The role of microRNAs in the involvement of vascular smooth muscle cells in the development of atherosclerosis
Abstract
MicroRNAs (miRNAs) are a class of nonprotein-encoding RNAs of ~22 nucleotides in length that bind to or complement each other with a target gene messenger RNA (mRNA) to promote mRNA degradation or inhibit translation of the target mRNA. The protein required [such as Toll-like receptor (TLR) proteins] is controlled at an optimal level. By affecting protein translation, miRNAs have become powerful regulators of biological processes, including development, differentiation, cell proliferation, and apoptosis. MiRNAs are involved in the regulation of proliferation, migration, and apoptosis of vascular smooth muscle cells (VSMCs), thereby affecting the formation of atherosclerosis (AS). In recent years, the role and mechanism of miRNAs involved in AS development in VSMCs have been studied extensively. In the current study, the results and progress in miRNA research are reviewed.
Abbreviations
-
- 3′-UTR
-
- 3′-untranslated region
-
- AAA
-
- abdominal aortic aneurysm
-
- AKT
-
- protein kinase B
-
- AP-1
-
- activator protein-1
-
- ApoE
-
- apolipoprotein E
-
- AS
-
- atherosclerosis
-
- BMP
-
- bone morphogenetic protein
-
- CAL
-
- carotid artery ligation
-
- CAS
-
- carotid stenosis
-
- CHD
-
- coronary heart disease
-
- Cor
-
- corticosterone
-
- Dex
-
- dexamethasone
-
- ECM
-
- extracellular matrix
-
- ELN
-
- elastin
-
- FBN1
-
- fibrin -1
-
- GCs
-
- glucocorticoids
-
- H2O2
-
- hydrogen peroxide
-
- HVSMCs
-
- human vascular smooth muscle cells
-
- IRAK1
-
- IL-1 receptor-associated kinase 1
-
- KLF5
-
- kruppel-like factor 5
-
- Lox
-
- lysyl oxidase
-
- LRP1
-
- low-density lipoprotein receptor-related protein-1
-
- MiRNAs
-
- microRNAs
-
- MMP
-
- matrix metalloproteinase
-
- mRNA
-
- messenger RNA
-
- mTOR
-
- mammalian target of rapamycin
-
- MYOCD
-
- muscle-specific transcriptional coactivator (MyoCDin)
-
- NF-κBp65
-
- nuclear factor-κBp65
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PDCD4
-
- programmed cell death 4
-
- PDGF
-
- platelet-derived growth factor
-
- PDGF-BB
-
- platelet-derived growth factor-BB
-
- PGE2
-
- prostaglandin E2
-
- PI3K
-
- phosphatidylinositide 3-kinases
-
- PTEN
-
- phosphatase and tensin homolog deleted on chromosome ten
-
- qRT-PCR
-
- quantitative real-time reverse transcription polymerase chain reaction
-
- RAOSMCs
-
- aortic smooth muscle cells
-
- ROCK1
-
- rho-associated, coiled-coil-containing protein kinase 1
-
- SMAD-1/4
-
- SMAD Family Member 1/4
-
- SV-SMCs
-
- saphenous veins smooth muscle cells
-
- TGF
-
- transforming growth factor
-
- TGF-β
-
- transforming growth factor-β
-
- TLR
-
- Toll-like receptors
-
- TRAF6
-
- TNF receptor-associated factor 6
-
- UC
-
- ursolic acid
-
- VSMCs
-
- vascular smooth muscle cells
-
- YY1
-
- ying yang 1
Introduction
Atherosclerosis (AS) is a chronic inflammatory disease of the large arteries that is the main pathological feature of coronary heart disease (CHD) and one of the most common causes of cardiovascular disease (Bruikman et al., 2017). Involved components include cells, inflammatory signaling pathways, cytokines, and inflammatory factors (Fujisawa et al., 2017). Among the identified risk factors, the proliferation of vascular smooth muscle cells (VSMCs) is a major factor in the pathogenesis of AS (Bennett et al., 2016). Previous studies have demonstrated that the phenotypic transformation of VSMCs is a key initial step in VSMC proliferation, and abnormal proliferation of VSMCs is closely related to the development of AS. In addition, the migration of VSMCs in the vessel wall can cause plaque formation, leading to an AS hardening progress (Pan et al., 2018); Furthermore, it has been demonstrated that the dedifferentiated phenotype of VSMCs is a key event in the pathogenesis of various cardiovascular diseases, including AS, and is also the cause of in-stent restenosis and hypertension (Rzucidlo et al., 2007). In recent years, an increasing number of studies have demonstrated that miRNAs play a functional role in the process of AS by regulating VSMCs proliferation, indeed miR-365 inhibits the proliferation of VSMCs by targeting cyclin D (Kim et al., 2014; Feinberg and Moore, 2016; Wang and Atanasov, 2019).
