SIRT1 expression is refractory to hypoxia and inflammatory cytokines in nucleus pulposus cells: Novel regulation by HIF-1α and NF-κB signaling
Abstract
Hypoxia and a marked increase in inflammatory cytokines are common hallmarks of intervertebral disc degeneration; these events disrupt the normal balance between extracellular matrix (ECM) degradation and synthesis in degenerative intervertebral discs. SIRT1, one of the NAD+-dependent class III histone deacetylases, controls cellular processes and is regulated by hypoxia and inflammatory cytokines in a cell-type-dependent manner. SIRT1 protects degenerative human nucleus pulposus cells against apoptosis. However, the role of SIRT1 in inflammation in intervertebral discs is still unclear. The current study showed that in rat NP cells, as in other cells, SIRT1 suppressed the induction of the mRNA expression of proteases that degrade ECM induced by TNF-α. Moreover, real-time PCR, transfection, and loss- and gain-of-function experiments revealed that SIRT1 mRNA and protein expression were refractory to hypoxia and HIF-1α. Additionally, SIRT1 mRNA and protein expression and the activity of the SIRT1 promoter were not affected by inflammatory cytokines but were sustained by NF-κB signaling in the presence or absence of TNF-α. In summary, the present study suggested that SIRT1 is not affected by hypoxia and inflammatory cytokines in rat intervertebral discs. Moreover, not HIF-1α but NF-κB signaling is critical for the maintenance of SIRT1 expression in NP cells under physiologic and pathophysiologic conditions.
Abbreviations
-
- AF
-
- annulus fibrosu
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- ECM
-
- extracellular matrix
-
- IL-1β
-
- interleukin-1β
-
- IVDD
-
- intervertebral disc degeneration
-
- NF-κB
-
- nuclear factor-κB
-
- NP
-
- nucleus pulposus
-
- SIRT1
-
- silent mating type information regulator 2 homolog 1
-
- TNF-α
-
- tumor necrosis factor α
Introduction
Low back pain, mainly resulting from intervertebral disc degeneration (IVDD), is one of the most common musculoskeletal disorders. Almost 80% of the population experiences some form of low back pain at some point (Luoma et al., 2000). The intervertebral disc consists of two distinct components: the outer, firm annulus fibrosus (AF) and the inner, gel-like core, the nucleus pulposus (NP). In the normal intervertebral disc, the extracellular matrix (ECM), including collagen II and proteoglycans, undergoes a process of remodeling that relies on a delicate balance between matrix synthesis and degradation. However, in a degenerative intervertebral disc, the normal balance is disrupted, and the ECM is broken down due to an increase of matrix-degrading proteinase activity (Gruber and Hanley, 1998).
Silent mating type information regulator 2 homolog 1 (SIRT1), a member of the NAD+-dependent class III histone deacetylases, deacetylates histones, and non-histone targets, including p53 and nuclear factor-κB (NF-κB) (Vaziri et al., 2001; Yeung et al., 2004). SIRT1 controls a broad range of cellular processes, including cell apoptosis, autophagy, and inflammation (Takayama et al., 2009; Moon et al., 2013; Ou et al., 2014). In human intervertebral discs, SIRT1 expression significantly increases with age and is elevated in the early degeneration stage (Zhang et al., 2011). The effect of SIRT1 on the activity of the ECM metabolism and the proliferation of NP cells depends on the grade of disc degeneration (Zhang et al., 2011). Moreover, SIRT1 protected degenerative NP cells against apoptosis by promoting autophagy or by phosphorylating Akt (Wang et al., 2013; Jiang et al., 2014).
