Tube formation in the first trimester placental trophoblast cells: Differential effects of angiogenic growth factors and fatty acids
Abstract
The study aims to investigate whether cytosolic fatty acid-binding protein-4 (FABP4) is involved in angiogenic growth factors- and fatty acid-induced tube formation in first trimester placental trophoblast cells, HTR8/SVneo. We determined the tube formation both at basal as well as stimulated levels in the absence and presence of inhibitors of FABP4 and VEGF signaling pathways. Basal level of tube formation was maximally reduced in the presence of 50 µM of FABP4 inhibitor compared with those by VEGF signaling pathway inhibitors (rapamycin, L-NAME, and p38 MAP kinase inhibitor). Whereas docosahexaenoic acid, 22:6n-3 (DHA)-, and VEGF-induced tube formation was maximally inhibited by p38 MAP kinase inhibitor (63.7 and 34.5%, respectively), however, leptin-induced tube formation was inhibited maximally by FABP4 inhibitor (50.7%). ANGPTL4 and oleic acid (OA)-induced tube formation was not blocked by any of these inhibitors. The FABP4 inhibitor inhibited cell growth stimulated by DHA, leptin, VEGF, and OA (P < 0.05) but was not affected by ANGPTL4. VEGF, leptin, and OA also increased FABP4 protein level in these cells, though the uptake of fatty acids by these cells was not affected by the presence of FABP4 inhibitor. Our data demonstrate that FABP4 may be involved in part in the basal level, and stimulated tube formation by VEGF, DHA, and leptin, whereas it has little or no effect in ANGPTL4- and OA-induced tube formation in these cells. Thus, FABP4 may play a differential role in fatty acids and angiogenic growth factors-mediated tube formation in the first trimester trophoblast cells in vitro.
Abbreviations
-
- ANGPTL4
-
- angiopoietin 4-like protein
-
- DHA
-
- docosahexaenoic acid, 22:6n-3
-
- FABP4
-
- cytosolic fatty acid-binding protein-4
-
- MTT
-
- 4,-dimethylthiazol-2-yl)-2,-diphenyl tetrazoliumbromide
-
- OA
-
- oleic acid
-
- VEGF
-
- vascular endothelial growth factor
Introduction
Angiogenesis is defined as a biological mechanism of new blood vessel formation from pre-existing ones and plays important roles in many processes including placentation (Khong and Brosens, 2011). Angiogenesis is critical to successful fetal outcomes, as the placental blood flow is dependent on placental vascularization. Lack of placental vascular development may contribute to inadequate cytotrophoblast invasion as observed in pre-eclampsia (Reynolds and Redmer, 2001). We have shown earlier that dietary fatty acids, vascular endothelial growth factor (VEGF), leptin, and insulin stimulate angiogenesis in the first trimester placental trophoblasts possibly via different mechanisms (Johnsen et al., 2011; Basak and Duttaroy, 2012, 2013a, b; Basak et al., 2013, 2015). Fatty acid-binding protein-4 (FABP4) also known as adipocyte FABP (A-FABP) or aP2 (Duttaroy, 2009) was shown to be involved in VEGF-mediated angiogenesis in endothelial cells (Ghelfi et al., 2013). Recent studies demonstrated that FABP4 as a novel target of VEGF and its receptors (VEGF/VEGFR2) pathway and a positive regulator of cell proliferation and angiogenesis in endothelial cells (Elmasri et al., 2012, 2009; Ghelfi et al., 2013). In fact, FABP4 plays a pro-angiogenic role in endothelial cells by promoting cell proliferation, migration, survival, lipid accumulation, and morphogenesis. FABP4 has a role in activation of several mitogenic pathways and expression of several key mediators of angiogenesis (Elmasri et al., 2009). In endothelial cells, FABP4 expression is induced by pro-angiogenic stimuli, such as VEGF and basic fibroblast growth factor (Elmasri et al., 2009). The VEGF-mediated expression of FABP4 was inhibited by siRNA-mediated knockdown of VEGFR2, whereas the VEGFR1 agonists, placental growth factors (PIGFs) had no such effect. FABP4 is primarily involved in most of the VEGF-mediated angiogenesis in endothelial cells (Elmasri et al., 2012; Harjes et al., 2014). The disruption of stem cell factor (SCF)/c-kit signalling pathway played a critical role in diminished VEGF-mediated angiogenic