The extracellular matrix (ECM) refers to a complex network composed of a variety of macromolecules surrounding the cells of a multicellular organism (Michel et al., 2010). The ECM is mainly composed of four types of components, namely collagen, non-collagen, elastin, proteoglycans, and glycosaminoglycans (Gao et al., 2014) (Figure 1). Typically, VSMCs are surrounded by a complex, highly structured ECM, consisting mainly of type I and III collagen, elastin, and proteoglycans (Stegemann et al., 2005). In chronic inflammation of AS, abnormal regulation of arterial VSMCs leads to increased dedifferentiation of VSMCs resulting in ECM synthesis in plaque areas (Chistiakov et al., 2015).
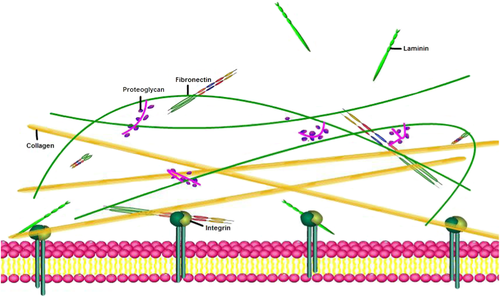
A schematic diagram of the extracellular matrix.
MiRNAs, or microRNAs, are endogenous, non-coding RNAs that are involved in the regulation of post-transcriptional gene expression in vivo, thereby regulating a variety of cellular characteristics, such as cell proliferation, cell migration, cell differentiation, and apoptosis (Barwari et al., 2016; Gao et al., 2016; Orlicka-Plocka et al., 2016). Abnormal miRNA expression has been shown to be associated with cardiovascular disease (Fan et al., 2017). The molecular mechanism by which miRNAs are involved in AS formation has gradually become a hot spot in cardiovascular research. In previous studies, it has been suggested that miRNAs may play a role in the phenotypic regulation of VSMCs and participate in the regulation of CHD (Cordes et al., 2009; Davis et al., 2009).
VSMC proliferation, migration, and apoptosis
miRNA-146a (miR-146a)
In previous studies, it has been confirmed that miR-146a is expressed in VSMCs and monocytes/macrophages, and acts on different target genes, including TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase 1 (IRAK1), and so forth, to regulate the development of AS (Cheng et al., 2013; Dong et al., 2013a). Dong et al. (2013a) demonstrated that miR-146a expression is increased in proliferative VSMCs, and that knockdown of miR-146a significantly inhibited the proliferation and migration of VSMCs in vitro, and induced apoptosis of VSMCs. Nuclear factor-κBp65 (NF-κBp65), proliferating cell nuclear antigen (PCNA), and Bax are key transcription factors and pro-inflammatory factors in vascular dysfunction and remodeling induction (Dong et al., 2013a). In previous experiments, it has been shown that the protein level of NF-κBp65 and PCNA was down-regulated after knockdown of miR-146a, while expression of the key pro-apoptotic molecule Bax was significantly increased; Moreover, western blot analysis showed that NF-κBp65 and PCNA were involved in miR-146a-mediated VSMC maturation and differentiation (Dong et al., 2013a). In addition, it has been shown that miR-146a can promote the proliferation and migration of VSMCs by up-regulating κBp65, while down-regulating the expression of Bax inhibited apoptosis of VSMCs (Dong et al., 2013a). MiR-146a can down-regulate the NF-κB activation through the negative feedback regulation of TRAF6 and IRAK1, and participate in the pathological process of AS and restenosis after vascular intervention through the regulation of NF-κB expression (Wu et al., 2016). Luo et al. revealed by microarray expression profiling that p53 protein in the p53 signaling pathway was up-regulated in rat VSMCs that were treated with a miR-146a inhibitor. However, p53 messenger RNA (mRNA) and protein expression levels in VSMCs transfected with miR-146a mimic significantly decreased, whereas mRNA and protein expression levels of p53 were increased in cells that were transfected with miR-146a inhibitor(Cyclo). Similarly, the expression levels of cyclin D1 mRNA and protein were significantly increased in VSMCs that were transfected with miR-146a, and the expression levels of cyclin D1 mRNA and protein were decreased after transfection with an miR-146a inhibitor, thereby suggesting that miR-146a may promote the proliferation of rat VSMCs by down-regulating p53 and up-regulating the expression of cyclin D1 (Luo et al., 2017) (Figure 2A).