The hallmarks of intervertebral disk degeneration are hypoxia and the apparent increase in proinflammatory cytokines, mainly tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β), which promote the expression of many catabolic enzymes (Takahashi et al., 1996; Burke et al., 2003; Xu et al., 2015; Ye et al., 2015). In hypoxia, the expression of SIRT1 increases, and it depends on HIF-1α (Chen et al., 2011; Hong et al., 2012). In addition, SIRT1 activation decreases the proinflammatory effects induced by TNF-α and IL-1β in many types of cells, including chondrocytes and fibroblasts (Zhu et al., 2011; Matsushita et al., 2013; Moon et al., 2013). However, the regulation of SIRT1 expression by inflammatory cytokines is cell type-dependent (Zhang et al., 2010; Serrano-Marco et al., 2012; Katto et al., 2013). For example, in vascular smooth muscle cells, TNF-α induces SIRT1 expression in a time- and dose-dependent manner, and the p65/RelA subunit of the transcriptional factor NF-κB mediates the TNF-α-induced elevated expression of SIRT1 (Serrano-Marco et al., 2012). Moreover, overexpression of several NF-κB subunits, including p65/RelA and p50, enhances the expression of SIRT1 in HEK293T-cells (Katto et al., 2013).
In the intervertebral disc, although SIRT1 protects degenerative human NP cells against apoptosis in vitro and plays a protective role in IVDD in vivo (Wang et al., 2013; Jiang et al., 2014; Xia et al., 2015), the SIRT1 expression response to hypoxia and inflammatory cytokines has not been completely elucidated. The overall objective of the present study was to reveal how SIRT1 is regulated in NP cells under inflammatory and hypoxia conditions.
Materials and methods
Reagents
To assay the promoter activity, the 5′-flanking region of the SIRT1 gene was inserted into the firefly luciferase reporter vector, pGL3-Basic (Promega), which contained no eukaryotic promoter or enhancer element. To construct the promoter, genomic DNA was isolated from 293T cells using a Gentra genomic DNA isolation kit (Qiagen, Valencia, CA, USA). PCR was used to amplify the 2.3 kb SIRT1 promoter (−2300/ + 15 SIRT1-Luc, forward primer, GGCGCCTCGAGTTGACATTTGCTATGAGC and reverse primer, CAAGCTTGTCTTGCCTGCCTCCTTGTAGGT) with Xho I and Hind III restriction enzyme sites. The plasmid pGL3-SV40 (Promega) contained the firefly luciferase gene driven by the SV40 promoter as a positive control. The plasmids RelA/p65 (no. 20012), RelB (no. 20017), p50 (no. 20018), c-Rel (no. 20013), and pCMX-IkBαM (no. 12330) developed by Dr. Inder Verma; HA-HIF-1α (no. 18955) developed by Dr. William G. Kaelin; and siHIF-1α (no. 21103) developed by Dr. Connie Cepko were obtained from the Addgene Company. The plasmids PLKO.1, pRL-TK, psPAX2, and pMD2.G were generously shared by Dr. D Xiao, Nanfang Medical University, China (Wang et al., 2014). The p65, IKKb, and HIF-1α shRNAs were provided by Dharmacon Research, Inc. (Lafayette, CO, USA). The knockdown sequences are 5′-AAACCCAGGGCTGCCTTGGAAAAG-3′ (p65 shRNA), 5′-ATGTTCAAGATATGAACCAGC-3′ (IKKβ shRNA), and 5′-AAACCCAGGGCTGCCTTGGAAAAG-3′ (HIF-1α shRNA). The Calbiochem Company (Danvers, MA, USA) provided SM7368 (an NF-κB inhibitor), sirtinol (a SIRT1 inhibitor), and resveratrol, and the Peprotech Company (Rocky Hill, NJ, USA) supplied the TNF-α and IL-1β. The rabbit IgG conjugated with horseradish peroxidase and the GAPDH, β-tubulin, SIRT1 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA) and HIF-1α antibody from R&D Company (Lorton, VA, USA).
The isolation, culture, and treatment of NP cells
In accord with the Institutional Review Board guidelines of Sun Yat-sen University, human NP tissue samples were obtained from patients suffering from thoracolumbar fractures. Each patient consented to the sample collection. The Experimental Animal Center of Sun Yat-sen University provided the Sprague–Dawley rats, and the Animal Care and Use Committee of the Sun Yat-sen University approved the experimental procedures.
