334; Aladdin) (2.5 μg/mL), 10 μM of LY294002 (an AKT inhibitor, S1105, Selleck), or Vehicle (DMSO) for 24 h.
Quantitative real-time (qRT) polymerase chain reaction (PCR)
Total RNA was extracted from liver tissues via TRIzol reagent (Invitrogen). First-strand complementary DNA (cDNA) was acquired using the RevertAid First Strand cDNA Synthesis kit (Fermentas, USA). Quantified analysis of TRIM25 messenger RNA (mRNA) was conducted using SYBR Green PCR Mix (Thermo Fisher Scientific, Shanghai, China) on ABI Prism 7300 SDS system (Applied Biosystem, Foster City, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize. Primer sequences of TRIM25 were: 5′-CCACCAGAGCACCATAGACC-3′ (forward); 5′-TGGGTAAGGCAGGGACAGG-3′ (reverse); Size: 191 bps. Primer sequences of GAPDH used in our study were: 5′-CACCCACTCCTCCACCTTTG-3′ (forward); 5′-CCACCACCCTGTTGCTGTAG-3′ (reverse); size: 110 bps.
Western blot analysis
Cells were lysed using RIPA (JRDUN, Shanghai, China) at 4°C, and then centrifuged at 12,000 rpm for 10 min. BCA protein assay kit (Thermo Fisher Scientific) was used to measure total protein content in supernatant, and 25 μg of which was separated on (sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and then transferred onto nitrocellulose membranes (Millipore, MA, USA). After blocking with 5% skim milk, the membrane was incubated with anti-TRIM25 antibody (#13773; Cell Signaling Technology [CST]), antibody against P-glycoprotein (P-gp) (Ab170904; Abcam), antibody against multiple drug-resistance protein 1 (MRP1) (Ab24102; Abcam), antibody against phosphatase and tensin homolog (PTEN) (#9188; CSt), antibody against Bcl2 (#15071; CST), antibody against Bax (#5023; CST), GAPDH (#5174; CST), antibody against AKT (#4035; CST), antibody against phosphorylated (p)-AKT (#4060; CST) at 4°C overnight followed by a horseradish peroxidase-conjugated antibodies (Beyotime, Shanghai, China) for another 1 hour at 25°C. Immunoreactive bands were analyzed by ECL system (GE Healthcare/Amersham Biosciences). ImageJ software (http://rsb.info.nih.gov/ij/, Bethesda, MD, USA) was used to quantify the band density.
Cell apoptosis assay
Cells were plated onto a six-well culture plate (3.0 × 105/well) and incubated overnight. After treatment, the cells were double-stained using fluorescein isothiocyanate-labeled recombinant annexin V (Annexin V-FITC)-apoptosis detection kit (Beyotime), and analysis by fluorescent microscope (BD Biosciences, Mountain View, CA, USA). Early apoptosis was Annexin (+)/PI(−), and presented in the lower right quadrant.
Isolation of primary HCC cell lines and treatment
Primary HCC cell lines were isolated from patients with HCC. In brief, after washing with phosphate-buffered saline (PBS), liver tissue (1.0 cm3) was cut into pieces, enzymatically digested, and then filtered using a 200-mesh sieve. The filtrate was centrifuged at 1,000 rpm for 10 min. Isolated human primary HCC cells were cultured at 37°C under 5% CO2 in a RPMI1640 medium (SH30027.02; HyClone) containing 20% (v/v) FBS (16000-044; Gibco). The primary HCC cells were treated with vehicle or EPI (2.5 μg/mL) for 24 h, and then cell apoptosis and TRIM25 protein expression was detected.
