Schisandrin B inhibits the proliferation of airway smooth muscle cells via microRNA-135a suppressing the expression of transient receptor potential channel 1
Abstract
Airway smooth muscle cell (ASMC) was known to involve in the pathophysiology of asthma. Schisandrin B was reported to have anti-asthmatic effects in a murine asthma model. However, the molecular mechanism involving in the effect of Schisandrin B on ASMCs remains poorly understood. Sprague–Dawley rats were divided into three groups: rats as the control (Group 1), sensitized rats (Group 2), sensitized rats and intragastric-administrated Schisandrin B (Group 3). The expression of miR-135a and TRPC1 was detected in the rats from three groups. Platelet-derived growth factor (PDGF)-BB was used to induce the proliferation of isolated ASMCs, and the expression of miR-135a and TRPC1 was detected in PDGF-BB-treated ASMCs. Cell viability was examined in ASMCs transfected with miR-135a inhibitor or si-TRPC1. The expression of TRPC1 was examined in A10 cells pretreated with miR-135a inhibitor or miR-135a mimic. In this study, we found that Schisandrin B attenuated the inspiratory and expiratory resistances in sensitized rats. Schisandrin B upregulated the mRNA level of miR-135a and decreased the expression of TRPC1 in sensitized rats. In addition, Schisandrin B reversed the expression of miR-135a and TRPC1 in PDGF-BB-induced ASMCs. Si-TRPC1 abrogated the increasing proliferation of ASMCs induced by miR-135a inhibitor. We also found that miR-135a regulated the expression of TRPC1 in the A10 cells. These results demonstrate that Schisandrin B inhibits the proliferation of ASMCs via miR-135a suppressing the expression of TRPC1.
Abbreviations
-
- AHR
-
- airway hyperresponsiveness
-
- ASMC
-
- airway smooth muscle cell
-
- NSCCs
-
- nonselective cation channels
-
- PDGF
-
- platelet-derived growth factor
-
- TRPC
-
- transient receptor potential
-
- VSMCs
-
- vascular smooth muscle cells
Introduction
It has been known that altered functions of airway smooth muscle cells (ASMCs) significantly contribute to the pathophysiology of asthma, which is marked by reversible and intermittent airway obstruction, airway inflammation, airway hyperresponsiveness (AHR), and airway wall remodeling (Yeganeh et al., 2013). A previous study shows that the proliferation of ASMCs is enhanced in patients with asthma, which is a feature of airway remodeling (Johnson et al., 2001). Regardless of the ASMCs proliferation, altered contractile responses of airway smooth muscle that result in AHR are considered the final pathway that causes the manifestation of various asthmatic symptoms (Chung and Sterk, 2000).
Mounting studies have provided unequivocal evidence that canonical transient receptor potential channels (TRPC) play important roles in physiological and pathological cellular responses in ASMCs (Nilius et al., 2007; Abramowitz and Birnbaumer, 2009). TRPC molecules may encode nonselective cation channels (NSCCs), which are present in ASMCs and are involved in the contraction, proliferation, migration, and gene expression of ASMCs (Wang and Zheng, 2011). Among the seven members of TRPC molecules, designated TRPC1 through TRPC7, TRPC1, and TRPC3 are found to be expressed in freshly isolated and primary mouse ASMCs and controlled the resting Vm and [Ca2+]i (Xiao et al., 2010). However, the molecular mechanism of TRPC molecules involving the proliferation of ASMCs remains poorly understood.
Schisandrin B is one of the major dibenzocyclooctadiene lignans extracted from the fruit of Schisandra chinensis Baill (Liu, 1989). Schisandrin B is widely used to treat hepatitis and myocardial disorders in traditional Chinese medicine (Liu, 1989). Previous studies have shown that Schisandrin B plays important roles in anti-inflammatory, antitumor, and antiamnesic (Ohkura et al., 1990; Yasukawa et al., 1992; Lee et al., 2010). Schisandrin B also showed the anti-proliferation of human lung adenocarcinoma cells by inducing cycle arrest and apoptosis (Lv et al., 2015). Recently, schizandrin was reported to show anti-asthmatic effects in a murine asthma model through reducing oxidative stress and airway inflammation (Lee et al., 2010).