responses in FABP4−/− endothelial cells, indicating FABP4 involvement in this process (Elmasri et al., 2012). It has been shown that the delta-like ligand (DLL) 4-NOTCH directly regulates FABP4 gene expression by binding of the FABP4 promoter in endothelial cells (Guba et al., 2002). The FABP4 response to VEGF is dependent on the NOTCH pathway, as inhibition of DLL4 binding to NOTCH and inhibition of NOTCH cleavage leads to FABP4 reduction in response to VEGF (Harjes et al., 2014). Furthermore, DLL4-NOTCH-induced FABP4 is dependent on the insulin-responsive FOXO1 transcription factor, providing a nodal point for the integration of angiogenic and metabolic signaling in endothelial cells. One of the metabolic changes often found during angiogenesis is their increased fatty acid synthesis and transport, lipid droplet formation, indicating possible involvement of fatty acid transport system. It is also well known that cells alter their metabolism to suit their needs for angiogenesis such as cell proliferation, invasion, and gene expression. Our previous data showed that long chain fatty acids favored energy-intensive tube formation process in the first trimester trophoblast cells (Johnsen et al., 2011; 2013a, b). FABP4 expression is induced by hypoxia that is essential for lipid accumulation in placental last trimester under increased lipid loads (Biron-Shental et al., 2008; Scifres et al., 2011). Recent data demonstrated that maternal serum FABP4 is independently associated with the subsequent development of pre-eclampsia. Elevated maternal serum FABP4 levels may also play a role in the pathogenesis of pre-eclampsia through pathways related to insulin resistance, inflammation, and abnormal lipid metabolism (Scifres et al., 2011). All these observations further warrant studies in order to understand the mechanisms as to how FABP4 regulates angiogenesis in the first trimester placenta. We demonstrated that leptin, docosahexaenoic acid, 22:6n-3 (DHA) and c9, t11-conjugated linoleic acid (c9, t11-CLA) stimulated FABP4 mRNA synthesis with concomitant enhanced tube formation in HTR8/SVneo cells (Johnsen et al., 2011; Basak et al., 2013; Basak and Duttaroy, 2012, 2013a). However, further study is required to ascertain the relationships between VEGF, angiopoietin 4-like protein (ANGPTL4), dietary fatty acids, and the roles of FABP4 in tube formation of the placental first trimester trophoblasts.
In this paper, we report for the first time about the differential effects of VEGF, leptin, ANGPTL4, and dietary fatty acids (OA and DHA) on FABP4 expression and its impact on tube formation in placental first trimester trophoblasts. Expression of FABP4 protein was associated with leptin, VEGF, and DHA-induced angiogenesis but not in ANGPTL4- and oleic acid (OA)-mediated tube formation of these cells. In addition, FABP4 may not be involved as the key regulator in these cells as observed in endothelial cells.
Materials and methods
Materials
The HTR8/SVneo trophoblast cell line was gifted by Dr. C.H. Graham, Queen's University, Canada. All the radiolabeled and unlabeled fatty acids were obtained as described previously (Johnsen et al., 2011; Basak and Duttaroy, 2013a). Recombinant human VEGFA and ANGPTL4 were purchased from R&D and Abnova (USA), respectively. Lactate dehydrogenase (LDH) assay kit was obtained from Roche Molecular Biochemical, Mannheim, Germany. Matrigel was from BD Biosciences, USA. Rapamycin (mTOR inhibitor) and FABP4 inhibitor (BMS309403) were obtained from Calbiochem, UK. p38 MAP kinase inhibitor (SB203580) and NOS inhibitor, L-Ng-nitro-L-arginine methyl ester (L-NAME), were obtained from Cell Signaling Technology, Inc., USA. Trypsin–EDTA, penicillin–streptomycin solution, 3-(4, 5-dimethyl thiazol-2-yl)-2,5 diphenyl tetrazoliumbromide (MTT) and RPMI-1640 medium and all other chemicals were obtained from Sigma Aldrich AS Norway.
Methods
Cell culture
The HTR8/SVneo cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum (Integro, Dieren, Holland), 2 mM L-glutamine, penicillin (50 units/mL), and streptomycin (50 µg/mL) at 37°C in 5% CO2 as described before (Johnsen et al., 2011).
