P2X receptors mediated abnormal interaction between satellite glial cells and neurons in visceral pathological changes
Abstract
The adenosine triphosphate (ATP)-gated P2X receptor cation channel family consists of permeable ligand-gated ion channels that expand on the binding of extracellular adenosine 5’-ATP. ATP-gated P2X receptors are trimer ion channels that assemble homo or isomer from seven cloned subunits. P2X receptors are discovered mostly in mammalian and are being found in an increasing number of non-vertebrates, such as zebrafish, bullfrog, and ameba. P2X receptors are involved in many physiological processes, including regulation of heart rhythm and contractility, and regulation of pain, especially chronic pain and glia integration. This review summarizes the current studies on the regulation of P2X receptors in abnormal neuronal-glial interaction and the pathological changes in viscera, especially in myocardial ischemia.
Abbreviations
-
- ATP
-
- adenosine triphosphate
-
- cDNA
-
- complementary deoxyribonucleic acid
-
- CGRP
-
- calcitonin gene-related peptide
-
- CK-MB
-
- creatine kinase-MB
-
- cTn-I
-
- cardiac troponin-I
-
- DRG
-
- dorsal root ganglia
-
- HR
-
- heart rate
-
- IL-6
-
- interleukin 6
-
- LDH
-
- lactic dehydrogenase
-
- SCG
-
- superior cervical ganglion
-
- SG
-
- stellate ganglia
-
- SGC
-
- satellite glial cell
-
- SP
-
- substance P
-
- TNF-α
-
- tumor necrosis factor-α
Introduction
P2X receptor can be found in all mammalian and a wide range of animal cells (Burnstock et al., 2010). It has a variety of functions, such as modulating the synaptic transmission between glial cells and presynaptic and post-synaptic nerve terminals, contracting the heart muscle cells, skeletal muscle cells, and various smooth muscle tissues, including blood vessels, vas deferens and bladder (Fowler et al., 2008). Since 1994, seven mammalian P2X complementary deoxyribonucleic acid (cDNA) (P2X1-P2X7) have been cloned (North, 2002). The expression of P2X receptor subtype on specific cell types has a certain subtype specificity. In this review, we have provided a connection between myocardial infarction and P2X receptors. Myocardial infarction often referred to as a heart attack, occurs when blood flow to a part of the heart decreases or stops, damaging the heart muscle. The most common symptom is chest pain or discomfort that can spread to the shoulders, arms, back, neck, or jaw (Valensi et al., 2011). Most myocardial infarctions are caused by coronary artery disease. Risk factors include high blood pressure, smoking, diabetes, lack of exercise, obesity, high cholesterol, poor diet, and excessive drinking (Mehta et al., 2015). Complete occlusion of the coronary arteries by atherosclerotic plaque rupture is often a potential mechanism of myocardial infarction. Myocardial ischemia can affect the function of cervical sympathetic ganglion cells through the afferent information of sensory nerve, and then enhance the activity of sympathetic ganglion neurons, causing sympathetic excitatory reflexes, such as increased blood pressure and increased heart rate (HR). 5’-Adenosine triphosphate (ATP) and P2X receptors are involved in the pathological changes of myocardial ischemia (Granado et al., 2015; Tu et al., 2016). This review summarizes the current studies on the regulation of P2X receptors on the pathological changes in viscera, especially in myocardial ischemia (Table 1).