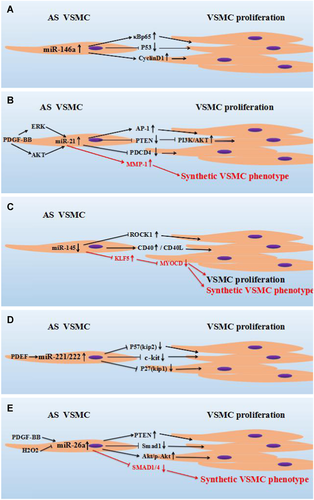
MiRNAs regulate the proliferation or phenotypic transformation of VSMCs by different molecules. (A) MiR-146a promoted the proliferation of VSMCs by up-regulating the expression of κBp65 and cyclin D, and promoted the proliferation of VSMCs by inhibiting expression levels of P53. (B) Circulating PDGF-BB regulated the expression of miR-21 by AKT and ERK signals. MiR-21 drives VSMCs into a synthetic phenotype by increasing MMP-1. PDCD4: Programmed Cell Death 4, AP-1: targeting activator protein-1. (C) The expression of miR-145 is decreased in AS, the decrease in miR-145 expression is accompanied by up-regulation of CD40 and ROCK1, and the proliferation of VSMCs is induced by up-regulated CD40 and ROCK1. In addition, miR-145 regulates VSMCs from a contractile state to a proliferative state through KLF5 and MYOCD. (D) PDGF-induced up-regulation of miR-221/222 expression in VSMCs, and miR-221/222 regulated the proliferation of VSMCs by inhibiting the expression of P57 (kip2), c-kit, and P27 (kip1). (E) Through the positive regulation of PDGF-BB and the negative regulation of H2O2, the expression of miR-26a in VSMCs was up-regulated, whereas the up-regulation of miR-26a regulated the increase in PTEN and Akt/p-Akt, and inhibited the expression of Smad1, thereby leading to the proliferation of VSMCs. H2O2, hydrogen peroxide; AKT, protein kinase B; AS, atherosclerosis; KLF5, kruppel-like factor 5; MiRNA, microRNA; MMP-1, matrix metalloproteinase 1; MYOCD; muscle-specific transcriptional coactivator (MyoCDin); PDCD4, programmed cell death 4; PDGF-BB, platelet-derived growth factor-BB; PTEN, phosphatase and tensin homolog deleted on chromosome ten; ROCK1, rho-associated, coiled-coil-containing protein kinase 1; VSMC, vascular smooth muscle cells.