Human and rat NP cells were obtained as described previously (Ye et al., 2013; Xu et al., 2015). After the Sprague–Dawley rats were euthanized with an overdose of pentobarbital and the lumbar intervertebral discs of the rats were collected, the rat NP cells were isolated. Using a microscope, the NP tissues were separated from the AF tissues and then cut into small pieces. Next, the small pieces were digested with pronase medium and cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C in a 5% CO2 incubator. After reaching 80% confluence, the NP cells were stimulated by hypoxia or TNF-α and IL-1β. For the inhibition experiments, 1 h before TNF-α or IL-1β, the inhibitors were added to the cell culture. RNA and protein were extracted from the cells at the specified times.
Transfections and dual-luciferase reporter assay
Rat NP cells were seeded in 48-well plates at a density of 3 × 104 cells/mL with 2% opti-medium 24 h before transfection. To observe the effect of hypoxia or the inflammatory cytokines TNF-α and IL-1β, cells were transfected with 250 ng of the SIRT1 promoter construct together with 250 ng pRL-TK plasmid. To explore the effect of NF-κB on SIRT1 promoter activity, cells were cotransfected with 50–200 ng of p65, p50, DN-NF-κB, or both p65 and p50 with or without the appropriate backbone vector and 150 ng SIRT1 reporter and 150 ng pRL-TK plasmid. In some wells, cells were treated with the NF-κB inhibitor (10 or 20 µM SM7368). To demonstrate the effect of HIF-1α on SIRT1 promoter activity, cells were cotransfected with 50–200 ng HA-HIF-1α and 100–300 ng siHIF-1α with 100 ng SIRT1 promoter plasmid and 100 ng pRL-TK. The transfection reagent Lipofectamine 2000 was used. The cells were harvested, and the firefly and Renilla luciferase activities were measured using the dual luciferase reporter assay kit (Promega) according to the manufacturer's instructions 48 h after transfection. All analyses were performed in triplicate.
Knockdown of p65, IKKβ, or HIF-1α
At a density of 3 × 106 cells per 10-cm plate, HEK 293T human embryonic kidney cells were seeded in normal culture medium (Ye et al., 2015). The cells were cotransfected with 9 µg p65, IKKβ, or HIF-1α shRNA plasmids; 6 µg psPAX2; and 3 µg pMD2.G. In the control, shRNA control sequences were used. After the transfection, the medium was replaced with normal culture medium. Medium containing lentiviral particles was obtained and, together with 6 mg/mL polybrene, used to replace the medium of the human NP cells. Finally, the cells were harvested, and the RNAs and proteins were extracted.
Real-time PCR
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Total RNA (2,000 ng) was copied with Superscript III Reverse Transcriptase (Invitrogen) to produce the single-stranded cDNA. Gene specific primers, cDNA, and Fast SYBR Green master mix from Applied Biosystems were mixed and incubated in the 7900HT Fast Real-Time PCR System (Applied Biosystems). Hprt and β-actin were used to normalize the expression of the rat and human samples, respectively. Each sample was analyzed in duplicate. The primers used were provided by Sangon, Inc., China (Table 1).
| Sense | Anti-sense | |
|---|---|---|
| Rat | ||
| Hprt | GCTGACCTGCTGGATTACAT | CCCGTTGACTGGTCATTACA |
| MMP3 | CAGGGAAAGTGACCCACATATT | CGCCAAGTTTCAGAGGAAGA |
| MMP9 | CCCAACCTTTACCAGCTACTC | GTCAGAACCGACCCTACAAAG |
| MMP13 | GAACCACGTGTGGAGTTATGA | GCATCTACTTTGTCGCCAATTC |
| ADAMTS4 | GGAGATCGTGTTTCCAGAGAAG | CAAAGGCTGGTAATCGGTACA |
| Human | ||
| β-actin | GGACCTGACTGACTACCTCAT | CGTAGCACAGCTTCTCCTTAAT |
| SIRT1 | TGGACAATTCCAGCCATCTC | GCGTGTCTATGTTCTGGGTATAG |
Western blots
After the treatment, cells were washed with ice-cold PBS twice and collected by scraping. Next, cells were treated with lysis buffer including 1 × protease inhibitor cocktail, NaCl (5 mM), NaF (200 µM), Na3VO4 (200 µM), and DTT (0.1 mM). Later, total cell proteins (30 ng), qualified using BCA reagent, were resolved on 10% SDS–PAGE gels and transferred by electroblotting to PVDF membranes. The membranes were blocked with 5% non-fat dry milk in TBST (50 mM Tris, pH 7.6, 150 mM NaCl, 0.1% Tween 20) and incubated overnight at 4°C with the anti-IKKβ (1:1,000), anti-SIRT1 (1:500), anti-HIF-1α, anti-β-tubulin (1:3,000), and anti-GAPDH (1:3,000). Finally, the membranes were incubated in the corresponding secondary antibody (1:1,000–1:5,000). The membranes were treated with ECL Plus 1 h later according to the manufacturer's instructions. Blot intensity was determined by densitometric analysis using Kodak 1D 3.6 software (Kodak, Rochester, NY, USA). The expression of GAPDH or β-tubulin, the internal control, was used to normalize the expression of the other proteins.
