Overexpression and one-step renaturation-purification of the tagged creatinine deiminase of Corynebacterium glutamicum in Escherichia coli cells
Abstract
The codA gene of Corynebacterium glutamicum PCM 1945 coding for a creatinine deiminase (CDI) (EC 3.5.4.21) has been amplified and cloned. The recombinant strain of Escherichia coli that overproduces the (His)6-tagged inactive CDI of C. glutamicum as inclusion bodies has been constructed. After solubilization of inclusion bodies in the presence of 0.3% N-lauroylsarcosine, the enzyme was renaturated and purified by a single-step procedure using metal-affinity chromatography. The yield of the (His)6-tagged CDI is ~30 mg from 1 L culture. The purified enzyme is sufficiently stable under the conditions designed and possesses an activity of 10–20 U/mg. The main characteristics of the tagged enzyme remained similar to that of the natural enzyme.
Abbreviations
-
- bp
-
- basepair
-
- CDI
-
- creatinine deiminase
-
- EDTA
-
- ethylenediaminetetraacetic acid
-
- IPTG
-
- isopropyl β-d-1-thiogalactopyranoside
-
- LB
-
- Luria–Bertani medium
-
- OPA
-
- o-phthaldialdehyde
-
- PCR
-
- polymerase chain reaction
-
- SDS-PAGE
-
- sodium dodecyl sulfate polyacrylamide gel electrophoresis
-
- Trx
-
- thioredoxin.
Introduction
Creatinine, the waste product of creatine, is produced in human muscles, transported via the bloodstream into the kidney and then excreted with urine. Thus, the serum creatinine level is an important indicator of the renal function and a valuable biomarker to control the hemodialysis procedure (Wyss and Kaddurah-Daouk, 2000; Sant et al., 2004). Currently, available chemical methods of creatinine detection possess several limitations due to insufficient sensitivity and low specificity (Zakalskiy et al., 2019). Despite the latter, these methods are widely used for assessments of patient conditions although the accuracy of such detection is crucial.
Usage of a creatinine deiminase (CDI) catalyzing hydrolysis of creatinine to N-methylhydantoin and ammonia could overcome these obstacles (Tabata et al., 1983; Wyss and Kaddurah-Daouk, 2000; Gottschalk et al., 2007; Zakalskiy et al., 2019). Certain microorganisms are able to produce CDIs. However, some of them display a low substrate specificity (Wyss and Kaddurah-Daouk, 2000; Zakalskiy et al., 2019). Earlier, CDI of Corynebacterium glutamicum (lilium) ATCC 15990 was purified and crystallized using a rather complicated protocol: fractionation with ammonium sulfate and protamine sulfate, column chromatography on diethylaminoethyl-cellulose, Sephadex G-100 and hydroxyapatite. The enzyme is specific to creatinine and exhibits a specific activity of 34 U/mg (Uwajima and Terada, 1976, 1977, 1980). Another CDI from the anaerobic microorganism BN11 with a specific activity of 78 U/mg appeared to be a more preferable enzyme to be used for creatinine quantification. Unfortunately, the yield of the enzyme was rather low (Gottschalk et al., 1991). Later, the Tissierella creatinini gene coding for CDI was expressed heterologously and the recombinant enzyme was purified and characterized (Gottschalk et al., 2007). Commercially available CDI has been used to develop different approaches for creatinine assay. However, the cost of the enzyme is rather high, possibly, due to a low protein production by the recombinant strain and a complex purification procedure (Gottschalk et al., 2007; Marchenko et al., 2016; Zakalskiy et al., 2019).
Cloning and expression of the corresponding gene in well-characterized host strains could address the issues mentioned above. Also, it can provide a possibility to fuse different tags to the N- or C-terminus of the recombinant protein to significantly simplify its purification. In most cases, overexpressed proteins are synthesized in an inactive, insoluble form as inclusion bodies. Therefore, the purification procedure will require additional steps of solubilization and renaturation. There are numerous protocols developed for solubilization of inclusion bodies and subsequent enzymes’ renaturation (Burgess, 2009). Despite that, solubilization and renaturation of a particular enzyme can be challenging and might demand “fine tuning” of a procedure (temperature, buffer composition, solubilization agents, a procedure of removing them, etc.) to obtain an active and stable preparation (Fayura et al., 2013). Additionally, the oligomeric structure of an enzyme (for instance CDI) further complicates correct reassembly of its active form.
Here we report on construction of the Escherichia coli recombinant strain overproducing tagged CDI of C. glutamicum. Protein expression, solubilization, and renaturation conditions were studied. As a result, we developed an efficient procedure for one-step refolding-purification of the tagged, apparently oligomeric CDI.