Cellular viability and proliferation assay
Cell viability and proliferation was performed as a measure of cellular growth and differentiation as described before (Basak and Duttaroy, 2013a). Cells were incubated with different angiogenic modulators including VEGF (10 ng/mL), leptin (25 ng/mL), angiopoietin-4 like protein (ANGPTL4) (40 ng/mL), OA (50 µM), and DHA (50 µM) in the absence and presence of different inhibitors such as rapamycin (20 nM), p38 MAP kinase inhibitor (5 μM), L-NAME (2 mM), and FABP4 inhibitor (50 µM). 3-(4,-Dimethylthiazol-2-yl)-2,-diphenyl tetrazoliumbromide (MTT) was used to detect viable proliferating cells. The absorbance was read at 562 nm.
Uptake of radiolabeled fatty acids by HTR8/SVneo cells: Effect of FABP4 inhibitor
Typically, radiolabeled fatty acid was dissolved in serum-free RPMI containing fat-free BSA to which appropriate quantities of the corresponding unlabeled fatty acid were added in order to achieve the desired final concentrations, as described (Basak and Duttaroy, 2013a). The fatty acid uptake was carried out as described before (Basak and Duttaroy, 2013a). The cells were pre-incubated with FABP4 inhibitor (50 μM) for 1 h, followed by 3 h incubation with 14C fatty acids with 100 µM of radiolabeled fatty acids of ([14C]Oleic acid, [14C]Linolenic acid, [14C]Arachidonic acid, [14C]Eicosapentaenoic acid, and [14C]Docosahexaenoic acid [specific activity 1,000–2,000 cpm/nmol]). Fatty acid uptake was stopped by the addition of an ice-cold solution of 0.5% fatty acid-free BSA and the cells were washed twice with 0.5% fatty acid-free BSA and twice with PBS to remove any surface-bound fatty acid. The cells were dissolved by the addition of 1 mL of 0.1 M NaOH and left overnight at 4°C. Cells were then scraped and 300 µL aliquots of cell homogenate were transferred into scintillation vials containing 2 mL of scintillation cocktail. The radioactivity was determined using a scintillation counter. Data were expressed as picomol of fatty acid taken up/µg of cellular protein.
Tube formation assay
Cellular angiogenesis was measured in vitro based on tube formation on an extracellular matrigel, as described before (Johnsen et al., 2011). The cells were seeded (5 × 104 cells/well/24-well plate) on matrigel (growth factor reduced) and FABP4 inhibitor (50 µM), rapamycin (2 0nM), p38 MAP kinase inhibitor (5 μM), L-NAME (2 mM) or all the inhibitors were added to the cells in designated wells. In other experiments, angiogenic factors such as DHA (50μM), VEGF (10 ng/mL), leptin (25 ng/mL), ANGPTL4 (40 ng/mL), or OA (50 μM) were added in separate wells with or without mentioned inhibitors (same concentrations) to observe relative effects on tube formation. The wells were captured after 16 h by an inverted microscope at 40× magnification (Nikon TS100F, Japan). Capillary tube length was quantified and expressed in pixel (Johnsen et al., 2011). Images were captured from the central view of at least five different fields per well and extreme edges were excluded due to gel meniscus formation. Adobe Photoshop (version CS4) was used to quantify tubule length of the capillary network formation. The results were expressed pixel or as % over control using the formula: % over control = the mean length of total tubes (assay groups) × 100/mean length of tubes (control groups).
Western blot analysis of FABP4 expression
HTR8/SVneo cells were pre-incubated in the absence and presence of VEGF (10 ng/mL) and leptin (25 ng/mL) and fatty acids (50–100 μM) for 24 h. Cells were lysed with 200 μL of radioimmuno precipitation assay (RIPA) buffer followed by sonication and centrifugation as described previously (Basak et al., 2015). The supernatants were estimated for protein levels with BCA protein assay kit (Pierce, USA) and 10 μg of protein/lane was resolved by SDS–PAGE (12%) prior to their transfer to polyvinylidene difluoride membranes (Immobilon-P, Millipore Corp.). Immediately after blocking, membranes were immunoblotted with antibodies against anti FABP4 (1:5,000, PA-530591, Thermo Scientific Pierce, USA), anti β-actin (1:5,000; ab-8227, Abcam) and incubated with peroxidase conjugated goat anti-rabbit IgG (1:10,000, 31460 Thermo Scientific Pierce, USA). The blots were detected by using enhanced chemiluminescence substrate (Cat-32132, Pierce, USA). Immunoblot signals were captured by Storm860 phosphor imager and quantified by Image Quant software (GE healthcare).