Statistical analysis
All experiments were performed in triplicate. All data were presented as the mean ± SE. Statistical analyses were performed using the SPSS 13.0 software, and P-values less than 0.05 were considered significant.
Results
SIRT1 attenuates the mRNA expression of the catabolic factors induced by TNF-α in rat NP cells
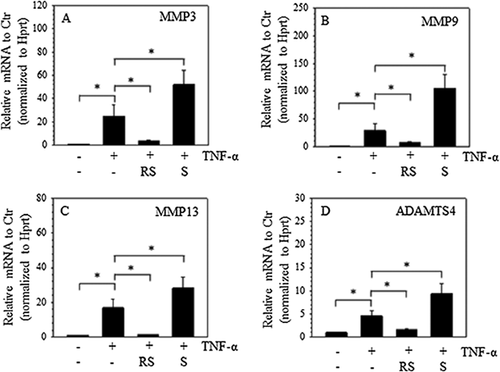
To demonstrate the effect of SIRT1 on the expression of TNF-α-induced catabolic genes in rat NP cells, sirtinol, a SIRT1 inhibitor, and resveratrol, a SIRT1 activator, were used (Howitz et al., 2003; Kaeberlein et al., 2005). The results showed that stimulation with TNF-α (50 ng/mL) increased the mRNA expression of MMPs, including MMP3, MMP9, and MMP13. Moreover, SIRT1 inhibition with 50 µM sirtinol increased the effect of TNF-α, whereas resveratrol (100 µM) suppressed the induction of MMP mRNA expression by TNF-α (Figures 1A–1C). Similarly, the ADAMTS4 mRNA expression was induced by TNF-α, and the SIRT1 inhibitor promoted the effect of TNF-α. Resveratrol, however, decreased the induction of ADAMTS4 mRNA expression by TNF-α (Figure 1D).
SIRT1 expression is refractory to hypoxia or HIF-1α in NP cells
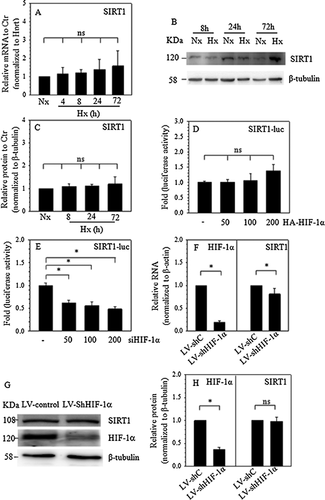
To investigate the role of hypoxia and HIF-1α on SIRT1 expression, real-time PCR and Western blot experiment were performed, and real-time PCR showed that hypoxia had no effect on SIRT1 mRNA expression (Figure 2A). Western blot (Figure 2B) and corresponding densitometric analysis (Figure 2C) revealed that hypoxia cannot increase or decrease the SIRT1 protein expression. In NP cells, hypoxia regulates the cell function through HIF-1α (Gogate et al., 2011). JASPAR database analysis (http://jaspar.genereg.net) revealed that there was many putative HIF-1α-binding motifs in the promoter and many putative binding sites (>85%). To investigate the role of HIF-1α in the transcriptional regulation of SIRT1, HIF-1α subunits and the SIRT1 reporter were cotransfected into rat NP cells. Like the hypoxia mRNA results, the activity of the SIRT1 promoter was refractory to 50, 100, and 200 ng HIF-1α subunits (Figure 2D). However, the data from the cotransfection with siHIF-1α showed that the SIRT1 promoter activity was suppressed in a dose-dependent manner (Figure 2E). In addition, a HIF-1α loss-of-function study with shRNA transduction was used to confirm the involvement of HIF-1α in controlling the SIRT1 expression of human NP cells. As expected, compared to the control cells, shHIF-1α transduction resulted in approximately an 80% decrease of HIF-1α RNA. Accordingly, SIRT1 mRNA expression in HIF-1α-suppressed human NP cells decreased significantly, to ∼20% of that in the control cells (Figure 2F). However, Western blot and corresponding densitometric analysis showed that, compared to the control cells, HIF-1α knockdown (∼65%) cannot lead to the significant suppression of SITR1 protein expression (Figures 2G and 2H).