Patient-derived xenograft (PDX)
Animal experiments were approved by the Animal Care Committee of The People's Hospital of Funing County. PDX model was established as previously described (Jiang et al., 2016). In brief, HCC tissues from patients were cut into 3 mm3 pieces and transplanted subcutaneously into male 4-week-old nude mice (SLAC Laboratory Animal Center, Shanghai, China). TRIM25 protein expression levels of these HCC tissues were determined by western blotting. After tumor formation, the mice were injected with EPI (1 mg/kg/day) or Vehicle (DMSO) every 3 days and were divided into two groups (n = 5 per group). Tumor volume was measured by the following formula: Tumor volume = 1/2 × width2 × length. After 33 days, the tumors were harvested and weighed.
Statistics and data analysis
Data were described as mean ± standard error of the mean (SEM) deviation of three independent experiments. Comparisons between the two groups were determined by Student's t test, and P ≤ 0.05 was considered as a significant difference.
Results
Enhanced expression of TRIM25 in human HCC tissues and cell lines
mRNA expression of TRIM25 was measured in 25 pairs of HCC tissues and their matched precancerous tissues. Figure 1A indicated that TRIM25 mRNA was significantly higher in tumor tissues when compared with non-tumorous tissues (P < 0.0001). IHC was performed on five pairs of HCC and precancerous tissues and confirmed the enhanced expression of TRIM25 protein in HCC tissues (Figure 1B). Analysis of TCGA HCC data set through UALCAN confirmed the increased expression of TRIM25 mRNA in HCC tissues (Figure 1C). Enhanced protein levels of TRIM25 were also observed in five HCC cell lines SK-HEP-1, Hep3B, HepG2, Huh7, and SMMC-7721 cells, compared with L02 cells (Figure 1D). In addition, immunofluorescence staining was performed on two representative HCC cell lines, HepG2 (with higher TRIM25 expression) and Huh7 (with lower TRIM25 expression), and L02, and the results showed that TRIM25 expressed in the cytoplasm and TRIM25 expression was elevated in HCC cells (Figure S1).
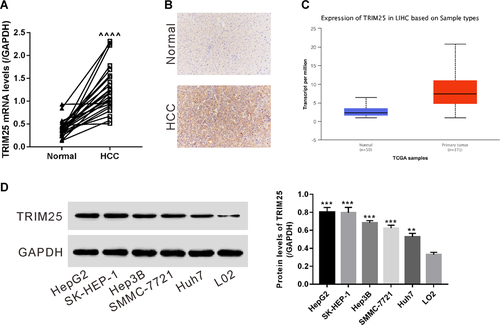
Knockdown of TRIM25 increased the sensitivity of HCC cells to EPI
Then we assessed the IC50 (half-maximal inhibitory concentration) of EPI in HepG2 (with higher TRIM25 expression) and Huh7 (with lower TRIM25 expression). HepG2 cells showed a higher IC50 as compared with Huh7 (Figure S2).
HepG2 cells were infected with virus expressing TRIM25 shRNAs or control shRNA. Protein expression of TRIM25 was remarkably decreased by all of shRNAs (shTRIM25-1, -2, -3) (Figure 2A). shTRIM25-3 with the highest efficiency was chosen for further assays. At 24 h after infection, HepG2 cells were treated with 0.5 μg/mL of EPI or Vehicle for 24 h, double-stained with PI and Annexin V and then subjected to flow cytometry analysis. As shown in Figure 2B, after EPI treatment, the percentages of viable cells (PI− Annexin V−) were significantly decreased in both shNC- and shTRIM25-infected cells, while the percentages of apoptotic cells (PI− Annexin V+) were remarkably increased. shTRIM25 infection resulted in a significant reduction in cell viability and an increase in cell apoptosis. More important, EPI-induced pro-apoptotic effects were strengthened with additional shTRIM25 treatment, suggesting that TRIM25 knockdown enhanced the sensitivity of HCC cells to EPI.