Quantitative estimation of gene expression by real-time PCR
Total RNA was isolated from the HTR8/SVneo cells using TRI reagent (Sigma T9424) as per the instruction of the supplier. Total RNA was purified with DNase I (Sigma AMPD1) and cDNAs were synthesized using iScript cDNA synthesis kit (Biorad #1708891). Reverse transcription of cDNA was performed by power SYBR green PCR master mix (Life Technologies Part no. 4367659) along with predesigned primers, KiCqStart® SYBR® green (Sigma) (Table 1). Real time PCR was carried out in ABI 7500 (Life Technology, USA). The Ct value of an endogenous control gene TBP (TATA-binding protein) was subtracted from the corresponding Ct value for the target gene resulting in the delta Ct value which was used for relative quantification of gene expression by the comparative Ct method (2−ΔΔCt).
| SL. no. | Primer ID | Gene symbol | Gene ID | Gene name | Nucleotide sequences (5′–3′) | Ref_seqID |
|---|---|---|---|---|---|---|
| 1 | H_PLIN2_1 | ADRP | 123 | Adipose differentiation-related protein | F 5′–GTTCACCTGATTGAATTTGC–3′ | NM_001122 |
| R 5′–GAGGTAGAGCTTATCCTGAG–3′ | ||||||
| 2 | H_ACSL3_3 | ACSL3 | 2181 | Acyl-CoA synthetase long-chain family member 3 | F 5′–GAGAGGAAGATGTCTACATTG–3′ | NM_004457 |
| R 5′–CTGATCTGCTAAAGTCTGTG–3′ | ||||||
| 3 | H_ACSL5_3 | ACSL5 | 51703 | Acyl-CoA synthetase long-chain family member 5 | F 5′–CATCCTTAGTAGGAGTGGTG–3′ | NM_016234 |
| R 5′–TTTAAGGCCACTTTCTTTCC–3′ | ||||||
| 4 | H_FABP4_1 | FABP4 | 2167 | Fatty acid-binding protein 4 | F 5′–CAAGAGCACCATAACCTTAG–3′ | NM_001442 |
| R 5′–CTCGTTTTCTCTTTATGGTGG–3′ | ||||||
| 5 | H_LPL_1 | LPL | 4023 | Lipoprotein lipase | F 5′–ACACAGAGGTAGATATTGGAG–3′ | NM_000237 |
| R 5′–CTTTTTCTGAGTCTCTCCTG–3′ | ||||||
| 6 | H_TBP_1 | TBP | 6908 | TATA-binding protein | F 5′–GCCAAGAGTGAAGAACAG–3′ | NM_003194 |
| R 5′–GAAGTCCAAGAACTTAGCTG–3′ | ||||||
| 7 | H_HIF1A_2 | HIF1A | 3091 | Hypoxia inducible factor 1 | F 5′–GAAACTACTAGTGCCACATC–3′ | NM_001243084 |
| R 5′–GGAACTGTAGTTCTTTGACTC–3′ |
Statistics and data analysis
All the values are presented as mean and standard errors of mean (SEM). Level of significance was calculated by using Student's t-test. A P-value of <0.05 was considered statistically significant. Statistical significance for MTT data was determined using the Holm–Sidak method, with α = 5.000%.