Materials and methods
Cloning of the codA gene from C. glutamicum into the expression vector pET32a
The open reading frame of the codA gene was amplified as a 1,362 bps long DNA fragment using primers codA-for (5′-TACTATGGATCCATGAACACCATTCATCACCATCACCATCACATGACATGTAGAAATGGA-3′) and codA-rev (5′-TAATAATGCGGCCGCTTAGATGTTCCAGTCCAC-3′) and total DNA C. glutamicum PCM 1945 as a template. The primers contained BamHI and NotI restriction sites as well as 5′-terminal DNA sequence encoding a (His)6-tag to be fused to the N-terminus of the protein.
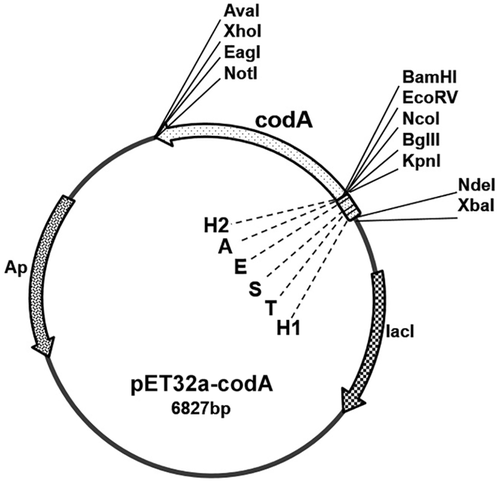
The resulting DNA fragment containing the tagged codA gene was cleaved with restriction endonucleases BamHI and NotI (Thermo Fisher Scientific Inc.) and cloned into the same sites of the expression vector pET32a (Novagen). Then, the NdeI fragment carrying the Trx Tag was cut out from the constructed plasmid. The structure of the pET32a-codA plasmid containing the gene coding for the tagged CDI is shown schematically in Figure 1. The nucleotide sequence of the cloned modified gene codA was verified by sequencing. The plasmid pET32a-codA was introduced into the E. coli strain BL21 (DE3) by electroporation.
Isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible expression of CDI in E. coli
Transformants of the E. coli BL21(DE3) were grown overnight at 37°C in LB medium containing ampicillin (50 mg/L). Next morning, the culture was diluted with fresh medium with the antibiotic to OD600 = 0.05 and the incubation was continued to OD600 = 0.4–1.0 (about 3 h). The CDI expression was induced with 0.4 mM IPTG for 2–3 h at 30°C before the cells were harvested by centrifugation (6,000g for 15 min at 4°C).
Purification of (His)6-tagged recombinant CDI
Preparation and solubilization of inclusion bodies were carried out according to the protocol of the Protein Refolding Kit (Novagen) with several modifications.
The induced cells were resuspended in 20 mM Tris-HCl buffer, pH 7.5, containing 1 mM ethylenediaminetetraacetic acid (EDTA) and 0.1% Triton X-100, treated with lysozyme (100 μg/mL) at 30°C for 15 min, and sonicated on ice until complete cellular lysis followed by a substantial decrease of the solution viscosity. The inclusion bodies were isolated from the crude cell lysate by centrifugation at 10,000g for 10 min at 4°C and washed twice with 20 mM Tris-HCl buffer, pH 7.5, containing 1 mM EDTA and 0.1% Triton X-100.
The inclusion bodies were resuspended in 50 mM Tris-SO4 buffer, pH 9.4, supplemented with 0.3% N-lauroylsarcosine, and incubated at room temperature for 30 min with gentle stirring. The supernatant containing the solubilized protein was clarified by centrifugation at 10,000g for 10 min at room temperature. The protein preparation from 200 mL of culture was stirred with 1 mL of settled Ni-NTA Superflow beads (Qiagen) for 1 h. The suspension was loaded to a column (0.6 × 7 cm) with the bottom outlet capped and beads were settled for 5–7 min. After removal of unspecific bound proteins by washing with 50 mM sodium phosphate buffer, pH 8.0, containing 300 mM NaCl and 2 mM β-mercaptoethanol, the tagged CDI was eluted by a step gradient of imidazole (20, 50, 100, 150, and 200 mM solution in the same buffer). The fractions exhibiting the highest CDI activity were pooled together, dialyzed against 50 mM sodium phosphate buffer, pH 8.0, supplemented with 300 mM NaCl and 2 mM β-mercaptoethanol, and used as the final CDI preparation. This procedure was developed after several preliminary experiments as described below.