miRNA-21 (miR-21)
MiRNA-21 exhibited high expression levels in VSMCs, and is also up-regulated in many cardiovascular diseases and is closely related with AS (Weber et al., 2010). Furthermore, studies have shown that overexpression of phosphatase and the tensin homolog deleted on chromosome ten (PTEN) inhibited PDGF-induced phosphorylation of p70 (s6k), Akt, and glycogen synthase kinases-3-α and β. Overexpression of PTEN significantly inhibited basal and PDGF-mediated proliferation and migration of VSMCs (Huang and Kontos, 2002), which may be a potential target for VSMC proliferation/AS treatment. It has previously been reported that the PTEN expression is regulated by miRNA-21 (Dong et al., 2013b), indeed Maegdefessel et al. (2012) suggested that the overexpression of miR-21 significantly reduced the expression of PTEN proteins, resulting in increased phosphorylation and activation of AKT, a component of pro-proliferative and anti-apoptotic pathways. Jiang et al. (2016) used ursolic acid (UC) to induce anti-proliferative effects in rat primary VSMCs. It has been demonstrated that miR-21 was significantly expressed at low levels, while PTEN expression levels (both mRNA and protein levels) were significantly increased. Furthermore, PTEN induced the proliferation of rat primary VSMCs and PTEN overexpression significantly decreased the expression of PI3K, indicating that miRNA-21 potentially interfered with the expression of PTEN and affected the proliferation of VSMCs through downstream PI3K/Akt/ mTOR signaling molecules (Jiang et al., 2016). Using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR), Lin et al. (2009) demonstrated that miR-21 was up-regulated in cardiomyocytes after treatment with hydrogen peroxide (H2O2). H2O2-induced cardiomyocyte death and apoptosis were increased by miR-21 inhibitors and decreased by pre-miR-21. Programmed cell death 4 (PDCD4) is regulated by miR-21, and is a direct target of miR-21 in cardiomyocytes. In addition, activator protein-1 (AP-1) is a downstream signaling molecule of PDCD4 that is involved in miR-21 mediated effects on cardiomyocytes. Moreover, Cheng et al. (2009) used microarray analysis to demonstrated that miRNAs were abnormally expressed in VSMCs after treatment with H2O2. H2O2-induced VSMCs apoptosis and death were increased by treatment with miR-21 inhibitors and decreased by pre-miR-21. PDCD4 is a direct target of miR-21 and is involved in miR-21 mediated effects on VSMCs. Furthermore, Activin 1 is a downstream signaling molecule of PDCD4 in miR-21 regulated VSMCs (Cheng et al., 2009). Platelet-derived growth factor-BB (PDGF-BB) can induce cell dedifferentiation and transform into a synthetic type, which increased cell proliferation and migration. Alshanwani et al. show that PDGF-BB increased the expression of miR-21 through the P13K/AKT pathway, while overexpression of miR-21 altered the phenotypic and functional changes of saphenous vein smooth muscle cells (SV-SMCs); matrix metalloproteinase 1 (MMP-1) is a target gene of miR-21 in the SV-SMCs identified by microarray, and overexpression of miR-21 leads to an increase in the expression level of MMP-1 mRNA, which in turn drives SMCs into a synthetic form, thereby further promoting the development of AS (Gan et al., 2013; Alshanwani et al., 2018) (Figure 2B).
miRNA-145 (miR-145)
MiR-145 is a key regulator of VSMCs phenotype conversion, which can transform VSMCs from a contractile phenotype to a synthetic phenotype, thereby increasing the dedifferentiation, proliferation, and migration ability of VSMCs, promoting the development of AS or causing blood vessel restenosis (Zhang, 2009; Higashi et al., 2015). In the mouse carotid artery ligation (CAL) model, Han et al. (2018) demonstrated that the expression of miR-145 was significantly decreased when compared with miR-145 expression in the carotid artery of the control group. Overexpression of miR-145 in VSMCs was found to significantly inhibit the proliferation of VSMCs in vitro. In vivo, overexpression of miR-145 significantly inhibited neointimal formation in the CAL model, indicating that miR-145 expression levels were significantly reduced in carotid stenosis (CAS) and played a key role in CAS by regulating the proliferation of VSMCs. Chen et al. (2018) also showed that high concentrations of glucose can stimulate the proliferation and migration of VSMCs, and that overexpression of miR-145 eliminated these changes in high glucose-induced VSMCs. Further studies revealed that overexpression of miR-145 significantly decreased ROCK1 mRNA and protein expression levels, and that miR-145 directly targeted the 3′-untranslated region (3′-UTR) of ROCK1, thereby inhibiting ROCK1 expression through interaction, indicating that the overexpression of miR-145 attenuated high glucose-induced VSMC proliferation and migration by reducing ROCK1 expression (Chen et al., 2018). In vitro studies have shown that CD40/CD40L interaction leads to activation of VSMCs and expression of its adhesion molecules, which is an important step in AS initiation (Mach et al., 1997; Lutgens et al., 1999). The decrease in miR-145 level is accompanied by up-regulation of CD40, suggesting that the role of miR-145 in regulating the VSMC phenotype partly depends in part on CD40 (Schonbeck et al., 2002; Guo et al., 2016). Zhang et al. show that kruppel-like factor 5 (KLF5) is a target gene of miR-145. In the atherosclerotic aorta, miR-145 was expressed at a low level, and the expression of its target gene, KLF5, was significantly increased, whereas the expression level of the smooth muscle-specific transcriptional coactivator (MyoCDin, MYOCD) was significantly decreased. Transduction of VSMCs with an miR-145 inhibitor revealed a significant proliferative state of VSMCs, combined with a decreased expression of calmodulin and α-SMA. These findings demonstrated that in AS, miR-145 regulated the VSMCs phenotype from a contractile state to a proliferative state via regulated KLF5 and MYOCD (Cheng et al., 2009; Zhang et al., 2016), and induced the formation of AS (Figure 2C).