Then, the protein levels of cell apoptosis- and drug-resistance-associated proteins were determined by Western blot. As shown in Figure 2C, TRIM25 knockdown and EPI exposure increased the expression of Bax, while decreased the expression of P-gp, MRP1 and Bcl2. Enhanced AKT activity is an important reason for the acquired resistance to antitumor agents (Cassinelli et al., 2013). Our data suggested that TRIM25 knockdown and EPI exposure downregulated p-AKT while upregulated PTEN when compared with shNC (Figure 2D). Additional shTRIM25 treatment also strengthened the effects of EPI. These data suggested that AKT signaling may be involved in the sensitivity of HCC cells to EPI.
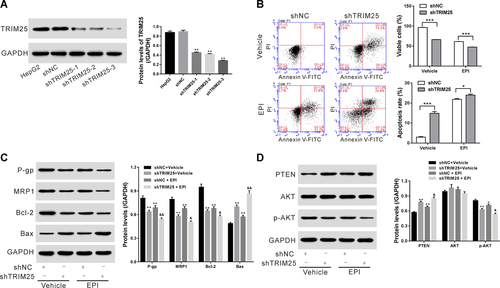
TRIM25 overexpression increased the resistance of HCC cells to EPI
Huh7 cells were infected with lentivirus expressing TRIM25 or Vector, and Western blot results showed that protein expression of TRIM25 was increased by lentivirus expressing TRIM25 (Figure 3A). At 24 h after infection, Huh7 cells were treated with 0.5 μg/mL of EPI for 24 h, and flow cytometry analysis showed that overexpression of TRIM25 decreased the sensitivity of Huh7 cells to EPI, as evidenced by obviously reduced cell apoptosis (Figure 3B), decreased expressions of Bax, while increased expression of P-gp, MRP1, and Bcl2 when compared with Vector (Figures 3C and 3D). Taken together, these data supported that TRIM25 overexpression enhanced the resistance of HCC cells to EPI.
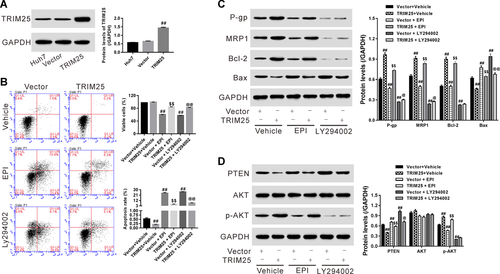
The AKT pathway was involved in the functions of TRIM25 in human HCC cells
To study whether AKT pathway mediated the functions of TRIM25 in human HCC cells, LY294002 (a specific synthetic AKT inhibitor) was introduced to treat Huh7 cells with TRIM25 overexpression. Our data suggested that TRIM25 overexpression inhibited apoptosis (Figure 3B), increased expression of P-gp, MRP1, and Bcl2 while reduced PTEN and Bax, but promoted p-AKT (Figures 3C and 3D), and the changes of which were reversed by additional LY294002 treatment, suggesting that TRIM25 regulated apoptosis of HCC cells by targeting AKT signaling.
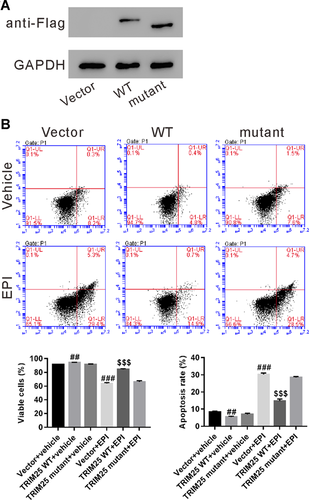
The E3 ligase activity of TRIM25 was required for resistance of HCC to EPI
TRIM25 possesses E3 ligase activity (Zang et al., 2017; Lee et al., 2018; Li et al., 2018). To explore the involvement of E3 ligase activity of TRIM25 in EPI resistance, Huh7 cells were transfected with plasmid expressing FLAG-tagged TRIM25 (WT) of E3 ligase-defective TRIM25 mutant (ΔRING). Figure 4A showed the expression of FLAG-tagged TRIM25 (WT and mutant). At 24 h after transfection, Huh7 cells were treated with 0.5 μg/mL of EPI for 24 h. As shown in Figure 4B, overexpression of TRIM25 mutant had no effect on EPI resistance. Taken together, these data supported that TRIM25 overexpression enhanced the resistance of HCC cells to EPI via E3 ligase.