CDI activity assay
CDI activity was determined in 50 mM K, Na-phosphate buffer, pH 7.5, containing 5 mM creatinine in a final volume of 1 mL. After incubation for 15–30 min at 37°C, the formation of ammonium was monitored fluorometrically after derivatization with o-phthaldialdehyde (OPA) in the presence of sodium sulfite, as described (Kuo et al., 2005, Stasyuk et al., 2016). One unit (U) of enzyme activity was defined as the amount of enzyme releasing 1.0 μmol of ammonium per 1 min under the above-mentioned conditions.
Enterokinase treatment
Treatment of the tagged CDI with enterokinase was carried out according to the recommended protocol (Sigma-Aldrich) with some modifications. One microgram of the purified tagged CDI was incubated with 0.2 units of enterokinase in 50 mM Tris-HCl buffer, pH 7.0, containing 2 mM CaCl2, in a final volume of 1.2 mL at 25°C. Thirty microliters of aliquots were withdrawn during the time course of incubation, frozen in liquid nitrogen and stored at −70°C.
Electrophoretic analysis of protein samples
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out as described (Laemmli, 1970). The gel was stained in 0.25% w/v Coomassie brilliant blue R-250 prepared in 50% v/v methanol and 10% v/v acetic acid. The gel was destained in 15% v/v ethanol and 7% v/v acetic acid.
Results and discussion
Cloning of C. glutamicum codA gene into E. coli expression vector pET32a
Database searches in the C. glutamicum ATCC 13032 genome sequence revealed the presence of a gene coding for a predicted 423 amino acids CDI. The gene was designated as codA (Kalinowski et al., 2003).
The open reading frame of codA gene of C. glutamicum PCM 1945 was cloned (in frame) into a pET32a vector, as described in Materials and Methods section. The Trx Tag encoding sequence was removed from the constructed plasmid to reduce changes to the N-terminal amino acid sequence, which could be involved in formation of an active oligomeric enzyme (Battchikova et al., 1996). The resulting plasmid pET32a-codA harbored the modified codA gene encoding C. glutamicum CDI polypeptide with several short tags fused to its N-terminus (Figure 1). The remaining tags were intended to provide additional approaches for protein purification and analysis.
Expression and purification of the tagged CDI
To induce the expression of CDI, cells of the E. coli strain BL21(DE3)/pET32a-codA carrying the constructed plasmid were incubated in the IPTG-containing selective growth medium following the protocol of the pET System Manual (Novagen). The highest expression level of the enzyme was achieved after 3 h of induction at 30°C. Increasing the temperature of induction to 37°C resulted in a lower yield of the enzyme (data not shown). The recombinant protein was accumulated as inclusion bodies in E. coli cells. The obtained protein preparation from these inclusion bodies contained an inactive CDI contaminated by other bacterial proteins (<40–50%; Figure 2A, lane 5).
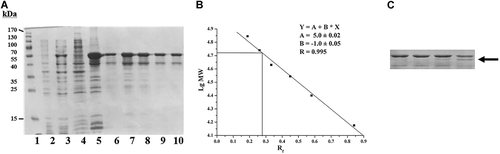
At first, the isolated inclusion bodies were dissolved in 50 mM sodium phosphate buffer, pH 8.0, containing 300 mM NaCl and 2 mM β-mercaptoethanol, supplemented with either 6 M urea or 6 M guanidine hydrochloride. Neither dialysis, nor fast dilution both of the protein solutions with the same buffer, nor chromatography on Ni-NTA resin (as described above) resulted in the enzyme reactivation (data not shown). Thus, we applied N-lauroylsarcosine as a solubilizing agent (see Materials and Methods section) and the obtained CDI preparation was used to refold the enzyme. No activity was detected after extensive dialysis against the same buffer without detergent. Fast dilution of the mixture with the same buffer without detergent resulted in protein aggregation, however, traces of CDI activity were detected. Decrease of the detergent concentration to 0.1–0.2% in the dissolving mixture did not lead to additional protein activation (data not shown). As the recombinant protein contained two (His)6-tags fused to the N-terminus of CDI, we used Ni-NTA chromatography to purify the protein and simultaneously remove the detergent as described in Materials and Methods section. The targeted CDI displayed high binding affinity to Ni-NTA resin. Only traces of the CDI were found in flow-through fractions after washing the column. The enzyme was then eluted by imidazole step gradient. The CDI appeared as a broad peak at 20–150 mM imidazole (Figure 2A, lane 6–10). The obtained highly purified enzyme preparation (Figure 2A, lane 7–8) exhibited a specific activity of 10–20 U/mg similar to the natural CDI purified from C. glutamicum ATCC 15590 (Uwajima and Terada, 1980). These results suggested that additional tags fused to the N-terminus did not affect renaturation of CDI, which appears as a homotetramer. The yield of CDI protein was ~30 mg/L of culture.