miRNA-221 and miRNA-222 (miR-221/miR-222)
In contrast to miR-145, the expression levels of miR-221 and miR-222 were increased in the neointimal injury response. In vascular biology, miR-221 and miR-222 have been shown to be important players in the proliferation of VSMCs (Chistiakov et al., 2015). Liu et al. (2012b) showed that inhibition of miR-221/222 expression increased endothelial regeneration and neointimal formation, therefore, miR-221/222 may be a promising therapeutic target for AS patients. In previous studies, the relationship between miR-221/222 and targets involved in the pathogenesis of AS has been studied (Xue et al., 2015). MiR-221/222 regulates multiple functions of cell proliferation through multiple targets including p27 (Kip1), p57 (Kip2), and c-kit (Liu et al., 2009). Overexpression of p27 and p57 inactivated the cyclin-CDK complex and led to cell cycle arrest, which negatively correlated with VSMCs proliferation (Yu and Li, 2014). Bildirici et al. (2018) demonstrated that the expression of miR-221 was increased (more than six times) in blood samples from patients with significant AS, and the proliferation of VSMCs was promoted by inhibiting its targets p27 (Kip1) and p57 (Kip2). Furthermore, it was discovered that in vivo knockdown of miR-221/miR-222 weakened the inhibition of p27 (Kip1) and p57 (Kip2), which inhibited the proliferation of VSMCs. When the expression of miR-221/222 in VSMCs increased, the expression level of p27Kip1 decreased, the proliferation and migration of VSMCs increased, and the intimal thickening increased after arterial injury (Liu et al., 2012a). Platelet-derived growth factor (PDGF) is an effector that reduces expression of the target genes c-Kit and p27Kip1, and down-regulates the expression of VSMC specific contractile genes (Liu et al., 2012b; Chistiakov et al., 2015). In addition, miR-221/222 also targets c-Kit or the anti-PI3K signaling molecule PIK3R1 and causes down-regulation of angiogenesis (Dai et al., 2007b; Chistiakov et al., 2015). It was found that PDGF can induce the expression of miR-221 and miR- 222 in VSMCs. Similarly, in vitro studies have shown that miR-221 and miR-222 are involved in the regulation of PDGF-mediated proliferation of VSMCs (Davis et al., 2009) (Figure 2d).