TRIM25 knockdown inhibited PTEN ubiquitination
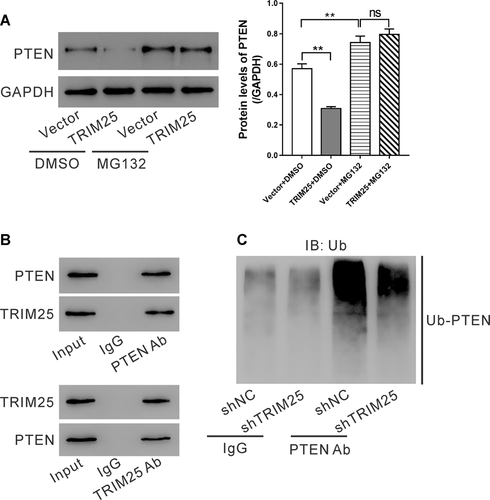
TRIM25 possesses E3 ligase activity (Zang et al., 2017; Lee et al., 2018; Li et al., 2018). TRIM25 expression levels affected the protein expression of PTEN. Thus, we hypothesized that TRIM25 may regulate PTEN protein via ubiquitination-proteasome pathway. As expected, MG132, a proteasome inhibitor (Seol, 2011), blocked TRIM25-caused reduction in PTEN protein levels (Figure 5A), suggesting a proteasome-dependent manner during the regulation of TRIM25 on PTEN protein expression. Further, the interaction between TRIM25 and PTEN was confirmed by IP experiments with antibody against TRIM25 or PTEN (Figure 5B). IP experiments with cell lysate from HepG2 expressing TRIM25 shRNA or control shRNA suggested that TRIM25 knockdown inhibited PTEN ubiquitination (Figure 5C). These data demonstrated that TRIM25 regulated PTEN protein via ubiquitination.
TRIM25 expression levels affected the effectiveness of EPI in treating HCC
Primary HCC cancer cells were isolated from 12 patients and treated with EPI or Vehicle for 24 h. Cell apoptosis was assessed by Annexin V–PI staining. As shown in Figure 6A, these cells could be divided into EPI-sensitive group (A) and EPI-resistant group (B) according to the apoptotic rate. The protein levels of TRIM25 were then detected. As shown in Figure 6B, cells in EPI-resistant group (B) had a relative higher levels of TRIM25 compared with those in EPI-sensitive group (A) (P < 0.0001). The levels of drug-resistance associated protein (P-gp and MRP1) were measured. Our data showed that EPI-sensitive primary HCC cell lines (A1, A2, and A3) expressed relative low levels of P-gp and MRP1 (Figure 6C). Taken together, these data supported that TRIM25 expression might be associated with the resistance to EPI within HCC cells.
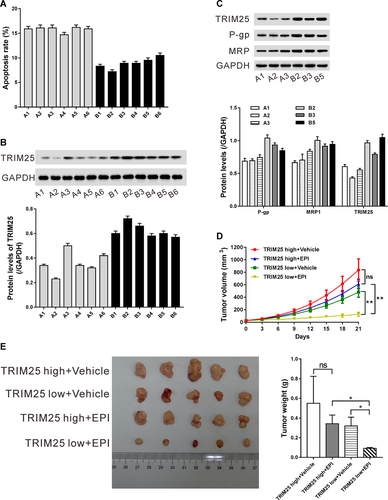
In addition, HCC tissues were collected to establish PDX model. The formed xenografts were defined as TRIM25 high and TRIM25 low according to the expression levels of TRIM25, and treated with EPI or Vehicle. As shown in Figures 6D and 6E, no significant difference was observed in tumor growth rate, tumor size, and tumor weight between EPI and Vehicle treatment in TRIM25 high PDX. EPI treatment was more efficient in TRIM25 low PDX than in TRIM25 high PDX. Taken together, these data suggest that EPI may be more effective in HCC with lower levels of TRIM25 than in those with higher levels of TRIM25.