The identity of the isolated protein with C. glutamicum CDI was additionally proved by mass-spectrometry of the random set of tryptic peptides of the enzyme, as described for arginase (Zakalskiy et al., 2012). On the basis of the results of two-dimensional gel electrophoresis, the molecular weight of the CDI subunit is 46.3 kDa (Bendt et al., 2004). Using the sedimentation equilibrium method, the molecular weight of purified CDI of C. glutamicum ATCC 15590 was shown to be 195 kDa (Uwajima and Terada, 1980). Taking together these data, it was suggested that the active enzyme consists of four identical subunits encoded by gene codA.
The molecular weight of the recombinant tagged CDI subunit was calculated as 52.5 kDa using elecrophoretic mobility in SDS-PAGE (Figure 2B). Digestion of purified active CDI with enterokinase produced a protein of the expected molecular weight (~47 kDa; Figure 2C). The obtained results well correlated with the published data regarding the C. glutamicum ATCC 13032 CDI molecular weight (Bendt et al., 2004). Treatment with enterokinase was used in preliminary analytical experiments only. It caused a significant decrease in the amount of the targeted protein, this may be due to unspecific proteolysis (Figure 2C). Thus, we did not check the enzymatic activity after the enterokinase digestion and omitted this stage from all subsequent experiments.
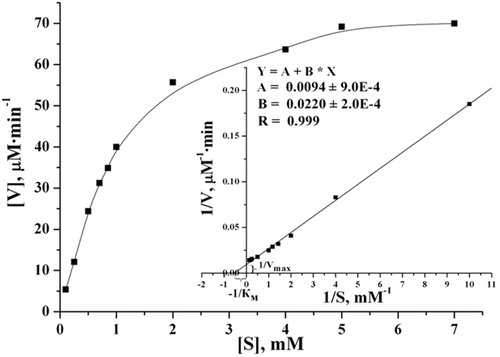
The purified tagged CDI possesses a relatively high affinity to creatinine under conditions reported for the natural enzyme (Uwajima and Terada, 1980). At 37°C and pH 7.5, KM for creatinine was determined as 1.96 ± 0.075 mM and Vmax was 114.6 ± 3.2 μM/min (at enzyme concentration of 5 μg/mL; Figure 3). Taking into account that the molecular weight of the native enzyme is ~195 kDa (Uwajima and Terada, 1980) the turnover number for the recombinant CDI was calculated as kcat = 74.5 ± 3.6 s−1. For comparison, CDI from C. glutamicum ATCC 15990 has KM for creatinine as 1.27 mM (at 37°C and pH 7.0) and Vmax as 45.4 μmol/min/mg of protein (that corresponds to kcat = 148 s−1) (Uwajima and Terada, 1980). With immobilized CDI, isolated from C. glutamicum, the KM value for creatinine was shown to be 1.9 mM (Tabata et al., 1983).
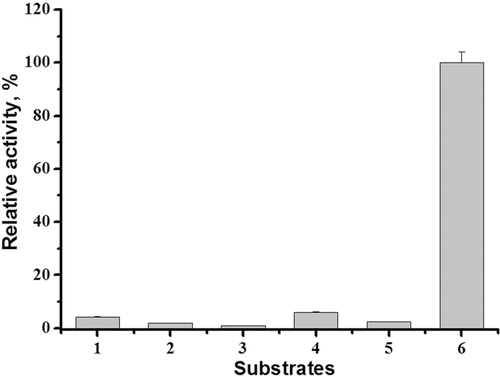
The purified tagged CDI, similar to the enzyme from C. glutamicum ATCC 15590 is specific for creatinine (Uwajima and Terada, 1980). It does not hydrolyze creatine, cytosine, cytidine, cytidine monophosphate, and cytidine triphosphate (Figure 4).