Results
Basal tube formation in HTR8/SVneo cells: Effect of inhibitors of FABP4 and VEGF-mediated angiogenic signaling pathways
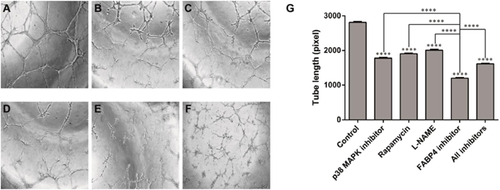
The basal tube formation (as a measure of in vitro angiogenesis) was performed on matrigel in the presence and absence of various inhibitors of VEGF signaling pathways and FABP4 in order to evaluate the effect of these inhibitors on angiogenesis in HTR8/SVneo cells. Inhibitors used were rapamycin (mTOR inhibitor, 2 nM), SB203580 (p38 MAP kinase inhibitor, 5 μM), L-NAME (eNOS inhibitor, 2 mM), FABP4 inhibitor (BMS309403, 50 μM). Basal tube formation and the effects of inhibitors were measured by tube length. Total length of tubular network as well as number of branches and connection points were significantly inhibited upon the treatment compared with the basal tube formation (control, P < 0.05). Figure 1 shows the effect of different inhibitors on basal tube formation capacity of the HTR8/SVneo cells. All these inhibitors blocked tube formation significantly but FABP4 inhibitor mediated its inhibitory effect on the tube formation to the greatest extent (P < 0.0001, unpaired t-test). Tube formation was maximally inhibited in the presence of FABP4 inhibitor compared with other inhibitors (rapamycin, L-NAME, and p38 MAP kinase inhibitor) at their respective potent doses in these cells. Comparison between the inhibitors on tube length showed differential inhibitory effects of these compounds. Inhibitory potencies on basal tube formation was in the order of FABP4 inhibitor (57.3%; 1200 ± 14.43, x = 3), p38 MAP kinase inhibitor (36.7%; 1778 ± 17.40, n = 3), rapamycin (32.2%; 1905 ± 10.41, n = 3), and L-NAME (28.5%; 2008 ± 22.05, n = 3). Presence of all inhibitors could not, however, block the tube formation (42.5%; 1615 ± 7,638, n = 3) more than that by FABP4 inhibitor alone (57.3%; 1200 ± 14.43, n = 3).
Inducer-mediated tube formation in the first trimester trophoblast cells, HTR8/SVneo: Effects of angiogenesis signaling pathway inhibitors
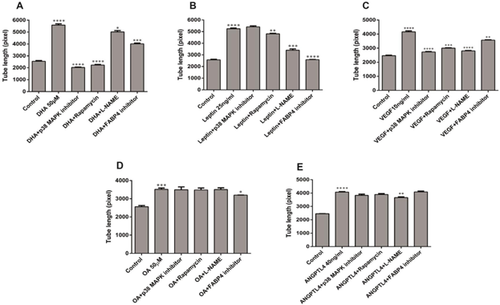
Effects of inhibitors (rapamycin, L-NAME, p38 MAP kinase inhibitor, and FABP4 inhibitor) on stimulated tube formation in the presence of DHA, leptin, VEGF, OA, or ANGPTL4 in these cells are shown in Figure 2. DHA-induced tube formation was inhibited in the order: p38 MAP kinase inhibitor (63.7%; 2025 ± 38.19, n = 3) > rapamycin (60.1%; 2225 ± 58.38, n = 3) > FABP4 inhibitor (28.1%; 4015 ± 67.14, n = 3) (Figure 2A). However, L-NAME inhibited the least (10.2%; 5015 ± 110.6, n = 3), P < 0.05. Figure 2B shows the inhibition of leptin-induced tube formation by these inhibitors. Unlike DHA and VEGF, FABP4 inhibitor blocked leptin-stimulated tube formation to the largest extent (50.7%; 2590 ± 16.07 n = 3) followed by L-NAME (35.2%; 3400 ± 117.3, n = 3), whereas p38 MAP kinase inhibitor had no effect. VEGF-induced tube formation was inhibited in the order of p38 MAP kinase inhibitor (34.5%; 2725 ± 38.19, n = 3) > rapamycin (27.8%; 3000 ± 28.87, n = 3) > FABP4 inhibitor (14.2%; 3567 ± 22.05, n = 3), P < 0.005 (Figure 2C). With the exception of FABP4 and L-NAME inhibitor, ANGPTL4 and OA-induced tube formation was not inhibited by majority of these inhibitors of VEGF signaling mediators (Figures 2D and 2E). FABP4 inhibitor significantly blocked the tube formation stimulated by DHA (P < 0.005), leptin (P < 0.0001), VEGF (P < 0.005) and to some extent OA-stimulated tube formation (P = 0.0165), whereas it did not considerably affect the tube formation induced by ANGPTL4 (P = 0.90003).