miRNA-26a (miR-26a)
MiR-26a is a highly conserved post-transcriptional regulator of various cellular processes, including proliferation, differentiation, migration, and apoptosis. MiR-26a promoted VSMC proliferation, inhibited cell differentiation and apoptosis, and altered the TGF-β pathway signaling conduction (Leeper et al., 2011). Tan et al. (2017) reported that miR-26a is involved in regulating the proliferation of VSMCs in neointimal hyperplasia. PDGF-BB is a key regulator in the VSMCs phenotypic transformation (Raines, 2004). Yang et al. (2017) show that after treatment of VSMCs with PDGF-BB, the differentiation marker gene (such as α-SMA) in VSMCs was significantly decreased. Upon transfection of miR-26a in VSMCs, miR-26a partially abolished the VSMC differentiation marker gene PDGF-BB expression. Moreover, PDGF-BB significantly promoted the proliferation and migration of VSMCs, while knockdown of miR-26a significantly inhibited the effect of PDGF-BB on VSMCs proliferation and migration. Thus, these results confirmed that miR-26a regulated the transformation of the PDGF-BB-mediated VSMCs phenotype. Smad1 is a key signal transduction molecule of the bone morphogenetic protein (BMP)/transforming growth factor-β (TGF-β) pathway and is involved in the induction of the VSMC contractile phenotype (Lagna et al., 2007). MiR-26a targets the 3′-UTR of Smad1 mRNA and down-regulates Smad1 expression in VSMCs (Icli et al., 2013). In previous studies,it was shown that Smad1 protein expression was significantly decreased in balloon-injured rat arteries and PDGF-BB-treated VSMCs. Anti-miR-26a attenuated the inhibitory effect of PDGF-BB on Smad1 expression; Smad1 overexpression partially reversed PDGF-BB inhibited differentiation markers in VSMCs, while Smad1 knockdown partially rescued phenotypic changes in VSMCs in cells transfected with anti-miR-26a (Yang et al., 2017). Thus, it was confirmed that miR-26a had a regulatory effect on PDGF-BB-mediated Smad1 expression in VSMCs. Peng et al. (2018) show that miR-26a expression was down-regulated in VSMCs after H2O2 stimulation. However, up-regulation of miR-26a attenuated H2O2-induced cell damage by enhancing cell viability, and inhibiting caspase-3 activity, apoptosis, and reactive oxygen species production. PTEN is a direct target of miR-26a in VSMCs, and overexpression of PTEN by a pcDNA-PTEN plasmid significantly inhibited the protective effect of miR-26a overexpression on H2O2-induced cell damage. Further studies revealed that miR-26a mediated anti-VSMCs apoptosis by activating the AKT/mTOR pathway [e.g., phosphorylation (p-) of AKT and up-regulation of p-mTOR to reduce apoptosis in VSMCs]. The Akt inhibitor API-2 can reverse the anti-apoptotic effect of miR-26a on VSMCs, thereby having a protective effect on VSMCs. Together, these results indicated that miR-26a protected VSMCs from H2O2-induced damage by activating the PTEN/AKT/mTOR pathway (Peng et al., 2018). SMAD1 and SMAD4 are two TGF-β and BMP-associated differentiation-promoting factors, and overexpression of miR-26a inhibited VSMCs differentiation and helped maintain cell balance in both synthetic and contractile states. In addition, miR-26a controlled phenotypic transitions by directly targeting SMAD1 and SMAD4. Inhibition of miR-26a increased gene expression of SMAD1 and SMAD4, while overexpression of miR-26a inhibited SMAD1 (Leeper et al., 2011; Yu and Li, 2014) (Figure 2e).
Other miRNAs
Another highly expressed miRNA in the vasculature is miR-133, which is down-regulated following vascular injury and proliferating VSMCs. MiR-133 inhibits the proliferation of VSMCs and inhibits the conversion of VSMCs to a synthetic phenotype by inhibiting PDGF and the transcription factor Sp-1. Therefore, the overexpression of miR-133 in injured arteries reduced neointimal formation and reduced the proliferation of VSMCs (Torella et al., 2011). In a recent study, it was shown that overexpression of miRNA-365b-3p up-regulated P21 and P27 expression and induced cell cycle arrest in the G1phase and apoptosis (Wang et al., 2013). Qu et al. demonstrated that miRNA-365b-3p directly binds to the 3′-UTR of ADAMTS1 mRNA in human vascular smooth muscle cells (HVSMCs), suggesting that ADAMTS1 is a target gene of miRNA-365b-3p; PDGF-BB significantly promoted the proliferation and migration of HVSMCs, and it was shown that the expression of disintegrin and metalloproteinase of ADAMTS1 were elevated, whereas the expression of miRNA-365b-3p was inhibited following 6 h of treatment of HVSMCs with PDGF-BB. In HVSMCs transfected with miRNA-365b-3p analogs, the proliferation of HVSMCs was significantly attenuated. Likewise, overexpression of miRNA-365b-3p directly targeted down-regulation of ADAMTS1 and inhibited the proliferation of PDGD-BB-induced HVSMCs (Qu and Zhang, 2018). Yue et al. (2018) demonstrated that overexpression of miRNA-147b enhanced the proliferation and migration of VSMCs and increased Wnt/β-catenin signaling activity in VSMCs. By knocking down miRNA-147b, it has been shown that the proliferation and migration of PDGF-BB-treated VSMCs or VSMCs were significantly inhibited. In VSMCs, ying yang 1 (YY1) is the target of miRNA-147b. The increase of YY1 inhibits the proliferation and migration of VSMCs cells and attenuates the effect of miRNA-147b overexpression on the proliferation and migration of VSMCs. This study demonstrates that miRNA-147b affects VSMC proliferation and migration by targeting YY1 and regulating Wnt/β-catenin activity (Yue et al., 2018).