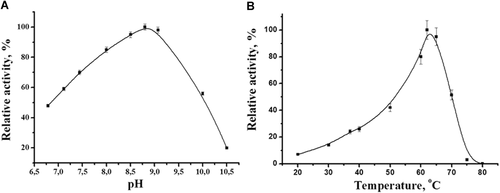
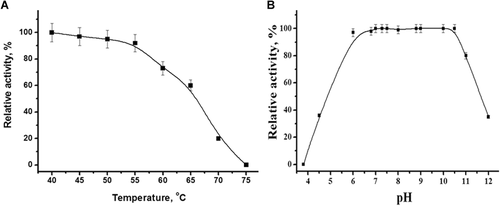
The purified tagged CDI exhibited the same activity at pH 7.9 in 50 mM K, Na-phosphate, 50 mM Tris-HCl and 50 mM Tris-SO4 buffers (data not shown). The highest activity was observed at pH 8.5–9.1 (Figure 5A). The temperature optimum was determined as 60–65°C (Figure 5B). It should be noted that the tagged enzyme retained 60% of its activity after prolonged incubation (60 min) at 65°C. The complete inactivation of the enzyme occurs at 75°C (Figure 6A). The enzyme was sufficiently stable over a wide range of pH from 6.0 to 10.5, while being incubated for 24 h in buffers of different composition (Figure 6B). These data well correlate with previously reported properties of the natural enzyme that possessed a broad pH optimum of 7.5–10.0, maximum activity at 60°C, and relatively high thermo- and pH-stability (Uwajima and Terada, 1980).
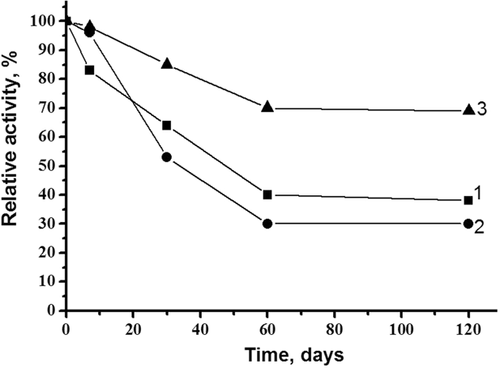
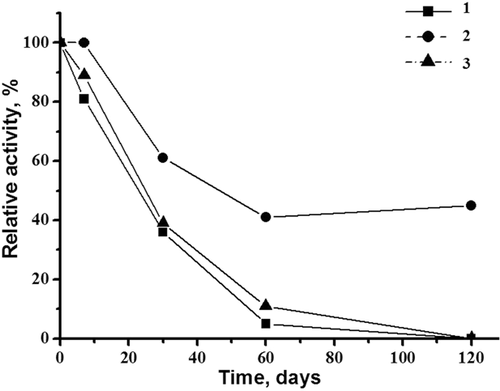
The tagged CDI appeared to be stable at 5°C in 50 mM K, Na-phosphate buffer, pH 7.5 for a week (Figure 7). During prolonged storage at −20°C in the same buffer, containing 20% glycerol, the enzyme lost only 30% of its activity within 4 months. Trehalose was found to be the most effective stabilizing agent during CDI lyophilization. This ensured maintenance of the enzyme's activity at 40% over 120 days of storage of the dried powder at 4°C (Figure 8). Additionally, CDI preparations can also be stored without significant loss of activity for at least 4 months as a suspension in 50 mM K, Na-phosphate buffer, pH 7.5 at 70% saturation with ammonium sulfate (data not shown) as was reported for the natural enzyme (Uwajima and Terada, 1980).
Thus, the main properties of the tagged enzyme, that is, specificity and affinity to creatinine, pH and temperature optimum, stability are similar to those of the natural CDI from C. glutamicum ATCC 15990. In addition, the recombinant enzyme can be more easily purified and with a higher yield.
Conclusions
The most prominent advantages of the proposed CDI preparation are a high yield of the enzyme, simple isolation of inclusion bodies, containing the enzyme, a possibility to apply the developed one-step procedure for renaturation-purification of the enzyme and its high stability under conditions designed. The main properties of the purified tagged CDI of C. glutamicum are similar to that of the natural enzyme. These findings can be used to develop a cost-efficient large-scale procedure for production of a highly specific targeted CDI.
Acknowledgment and funding
The authors are grateful to Dr. Daniel Broda (Rzeszow University, Poland) for helping to provide mass spectrometric studies of recombinant CDI. This work was financially supported by NAS of Ukraine (Program “Smart Sensory Devices of a New Generation Based on Modern Materials and Technologies”) and by the Ministry of Education and Science of Ukraine (projects Nos. 0119U100671, 0118U000297, and 0118U000809).
Author contribution
M.V.G., A.E.Z., and Y.R.B. conceived of the experiments and analyzed the data. A.E.Z., N.Ye.S., O.M.Z., and Y.R.B carried out the experiments. All authors contributed important ideas throughout the project and were involved in the preparation of the manuscript. All authors have read and approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.