Effects of inhibitors FABP4 and VEGF signaling pathways on cell growth and proliferation in HTR8/SVneo cells
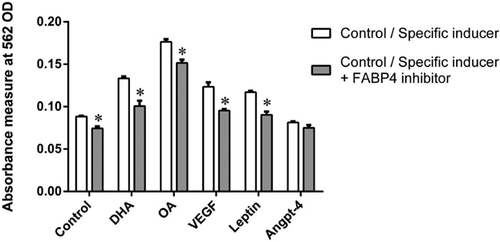
In order to elucidate further the roles of FABP4 on cellular growth and proliferation, HTR8/SVneo cells were cultured in the presence and absence of FABP4 inhibitor (BMS309403) along with VEGF, leptin, OA, and DHA (Figure 3). The growth and proliferation of HTR8/SVneo cells was inhibited by BMS309403 in the range of ∼15–25% when treated with DHA, OA, VEGF, and leptin as compared with their respective controls (P < 0.05). The cell proliferation inhibited by BMS309403 at basal level (control) was 16% (P < 0.05) without any treatment. FABP4 inhibitor did not affect the cellular growth stimulated by ANGPTL4.
Effect of FABP4 inhibitor on fatty acid uptakes in HTR8/SVneo cells
In order to understand the role of FABP4 in the fatty acid uptake of the first trimester trophoblast cells, we examined the uptake of ([14C]Oleic acid (OA), [14C]α-Linolenic acid (αLA), [14C]Arachidonic acid (AA), [14C]Eicosapentaenoic acid (EPA), and [14C]Docosahexaenoic acid (DHA) in the presence and absence of FABP4 inhibitor. The presence of FABP4 inhibitor did not affect the uptake of these radiolabeled fatty acids compared with control (data not shown).
Effects of VEGF on mRNA expression of lipid metabolic genes in HTR8/SVneo cells
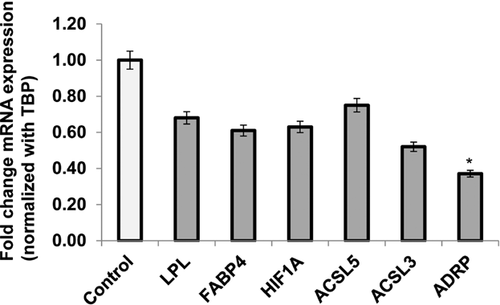
In order to determine whether VEGF-induced tube formation was associated with lipid metabolism of these cells, we measured the mRNA expression of FABP4 and other important lipid metabolic genes such as LPL, ACSL3, and ACSL5, ADRP, and HIF1α in the absence and presence of this angiogenesis inducer. Pre-incubation of HTR8/SVneo cells with VEGF (10 ng/mL) did not alter the expression of genes associated with fatty acid uptake, binding, and metabolic activities as compared with those in controls. However, there was a significant decrease (2.7 fold), P < 0.05) in the expression of ADRP, a gene encodes lipid droplet protein, in the first trimester trophoblast cells as compared with the control (Figure 4).
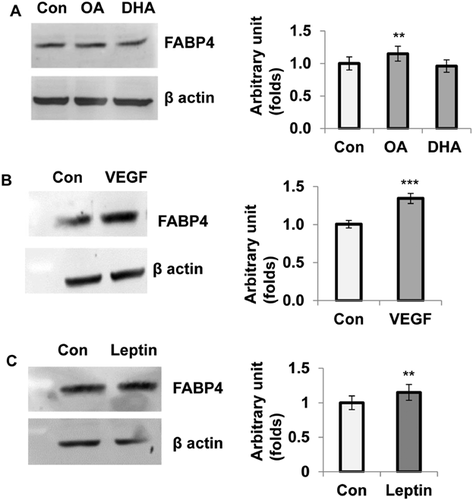
Effect of OA, DHA, VEGF, and leptin on FABP4 protein expression in HTR8/SVneo cells
In order to investigate the expression of FABP4 at protein level, HTR8/SVneo cells were pre-incubated with VEGF (10 ng/mL), leptin (25 ng/mL), DHA and OA (50 μM) for 24 h and harvested whole cell lysate for Western blotting. Figure 5 shows increased expression of FABP4 protein level in these cells in the presence of VEGF, leptin and OA compared with control. DHA, however, had no effect on FABP4 protein expression in these cells. Relative expression of FABP4 was increased significantly by VEGF (34%; P < 0.0001), leptin and OA (15%; P < 0.002) as compared with the control (Figures 5A–5C).