In summary, major miRNAs are involved in the development of AS, which is mediated by VSMC proliferation and phenotypic conversion (Figure 3) (Table 1).
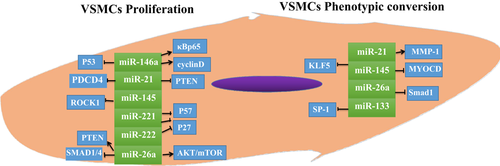
Proliferation and phenotype-specific gene expression in VSMCs is mediated by miRNAs. MiRNAs mediate proliferation and phenotypic transformation of VSMCs through a variety of molecules. Up- or down-regulation of miRNA expression affects the proliferation rate of VSMCs. KLF5, kruppel-like factor 5; miRNA, microRNA; MMP-1, matrix metalloproteinase 1; MYOCD, muscle-specific transcriptional coactivator (MyoCDin); NF-κBp65, nuclear factor-κBp65; PTEN, phosphatase and tensin homolog deleted on chromosome ten; VSMC, vascular smooth muscle cell.
| Function | MiRs | Targets | Expression |
|---|---|---|---|
| Promote proliferation | miR-21 | PTEN↓ | ↑ |
| miR-26a | PTEN ↑ SMAD-1/4↓ Akt↑ | ↑ | |
| miR-146a | κBp65 Cyclin D1↑ | ↑ | |
| miR-221/222 | P27(Kip1) P57(Kip2)↓ | ↑ | |
| Inhibit proliferation | miR-133 | SP-1↓ | ↑ |
| miR-145 | KLF5↑ | ↓ | |
| Promote contractile phenotype | miR-133 | SP-1↓ | ↑ |
| miR-145 | KLF5↑ | ↓ | |
| Promote synthetic phenotype | miR-26a | SMAD1/4↓ | ↑ |
| miR-221/222 | c-kit P27(Kip1) P57(Kip2)↓ | ↑ | |
| Dual effect | miR-21 | PDCD4↓ | ↑ |
| Promote migration | miR-26a | SMAD-1/4↓ | ↑ |
| miR-133 | SP-1↓ | ↑ | |
| Inhibit migration | miR-145 | KLF5↑ | ↓ |
- KLF5, kruppel-like factor 5; PDCD4, programmed cell death 4; PTEN, phosphatase and tensin homolog deleted on chromosome ten; SMAD-1/4, SMAD Family Member 1/; VSMC, vascular smooth muscle cell.
VSMC ECM synthesis
Zhao et al. (2015) showed that although the deletion of certain miRNAs in mice was not fatal, it was accompanied by reduced smooth muscle stress fiber formation and an increased rough endoplasmic reticulum, resulting in the disappearance of the contractile function of VSMCs cells, decreased migration and proliferation from the tunica media to the intima, and the secretion of a large amount of ECM components (Table 2).
| Function | MiRs | Targets | Expression |
|---|---|---|---|
| Inhibit ECM synthesis | miRNA-145 | TGF signal transduction genes↑ | ↓ |
| miRNA-29b | MMP↓ | ↑ | |
| miRNA-29c | ↑ | ||
| miRNA-205 | LRP1↓ | ↑ |
- ECM, extracellular matrix; LRP1, low-density lipoprotein receptor-related protein-1; MMP, matrix metalloproteinase; TGF, transforming growth factor; VSMC, vascular smooth muscle cells.