| Myocardial ischemia and myocardial infarction | Visceral hyperalgesia | |
|---|---|---|
| P2X3 | Influence the transmission of myocardial ischemic injury (Liang et al., 2010; Li et al., 2011; Shcherbatko et al., 2016; Chen et al., 2016). | P2X3 receptor activation evoked bladder afferent activity in the mouse urinary bladder enhanced by TRPV1 (Grundy et al., 2018). |
| P2X2/3 | Increase systolic blood pressure and HR (Ando and Sperlagh, 2013; Liu et al., 2015ab). | Induce channel opening, membrane depolarization and the onset of pain signaling (Shcherbatko et al., 2016). |
| P2X4 | Play a protective role in heart failure (Yang et al., 2014; Yang et al., 2015). | The upregulated expression of P2X4 receptor in microglia cells can regulate the occurrence of neuropathic pain through the release of BDNF (Hao et al., 2018). |
| P2X7 | Increase the cervical superior sympathetic activity (Kong et al., 2013). | Activate spinal microglia and increase the chronic pancreatitis-related visceral hyperalgesia (Liu et al., 2015aa). |
- BDNF, brain-derived neurotrophic factor; HR, heart rate; TRPV1, transient receptor potential vanilloid subfamily member 1.
The heart function regulated by superior cervical ganglia and stellate ganglia
Sympathetic preganglionic neurons are located in the medial-lateral column of the first to fifth thoracic segments of the spinal cord. Postcardiac sympathetic ganglion neurons are located in superior cervical ganglion (SCG) and stellate ganglia (SG). The axons of sympathetic postganglionic neurons organize the cardiac plexus and supply various parts of the heart, including the sinus node, the atrioventricular junction, the atrioventricular bundle, the atrial muscle, and the ventricular muscle. SCG and SG not only transmit sympathetic ganglion signals, but also play an integral role in the regulation of autonomic function (Tu et al., 2013; Liu et al., 2014; Zhang et al., 2015; Merino-Jimenez et al., 2018). Thus, the cervical sympathetic ganglia can be viewed as a neural circuit that receives sensory visceral information and controls output to cardiovascular targets. Early myocardial ischemia often leads to increased sympathetic activity. Abnormal sympathetic excitatory response leads to increased HR and blood pressure, aggravating the myocardial ischemic injury (Liu et al., 2013) (Figure 1). Upregulated P2X receptor promoted the activation of satellite glial cells (SGCs) in the cervical sympathetic ganglia, resulting in sensory-sympathetic coupling formation and releasing creatine kinase-MB (CK-MB), lactic dehydrogenase (LDH) and cardiac troponin-I (cTn-I), tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) then facilitating sympathoexcitatory action (Liu et al., 2013).
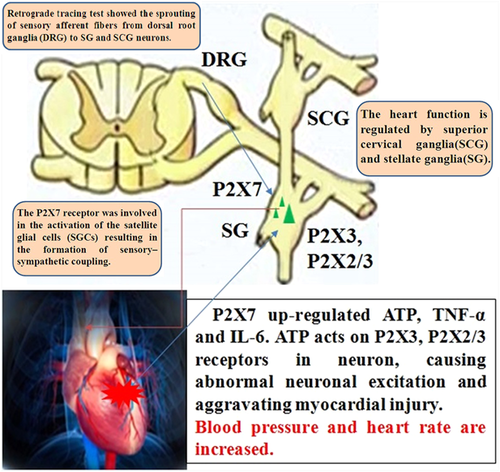
The heart function is regulated by superior cervical ganglia and stellate ganglia. Superior cervical ganglia (SCG) and stellate ganglia (SG) distribute the heart and regulate heart function. Retrograde tracing test showed the sprouting of sensory afferent fibers from dorsal root ganglia (DRG) to SCG and SG neurons. ATP, adenosine triphosphate; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α.
Upregulated P2X receptors mediate the abnormal interaction between neurons and glial cells in the cervical sympathetic ganglia
The ATP-gated P2X receptor cation channel family or P2X receptor family for short consists of permeable ligand-gated ion channels that open when extracellular ATP, which releases from the sympathetic ganglia and has a function in signal transduction, binds on P2X receptors (Burnstock, 2007; Burnstock et al., 2011; Tu et al., 2016). The upregulation of P2X3, P2X2/3, and P2X7 receptors in SCG and SG leads to the increase of blood pressure and HR by enhancing the activity of sympathetic postganglionic neurons (Ando and Sperlagh, 2013; Liu et al., 2015bb). Interaction between neurons and SGCs in sympathetic ganglia and the proliferation of P2X7 receptor increased the release of inflammatory factors by SGCs (Figure 2). After that, ATP acts on P2X3 receptor in neuron, causing abnormal neuronal excitation and aggravating myocardial injury.