Discussion
Cell tube network formation on matrigel occurs as a consequence of a number of necessary biological activities, including cell migration, proliferation, cell–cell junction formation, and cell elongation. However, these processes do not mimic the whole process of in vivo angiogenesis. We investigated the tube formation as a measure of angiogenesis as evident in early placentation process. The mechanisms that determine the angiogenic capacity of VEGF, leptin, and fatty acids in first trimester placental trophoblast cells may underlie important differences in the mechanism of actions between them. We previously demonstrated that DHA stimulated the expression of VEGF with concomitant increase in the cellular proliferation and tube formation (as a measure of angiogenesis) in the first trimester trophoblast cells, HTR8/SVneo (Johnsen et al., 2011; Basak and Duttaroy, 2013b). In contrast to DHA, other long chain fatty acids such as EPA, AA, OA, and CLA promote synthesis of ANGPTL4 and tube formation without affecting VEGF synthesis in these trophoblast cells (Johnsen et al., 2011; Basak and Duttaroy, 2013b). Based on these data, we proposed that different mechanisms of action of DHA and other long chain fatty acids on tube formation may operate in the tube formation of the first trimester trophoblast cells.
Recent studies have highlighted FABP4 as a novel target of VEGF and its mediators of the VEGF signalling pathway in endothelial cells (Cataltepe et al., 2012; Elmasri et al., 2012; Ghelfi et al., 2013). In addition, FABP4 has been reported as a positive regulator of cell proliferation and angiogenesis in endothelial cells (Ghelfi et al., 2013). We previously reported that fatty acids such as EPA, DHA, c9t11-CLA, and leptin stimulate mRNA expression of FABP4 in the first trimester trophoblast cells, HTR8/SVneo (Johnsen et al., 2011; Basak and Duttaroy, 2012, 2013a; Basak et al., 2013). However, the role of FABP4 on the tube formation mediated by VEGF, ANGPTL4, and fatty acids in these cells is not known.
In order to ascertain the role of FABP4, we investigated various aspects of angiogenesis processes such as cellular growth and proliferation, tube formation, and fatty acid uptake in the presence of different angiogenic factors, and FABP4 inhibitor in placental first trimester cells, HTR8/SVneo. In order to understand VEGF-FABP4 cross talk, we used several inhibitors of VEGF signaling pathway mediators such as rapamycin (mTOR inhibitor), P38 kinase inhibitor, and L-NAME (eNOS inhibitor) to further elucidate the mechanism of DHA, VEGF, and ANGPTL4 and fatty acid-mediated angiogenesis in these cells.
Angiogenesis is regulated by a complex interplay between pro-angiogenic and anti-angiogenic factors. In order to explore the involvement of VEGF signaling pathways that are activated downstream during angiogenesis, we used several inhibitors of VEGF signaling pathways mediators. A major signaling event downstream of VEGF is the activation of AKT which is regulated by phosphoinositide-dependent kinase 1 and mammalian target of rapamycin (mTOR) complex. mTOR is a serine/threonine kinase that regulates a diverse array of cellular processes, including cell growth, survival, metabolism, and cytoskeleton dynamics. Angiogenesis depends on Akt/mTOR and VEGF signaling cascade. Rapamycin is an inhibitor of mammalian target of mTOR. Inhibition of mTOR has been shown to block the actions of VEGF through both inhibition of VEGF synthesis and signal transduction (Guba et al., 2002; Del Bufalo et al., 2006). Rapamycin has been shown to block tube formation in endothelial cells (Luo et al., 2012). Placenta expresses high level of p38α and p38δ but not p38β and p38γ (Wang et al., 1997), whereas vascular endothelial cells co-express p38α and p38δ (Hale et al., 1999). VEGF activate p38 (Rousseau et al., 1997). p38 MAP kinase inhibitor (SB203580) has been shown to inhibit p38α and p38β (Lee et al., 1999) and VEGF-induced tube formation in different cell systems (Wu et al., 2006; Lin et al., 2015). Whereas, eNOS inhibitor (L-NAME) has been used in inhibition of angiogenesis in cells (Lin et al., 2015).