NF-κB signaling pathway controls SIRT1 transcription activity in NP cells
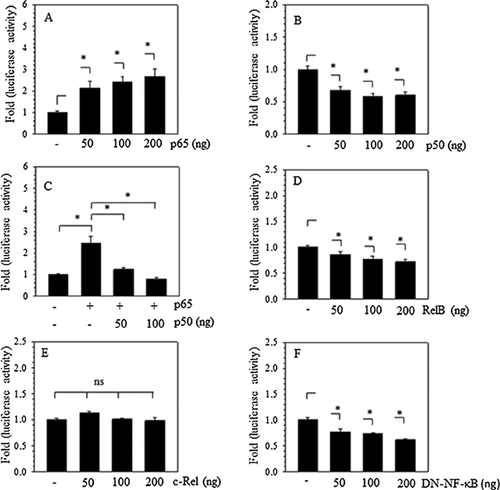
A marked increase in the proinflammatory cytokines TNF-α and IL-1β occurs during IVDD. Proinflammatory cytokines promote catabolic enzyme expression through NF-κB signaling (Ohba et al., 2009; Li et al., 2015). To probe the role of NF-κB in the transcriptional regulation of SIRT1, JASPAR database analysis was used for evidence of putative NF-κB binding motifs in the promoter, and at least five putative binding sites (>85%), including CGGAGTTTCG, from −313 to −322 bp, and CGGGGTTTCT, from −479 to −488 bp, were revealed. When NF-κB subunits were cotransfected with the SIRT1 promoter into rat NP cells, the data showed that cotransfection with p65 resulted in a dose-dependent increase, whereas p50 led to a dose-dependent suppression of SIRT1 promoter activity (Figures 3A and 3B). Moreover, p50 blocked the inductive effect of p65 (Figure 3C). In addition, RelB, a p50 subunit, suppressed the SIRT1 promoter (Figure 3D), whereas the activity of the SIRT1 promoter was refractory to the c-Rel subunit (Figure 3E). It should be noticed that the dominant negative NF-κB plasmid, IkBαM, reduced the activity of the SIRT1 promoter (Figure 3F).
SIRT1 mRNA and protein expression is controlled by the NF-κB signaling pathway but is refractory to inflammatory cytokines in NP cells
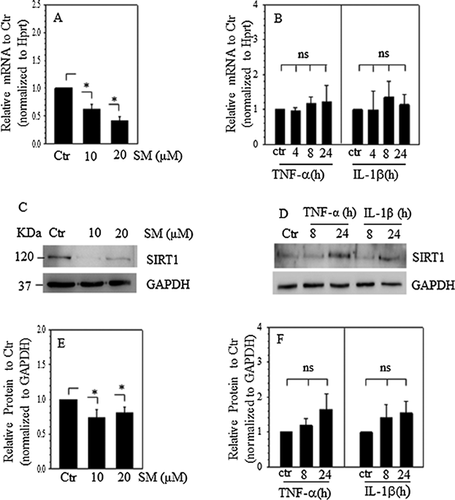
Because the SIRT1 transcription activity was controlled by NF-κB signaling pathway in NP cells, we evaluated the effect of NF-κB signaling and proinflammatory cytokines on SIRT1 mRNA and protein expression. The data showed that SIRT1 mRNA expression decreased in a dose-dependent manner after NF-κB signaling inhibition (Figure 4A). However, SIRT1 mRNA expression was refractory to stimulation by TNF-α and IL-1β (Figure 4B). In parallel, Western blots and subsequent densitometric analysis showed that an NF-κB inhibitor decreased the protein expression of SIRT1 (Figures 4C and 4E), whereas TNF-α and IL-1β had no effect on SIRT1 protein expression (Figures 4D and 4F).