miRNA-145
Transforming growth factor (TGF) is an inducer of smooth muscle differentiation, and previous studies have shown that TGF can activate the expression of miR-145. Zhao et al. (2015) demonstrated that miR-145 preferentially targets TGF signal transduction genes. In VSMCs, TGF activated stromal genes and smooth muscle-specific genes; however, miR-145-specific weakened stromal genes were decreased (Zhao et al., 2015). Lysyl oxidase (Lox) can cross-link the adjacent collagen trihelix, provide collagen fibrillary tensile strength, and promote neointimal growth through the chemotaxis activity of VSMC and monocytes, which is negatively regulated by miR-145 (Yu and Li, 2014). ApoE, which inhibited the expression of ECM gene, reduced the level of Lox by increasing the expression of miR-145 (Kothapalli et al., 2012; Yu and Li, 2014).
miRNA-29
The miR-29 family can accelerate fibrosis of various tissues by regulating downstream ECM genes (van Rooij et al., 2008), including a variety of collagenous homotypes (COL1A1, COL5A1, COL3A1) and aortic wall components, such as elastin (ELN) and fibrin -1 (FBN1). MMP is a type of proteolytic enzyme, whose activity is dependent on Zn2+ and Ca2+, and can degrade the ECM. An imbalance in ECM homeostasis may lead to several pathological conditions, including fibrosis and carcinogenic invasion. MMPs (such as MMP2 and MMP-9) have been identified as a direct target gene of miR-29b, and Han et al. (2018) showed that miR-29b can participate in the development of abdominal aortic aneurysm (AAA) by changing the ECM microenvironment and by inhibiting the expression of ECM-related proteins. It has also been shown that when compared with untreated cells, treatment of SMCs with prostaglandin E2 (PGE2) increased the expression of miR-29b, and thereby inhibited the expression of ECM-related genes. Glucocorticoids (GCs) can inhibit the expression of MMP-9 (Eberhardt et al., 2002), and differentially affect the expression of fibronectin and type IV collagen (Zhou et al., 1998; Gu et al., 2001). Chuang et al. (2015) demonstrated that dexamethasone (Dex) and corticosterone (Cor) increased miR-29c expression in aortic smooth muscle cells (RAOSMCs) in a time-dependent manner, and reduced protein expression of type III collagen (Col3A1), type IV collagen (Col4A5), ELN, and MMP2. In a nutshell, the anti-fibrosis effect of GCs on blood vessels is at least partly induced by miR-29c as miR-29c targets and down-regulates ECM-related protein components (Chuang et al., 2015).
miRNA-205
Chan et al. (2017) show that the expression of low-density lipoprotein receptor-related protein-1 (LRP1) was significantly down-regulated in VSMCs overexpressing miR-205 (P < 0.05), while the expression of LRP1 mRNA remained unchanged (Chan et al., 2017). In addition, the LRP1 silencing in VSMCs showed an exogenous MMP-9 clearance rate that was significantly lower (P < 0.05). In addition, pretreatment with an anti LRP1 antibody completely eliminated the MMP-9 clearance rate, indicating that the down-regulation of LRP1 in VSMCs may hinder the ability of MMP-9 around the cells, which results in excess MMP-9, thereby damaging the integrity of the vessel wall (Chan et al., 2017).
Studies on the effects of miRs on ECM synthesis by VSMCs are limited, therefore further research is needed.
Conclusion
AS is the pathological basis of various ischemic diseases. The phenotypic changes of VSMCs and induced proliferation are critical to the formation of AS. In recent years, with the deepening of basic research, increased evidence has indicated that various miRNAs participate in the induction of AS by affecting the transformation of the VSMCs phenotype, thereby playing an important role in regulating AS. Recent studies have shown that miRNAs can be used as potential targets for the treatment of AS, which can also be used to predict the progression of atherosclerotic disease and the occurrence of cardiovascular events (Lu et al., 2018). Further in-depth research should be pursued to identify potential molecular targets for atherosclerotic disease and provide novel evidence for the intervention, diagnosis, and treatment of clinical atherosclerotic disease.
Acknowledgments and funding
The author thanks Prof. Tang and Dr. Li from the Department of Cardiology, Zhongda Hospital affiliated to Southeast University for reviewing this review. This work was supported by research grant (H0203) from the Natural Science Foundation of China (NSFC).
Conflict of interest
The authors report no conflicts of interest.