We reported previously that tube formation is spontaneous at the basal level in HTR8/SVneo cell (Johnsen et al., 2011). Secretion of VEGF in the basal condition media of HTR8/SVneo cells in the matrigel indicates that VEGF predominantly drives tube formation at the basal level. At the basal level, inhibition of the tube formation in the HTR8/SVneo cells was observed in the following order: FABP4 inhibitor > P38 inhibitor kinase > rapamycin > L-NAME. Since effect of FABP4 inhibitor was more potent at basal level (or non-induced state) of HTR8/SVneo cells, it is reasonable to argue that FABP4 may be more involved in VEGF-mediated tube formation compared with other VEGF signaling mediators in these cells. However, inhibition of tube formation at the stimulated levels by these compounds such as p38 MAP kinase inhibitor, rapamycin, and L-NAME demonstrated differential effects on tube formation in first trimester trophoblast cells, HTR8/SVneo. Rapamycin and p38 MAPK inhibitor blocked both VEGF- and DHA-mediated tube formation in these cells. It was demonstrated that DHA-stimulated tube formation via VEGF (Johnsen et al., 2011). Therefore, as expected, the inhibitors of VEGF signaling pathways blocked VEGF-, DHA- and leptin-stimulated tube formation to a different degree without affecting ANGPTL4- and OA-induced tube formation. These data and others indicate that FABP4 is involved in VEGF-mediated tube formation of endothelial cells (Elmasri et al., 2012, 2009; Ghelfi et al., 2013). Our data showed that FABP4 was involved in cellular growth and proliferation, and its inhibitor blocked DHA-, VEGF-, and leptin-mediated tube formation in vitro but with different degrees. We showed that leptin-stimulated tube formation was not inhibited by the selective inhibitor of VEGF, indicating that its action was independent of VEGF and ANGPTL4 (Basak and Duttaroy, 2012). Leptin, however, significantly increased the expression of FABP4 and genes those are involved in angiogenesis pathways (Basak and Duttaroy, 2012).
This paper also reports for the first time that FABP4 protein expression is increased in the presence of VEGF in the first trimester trophoblast cells, HTR8/SVneo. Unlike protein level, the basal levels of VEGF and FABP4 mRNA expression were lower in HTR8/SVneo cells as compared to Ea.Hy926 (an endothelial cell line) as evidenced by the differential Ct values in these conditions (unpublished data). It is possible that VEGF-induced FABP4 expression at protein level contributed in the augmented tube formation of the first trimester trophoblast cells. FABP4 has been shown as essential for trophoblast lipid accumulation (Scifres et al., 2011). FABP4 delivers fatty acids to different intracellular compartments and thus effect fatty acid metabolism and also gene expression by delivering fatty acid ligands to the peroxisome proliferator-activated receptors (Duttaroy, 2009). VEGF down-regulated mRNA expression of ADRP in the HTR8/SVneo cells possibly sequestered fatty acids for metabolic activities instead of storage as a cellular energy. However, further work of ADRP expression at protein level and lipid droplets would be required in the first trimester cells for definitive conclusions.
FABP4 had less effect on ANGPTL4- and OA-induced tube formation compared with VEGF-mediated tube formation in these cells despite the fact that ANGPTL4 played a crucial role in angiogenesis, metabolism, and uptake of fatty acids particularly as an inhibitor of lipoprotein lipase activity (Georgiadi et al., 2010, Chi et al., 2015). FABP4, which is the principal and main target of VEGF-induced angiogenesis in endothelial cells (Elmasri et al., 2012, 2009; Harjes et al., 2014), may not be solely responsible for angiogenesis processes in these cells, as suggested by different experimental observations obtained from tube formation, cellular growth, and its mRNA and protein expression.
In conclusion, we demonstrate that the differential effects of VEGF, leptin, ANGPTL4, and fatty acids on FABP4 expression and its impact on angiogenesis (as measured as tube formation) in placental first trimester trophoblasts. Expression of FABP4 protein was associated with leptin-, VEGF-, and DHA-induced tube formation but not in ANGPTL4- and OA-mediated tube formation in these cells. In addition, at basal level of tube formation in HTR8/SVneo cells, FABP4–VEGF axis may be more involved in tube formation. However, FABP4 is not the key regulator in stimulated tube formation by DHA, VEGF, and leptin in these cells and has no or little involvement in ANGPTL4-, OA-mediated tube formation.
Acknowledgments and funding
This study was supported by the grant from the Thune Holst Foundation.