SIRT1 expression is controlled by the NF-κB signaling pathway in the present of TNF-α
To confirm that SIRT1 promoter activity is responsive to NF-κB signaling in the presence of TNF-α, the activity of the SIRT1 promoter fragment stimulated by TNF-α and IL-1β was detected, and the data revealed that TNF-α and IL-1β had no effect on the SIRT1 promoter in rat NP cells (Figure 5A). The results of cotransfecting NP cells with DN-NF-κB/IkBαM (Figure 5B) or treatment with the NF-κB inhibitor SM7368 (Figure 5C) in the presence of TNF-α revealed that both DN-NF-κB and the NF-κB inhibitor suppressed the activity of the SIRT1 promoter even with TNF-α treatment.
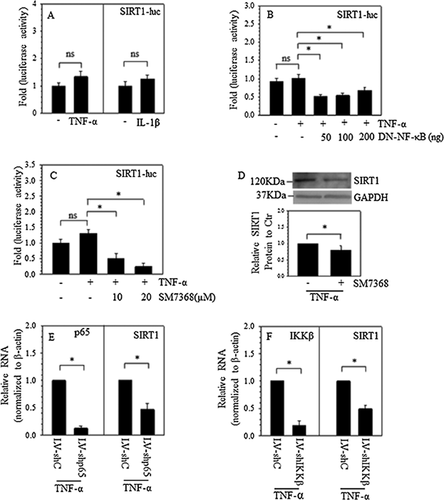
Furthermore, Western blots and subsequent densitometric analysis showed that in the presence of TNF-α, the NF-κB inhibitor also decreased the protein expression of SIRT1 (Figure 5D). Additionally, to observe the role of NF-κB signaling in controlling SIRT1 expression in human NP cells, p65 and IKKβ loss-of-function studies were performed using lentiviral delivery of gene targeting shRNA. As expected, compared to cells transduced with control shRNA, in human NP cells transduced with shp65, the mRNA expression of p65 significantly decreased (by ∼85%). In the presence of TNF-α, SIRT1 mRNA expression was reduced to ∼45% (Figure 5E) of the control cells. Similarly, in human NP cells transduced with shIKKβ, the mRNA expression of IKKβ significantly decreased (by ∼80%), and SIRT1 mRNA expression was reduced in the presence of TNF-α to ∼45% of that of the control cells (Figure 5F).
Discussion
The current investigation verified that SIRT1 controlled the expression of proteases that degrade ECM induced by TNF-α in rat NP cells. Moreover, it demonstrated that the expression of SIRT1 was refractory to hypoxia and HIF-1α. NF-κB signaling sustained the activity of the SIRT1 promoter in the presence or absence of TNF-α⋅ In addition, treatment with TNF-α and IL-1β had no effect on the expression of SIRT1 mRNA and protein.