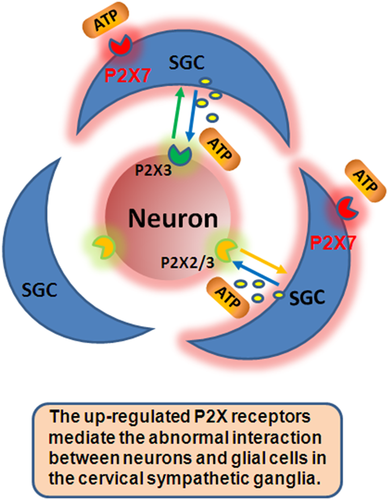
Upregulated P2X receptors mediate the abnormal interaction between neurons and satellite glial cells (SGCs) in the cervical sympathetic ganglia. The activation of SGCs facilitated the sympathoexcitatory reflex. ATP, adenosine triphosphate.
Glial cells express a variety of neurotransmitter receptors that can evoke physiological cellular signals and make cells sensitive to neurotransmitters in the extracellular space. The primary cell type of glial cells in most sympathetic ganglia is the SGC. SGC usually forms an envelope around a single neuron, which produces a unique functional unit composed of the neuron and its involved SGC (Liang et al., 2010). Previous studies in our laboratory showed that, at the condition after myocardial ischemia injury, the expression of P2X3 and P2X2/3 receptors in the SG and SCG was upregulated, thereby enhancing the post-sympathetic activity and increased blood pressure (Lohr et al., 2014; Arribas-Blazquez et al., 2019). Our study also showed that A-317491, an antagonist of P2X3 and P2X2/3 receptors, could reduce systolic blood pressure and HR and alleviate arrhythmia caused by myocardial ischemia (Li et al., 2010; Liang et al., 2010; Li et al., 2011). From our previous studies, we know that P2X3 can influence the transmission of the nociceptive signal (Shcherbatko et al., 2016; Fabbretti, 2019). ATP is released by SCG, and then ATP activates P2X3 and P2X2/3 receptors in SCG neurons to participate in the sympathetic excitatory response induced by myocardial ischemia (Liang et al., 2010; Li et al., 2011; Shcherbatko et al., 2016).
We have also found that the upregulation of the expression level of P2X7 receptor on the surface membrane of the SGCs promotes the activation of sensory-sympathetic coupling, which, in turn, promotes the sympathetic excitatory reflex (Kong et al., 2013; Tu et al., 2013; Liu et al., 2015aa). P2X7 receptor was expressed in the SGCs of SG and SCG. P2X7 receptor and tyrosine hydroxylase (TH, a sympathetic nerve marker) were co-expressed in the cervical sympathetic ganglia. Myocardial ischemia upregulated the expression of glutamine synthetase (GS, a marker of SGC) in the cervical sympathetic ganglia. Upregulated GS indicated the activation of SGCs. Enhanced P2X7 receptor was co-expressed with GS (Zuccarini et al., 2017). Retrograde tracing test showed the sprouting of calcitonin gene-related peptide (CGRP) or substance P (SP) sensory nerves (the markers of sensory afferent fibers) from dorsal root ganglia (DRG) to SCG neurons. The P2X7 receptor was involved in the activation of SGCs resulting in the formation of sensory-sympathetic coupling. The activation of SGCs facilitated the sympathoexcitatory action. There is increasing evidence that P2X7 receptor can lead the abnormal stimulation and increase the cervical superior sympathetic activity after myocardial ischemia injury (Kong et al., 2013). The activated P2X7 promotes the release of ATP to excite the P2X3 receptor in the neurons of the cervical sympathetic ganglia. Thus, the upregulated P2X receptors mediate the abnormal interaction between neurons and glial cells in the cervical sympathetic ganglia.