Discussion
TRIM25 serves as an oncogene in various cancers (Qin et al., 2016; Zhu et al., 2016; Takayama et al., 2018). In the current study, our data demonstrated that TRIM25 was aberrantly elevated in HCC tissues (Figures 1A–C), suggesting the clinical significance of TRIM25 in the diagnosis of HCC.
Escape from apoptosis contributes to chemoresistance of cancer cells (Delbridge & Strasser, 2015). Some studies have shown that TRIM25 silencing increased cell apoptosis and promoted chemo-sensitivity of human lung cancer cells (Qin et al., 2016, 2017). In our study, HepG2 cells with higher TRIM25 expression showed a higher IC50 to EPI as compared with Huh7, which was in line with a previous report (Zhang et al., 2017). Furthermore, we substantiated that knockdown of TRIM25 strengthen the anti-viable and pro-apoptotic activity of EPI in HepG2 cells (Figure 2), whereas overexpression of TRIM25 weakened the effects of EPI in Huh7 cells (Figure 3), supporting that suppression of TRIM25 increased the sensitivity of HCC cells to EPI. In addition, E3 ligase-defective TRIM25 mutant had no effect on EPI resistance, suggesting the importance of E3 ligase activity of TRIM25 (Figure 4). Notably, primary HCC cells, which were more sensitive to EPI-induced apoptosis had lower TRIM25 expression levels (Figures 6A and 6B), which was confirmed by using PDX model in vivo (Figures 6D and 6E). Thus, repression of TRIM25 could enhance the antitumor effect of EPI in HCC.
Elevated AKT activity enhanced the resistance of cancer cells to antitumor agents (Cassinelli et al., 2013). Downregulation of PTEN, the inhibitor of AKT activity, results in the promotion of proliferation, decline of apoptosis, and low chemotherapy efficiency in HCC cells (Lee et al., 2011). P-gp and MRP1, are known as two important drug efflux pumps, and their overexpression in cellular membrane leads to the failure of chemo-therapeutics (Tekchandani et al., 2017). LY294002, a well-known PI3K inhibitor, targets P-gp or MRP1 to antagonize chemoresistance (Abdulghani et al., 2006; Sedlák, 2006). Our data suggested that EPI treatment significantly suppressed P-gp, MRP1, and p-AKT, and increased PTEN expression in HCC cells. TRIM25 knockdown strengthened such effects of EPI (Figure 2). Primary HCC cells, which were more sensitive to EPI-induced apoptosis had lower expression levels of P-gp and MRP1 (Figure 6C). These data suggested that P-gp and MRP1 were the potential targets for TRIM25 to regulate EPI resistance of HCC cells. Besides, TRIM25 overexpression downregulated PTEN, and increased p-AKT, P-gp, and MRP1, which could be reversed by LY294002 (Figure 3). TRIM25 acted on AKT signaling to modulate the resistance of HCC cells to EPI. Lastly, TRIM25 was an ubiquitin E3 ligase for PPARγ and metastasis-associated 1 protein (Zang et al., 2017; Lee et al., 2018; Li et al., 2018). In the current study, we demonstrated that TRIM25 bound to PTEN and TRIM25 knockdown inhibited PTEN ubiquitination (Figure 5), and suggested a potential regulatory mechanism of TRIM25 on PTEN/AKT pathway.
In conclusion, our data indicated that TRIM25 was upregulated in patients with HCC. TRIM25 was identified as a novel chemoresistance-conferring gene in HCC, and inhibiting TRIM25 may represent an effective strategy to exert synergized effect in chemotherapy in this disease.
Acknowledgments and Funding
The study was supported by the grant from the Science and Technology Bureau of Yancheng (YK2017079).
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Our research was permitted by the Ethics Committee of The People's Hospital of Funing County.