Sirt1 plays a critical role in metabolism, anti-apoptosis, anti-ageing and anti-inflammation (Howitz et al., 2003; Takayama et al., 2009; Moon et al., 2013; Ou et al., 2014). In the intervertebral disc, Sirt1 downregulates the apoptosis of NP cells through autophagy, similar to the effect in chondrocytes (Takayama et al., 2009; Jiang et al., 2014). TNF-α and IL-1β increase the proteoglycan degradation through MMP3, MMP13, ADAMTS4 etc. Proteoglycan degradation contributes to the pathogenesis of IVDD (Vo et al., 2013; Ye et al., 2015). SIRT1 may prevent the release of proinflammatory mediators and protect against ECM degradation in osteoarthritis (Matsushita et al., 2013; Moon et al., 2013). Could SIRT1 play a similar role in intervertebral discs? Herein, our results demonstrated that, as it did in chondrocytes and bovine NP cells (Wuertz et al., 2011; Liu-Bryan and Terkeltaub, 2015), resveratrol (SIRT1 activator) repressed the induction of catabolic factors, including MMP3, MMP13 and ADAMTS4, induced by TNF-α in rat NP cells. Moreover, sirtinol (a common inhibitor of SIRT1) increased the expression of the catabolic factors. To some extent, SIRT1 is a protective factor in rat NP cells, as it is in other cells.
The regulation of Sirt1 by hypoxia is cell-type dependent. In Hep3B or HT1080 cells, hypoxia upregulates SIRT1 expression, whereas SIRT1 expression is suppressed by hypoxia in ovarian cancer cells (Chen et al., 2011; Sun et al., 2013). In addition, SIRT1 controls the expression of HIF-1α in a cell-dependent manner (Laemmle et al., 2012; Bae et al., 2013). Moreover, HIF-1α increases the expression of SIRT1 (Chen et al., 2011). In the process of IVDD, hypoxia regulates the activity of NP cells through HIFs (Gogate et al., 2011). The present study revealed that, unlike other cells, SIRT1 expression in rat NP cells was refractory to hypoxia. Moreover, HIF-1α overexpression had no effect on the expression of SIRT1 and, although markedly suppressed the activity of the SIRT1 promoter, HIF-1α knockdown cannot result in a pronounced decrease of SIRT1 mRNA and lead to a significantly decrease of SIRT1 protein in NP cells. Therefore, perhaps HIF-1α had no effect on the expression of SIRT1 in NP cells.
TNF-α and IL-1β are the key inflammatory cytokines associated with degenerative disc disease, and they induce the expression of catabolic factors through NF-κB signaling (Ohba et al., 2009; Ye et al., 2013; Li et al., 2015). Katto et al. (2013) showed that the putative binding sites for NF-κB within the SIRT1 promoter appear to be functional, and several NF-κB subunits increase the activity of the SIRT1 promoter. The heterodimer consisting of the p65 and p50 proteins is the most prevalent and most thoroughly studied dimer in NF-κB signaling (Rigoglou and Papavassiliou, 2013; Wang et al., 2015). In contrast to a previous report, our data showed that, in the NP cells, only p65 increased the activity of the SIRT1 promoter, whereas p50 or RelB decreased the SIRT1 promoter activity, and c-Rel had no effect. More importantly, DN-NF-κB suppressed the activity of the SIRT1 promoter.
Although TNF-α and IL-1β induced the expression of catabolic factors through NF-κB signaling, and NF-κB signaling regulated the activity of the SIRT1 promoter in other cells (Gogate et al., 2011; Serrano-Marco et al., 2012), our results revealed that TNF-α and IL-1β had no effect on the activity of the SIRT1 promoter in NP cells. Moreover, the expression of SIRT1 mRNA and protein was refractory to TNF-α and IL-1β. However, downregulation of NF-κB signaling through p65 and IKKβ knockdown or inhibitor treatment suppressed SIRT1 mRNA and protein expression in the presence or absence of TNF-α. Moreover, NF-κB signaling controlled the activity of the SIRT1 promoter in NP cells in the presence of TNF-α.
Conclusion
In this study, we provide new insights into the regulation of SIRT1 in NP cells. Neither hypoxia, nor inflammatory cytokines could decrease or increase the expression of SIRT1. Moreover, HIF-1α may have no effect on the expression of SIRT1. However, under physiologic and pathophysiologic conditions, NF-κB signaling are critical for the maintenance of SIRT1 expression in NP cells.
Acknowledgments and funding
This work was supported by grants from the National Natural Science Foundation of China (NO. 81572197), the Natural Science Foundation of Guangdong Province, China (NO. 10151008901000084), and the Science and Technology Planning Project of Guangdong Province, China (NO. 2014A020212058).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.