P2X receptors and myocardial ischemia or other visceral pathology
Myocardial ischemia and myocardial infarction
Coronary heart disease myocardial infarction is one of the common clinical diseases with high fatality rate. Myocardial ischemia produces a variety of chemicals, including ATP, which acts on the afferent nerves of the heart, exacerbating the incidence of myocardial infarction. Previous studies have shown that P2X3 receptor in the SCG and SG in the cardiac afferent nerve pathway is involved in the transmission of myocardial ischemic injury (Chen et al., 2016). The P2X7 receptor is a ligand-gated purine receptor activated by extracellular ATP and plays an important role in the physiology and pathophysiology of a series of diseases, including cardiovascular diseases (Granado et al., 2015; Martinez, 2019). P2X7 antagonist decreased the activation of P2X7 receptor on SGCs and inhibited primary sensory sprouting. Downregulation of the expression of the P2X7 receptor on the surface membrane of the SGCs decreased the activation of the sensory-sympathetic coupling, thus reducing the sympathetic excitatory reflex (Kong et al., 2013; Liu et al., 2013; Tu et al., 2013). By diminishing the activation of SGCs, P2X7 antagonist decreased the nociceptive transmission of sensory-sympathetic coupling between the cervical DRG nerves and the cervical sympathetic ganglion neurons after myocardial ischemia (Kong et al., 2013; Liu et al., 2013; Chen et al., 2018).
Acute myocardial infarction augmented the expression levels of microglia P2X7 receptor to stimulate a sympathoexcitatory response in model rats with acute myocardial infarction. Inhibiting pro-inflammatory cytokines, IL-1β and IL-6, and silencing the P2X7 receptor alleviated inflammatory responses and moderated the process of cardiac remodeling via influencing nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling (Thawkar and Kaur, 2019; Ding et al., 2019). Mice with the overexpression of cardiac-specific P2X4 receptor can be protected against heart failure (Yang et al., 2014). The existence of a physical P2X4 receptor nitric oxide synthase 3 (eNOS) interaction indicates the protective role of the endogenous cardiac myocyte P2X4 receptor in heart failure (Yang et al., 2015).
Visceral hyperalgesia
P2X3 and P2X2/3 receptors are expressed primarily in nociceptive neurons and increase in extracellular ATP concentrations under pathological conditions, such as tissue damage or visceral dilation, induce channel opening, membrane depolarization and the onset of pain signaling (Shcherbatko et al., 2016).
The co-expression levels of P2X7 receptor and glial fibrillary acidic protein (GFAP, a marker of SGCs) in DRG of the visceral pain model rats were significantly enhanced (Liu et al., 2015aa). Heat-sensitive moxibustion at Dachangshu acupoint decreased the upregulation of the P2X7 receptor and relieved the pathological injury of irritable bowel syndrome (IBS).
Colonic hypersensitivity induced the upregulation of P2X3, P2X4, and P2X7 receptors during the post-infectious period (Lashermes et al., 2018). Inhibited P2X3 receptors might reduce visceral hypersensitivity caused by IBS (Galligan, 2004; Weng et al., 2013; Deiteren et al., 2015; Hu et al., 2017). The increased expression levels of homomeric P2X4, P2X6, and P2X7 receptors and heteromeric P2X4/6, P2X4/7 receptors may relate to visceral hyperalgesia (Chen et al., 2016). After the downregulated expression of P2X2 and P2X3 receptors in DRG, the electroacupuncture treatment could decrease visceral hypersensitivity in rats with IBS (Weng et al., 2013). In 2012, Dr. Kanazawa found that the expression of P2X4 receptors in the posterior horn of the spinal cord increased in patients with IBS. Low-grade inflammation in the gut with IBS activates spinal microglia, resulting in upregulation of P2X4 receptor expression and cell activation (Keszthelyi et al., 2012). P2X4 receptor in microglia cells is involved in nociceptive transmission. After peripheral nerve injury, the upregulated expression of P2X4 receptor in microglia cells can regulate the occurrence of neuropathic pain through the release of brain-derived neurotrophic factor (BDNF) (Inoue, 2018).
Protease-activated receptor 4 (PAR4) is involved in the inhibition of visceral hyperalgesia. The downregulation of the P2X7 receptor in PAR4-activated mast cells inhibits visceral hyperalgesia via the MAPK signaling pathway (Hao et al., 2018). Chronic pancreatitis increased the co-expression levels of spinal P2X7 receptor and OX-42 (a microglia marker). Blocking the P2X7 receptor inhibited the activation of spinal microglia and reduced the chronic pancreatitis-related visceral hyperalgesia (Liu et al., 2015aa). Sensitization of vagal lung C-fibers is involved in the pathogenesis of airway hypersensitivity. P2X receptors are related to sensitization by pulmonary reactive oxygen species of rat vagal lung C-fibers (Ruan et al., 2014). Transient receptor potential vanilloid subfamily member 1 (TRPV1) and P2X receptors co-expressed in bladder sensory nerves and implicated in mechanosensation during bladder filling. P2X3 receptor activation evoked bladder afferent activity in the mouse urinary bladder enhanced by TRPV1 (Grundy et al., 2018).
Ischemia and reperfusion in bowel
Intestinal ischemia followed by reperfusion may occur following intestinal obstruction. Ischemia and reperfusion in the small intestine leads to structural changes accompanied by neuronal death. Following ischemia and reperfusion, neuronal density decreased by 22.6% in P2X7 receptor-immunoreactive neurons (Palombit et al., 2013). Following ischemia and reperfusion, the neuronal density decreased in the P2X2 receptor-immunoreactive neurons at 24 h and 1 week following injury compared with the densities in the control and sham groups (Marosti et al., 2014). Following ischemia/reperfusion in the intestine, a decrease in P2X2 receptor immunoreactivity in the cytoplasm and surface membranes of neurons of the myenteric and submucosal plexuses was found (Paulino et al., 2011). Therefore, P2X2 and P2X7 receptors were related to intestinal ischemia and reperfusion.
In summary
In this review, we summarize the heart function regulated by SCG and SG. It has been found that the upregulated P2X receptors mediated the abnormal interaction between neurons and glial cells in the cervical sympathetic ganglia. The P2X3 receptor in the SCG and SG was involved in the transmission of myocardial ischemic injury. The purinergic ATP-gated ion channels of P2X3 and P2X2/3 receptors were involved in nociceptive signaling and antagonists targeted on P2X receptors have shown promise in the clinic. The P2X4 receptors have protective roles in heart failure during myocardial ischemia and myocardial infarction, which results in the upregulation of P2X4 receptor expression and cell activation in visceral hyperalgesia. The P2X7 receptor was related to visceral hyperalgesia. P2X2 and P2X7 receptors were related to intestinal ischemia and reperfusion. Retrograde tracing test showed the sprouting of CGRP or SP sensory nerves (the markers of sensory afferent fibers) from DRG to SCG neurons. The P2X7 receptor was involved in the activation of SGCs resulting in the formation of sensory-sympathetic coupling. The activation of SGCs facilitated the sympathoexcitatory reflex. As time goes by, there will be more studies focus on P2X7 receptor than other P2X receptors due to its conclusive role in myocardial ischemia and other visceral pathology.
Funding
This work was supported by grants (Nos: 81870574, 8181101216, 81570735, 31560276) from the National Natural Science Foundation of China.
Conflict of interest
The authors declare that there is no conflict of interests.