Recent evidence have indicated that microRNAs (miRNAs) exert important posttranscriptional regulatory functions through binding to the complementary sites on target mRNA (Farh et al., 2005). Although ectopic expression of miRNAs has been involved in numerous human diseases, little is known about the functions of miRNA in smooth muscle. For example, miR-25 was reported to be involved in ASMs homeostasis and pathology by targeting kruppel-like factor 4 (KLF4) (Kuhn et al., 2010). Upregulation of miR-712*, miR-135a*, miR-714, and miR-762 in vascular smooth muscle cells (VSMCs) may be related to VSMC calcification through disrupting Ca2+ efflux proteins (Gui et al., 2012). In diabetic nephropathy, miR-135a promotes renal fibrosis via regulating TRPC1 (He et al., 2014). However, the role of miR-135a in asthma is not fully understood.
In present study, we demonstrate that Schisandrin B inhibits the proliferation of ASMCs, and miR-135a is strongly associated with this anti-proliferation effect via suppressing the expression of TRPC1.
Materials and methods
Animals and experiment group
NIH Guidelines for the Care and Use of Laboratory Animals were complied with in all experimental procedures, which were approved by People's Hospital Affiliated to Zhengzhou University. Thirty male Sprague–Dawley rats were used to establish the chronic asthmatic model and randomly divided into control group (n = 10), asthmatic group (n = 10), and Schisandrin B treatment group (n = 10). The ovalbumin (OVA) model was constructed as previously reported with some modification (Chen et al., 2013). Rats were sensitized with 10 mg of OVA through subcutaneous injection, as well as 200 mg of aluminum hydroxide (dissolved in 1 mL of 0.9% NaCl solution) and 1 mL inactivated Bordetella pertussis 1st and 8th days of the experiment. Rats injected with an equal volume of saline were used as controls. In Schisandrin B treatment group, the rats were similar to the asthmatic group but intraperitoneally injected with 80 mg/kg of Schisandrin B every day. Then, the next 6 weeks, the rats were excited by ultrasonic atomizing inhalation with 2% OVA for 30 min and repeated thrice a week. The whole experimental sustained 8 weeks. After the final challenge, the rats were sacrificed within 18–24 h in each group.
ASMCs isolation and culture
Rats were anesthetized and sacrificed by exsanguination according to the protocol approved by the Institutional Animal Care and Use Committees of People's Hospital Affiliated to Zhengzhou University. Primary rat ASMC cultures were prepared as described previously (Wei et al., 2013). Large bronchi were washed and airways without cartilages were used for the following experiment. Under a dissecting microscope, pure airway smooth muscle bundles were cut free from surrounding tissues. Then, the airway was cut open and the endothelium was disrupted by gently stripping the luminal surface with a blade. The tissue was cultured in 2–3 mL DMEM with 20% (v/v) fetal bovine serum and 100 U/mL of streptomycin and penicillin Gin a 5% CO2 humidified incubator at 37°C. The culture medium was replaced regularly (2–3 days) until cell confluence occurred (usually 8–10 days).
Airway resistance measurement
The inspiratory and expiratory resistances of the respiratory system were calculated through measuring airway reactivity to methacholine as described in a previous study (Yang et al., 2013). Rats were intravenously injected with methacholine (dissolved in 0.9% sodium chloride) at an initial dose of 0.0625 mg/kg. To obtain a response curve of lung resistance, the dose was increased twofold with each injection up to 1 mg/kg. The spacing intervals of injections were 5 min. Prior to the next methacholine injection, 50 μL of methacholine was administered over 3–4 s according to the return of resistance curves to the pre-methacholine level. Following the methacholine administration, response was measured immediately as the peak increase above the baseline.
Cell viability assay
Cell Counting Kit-8 (Takara, Dalian, China) was used to detect the cell viability. Briefly, cells were seeded into 96-well plates and preincubated in 5% CO2 at 37°C. Then, 10 μL of CCK-8 was added into each well. Absorbance was assessed using a microplate reader at 450 nm according to the manufacturer's instructions.
Western blot analysis
Cellular protein extracts were separated by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Bio-Rad, CA, USA) and transferred to nitrocellulose membranes (Millipore, MA, USA). Each membrane was incubated with a primary antibody against TRPC1 or β-actin (Abcam, Cambridge, UK) overnight at 4°C and subsequently incubated with appropriate secondary antibodies at room temperature for 2 h.
Quantitative real-time (RT) PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's protocols. Total RNA was reverse transcribed using a specific stem-loop primer and quantified by real-time PCR with the TaqMan MicroRNA assay kit (Applied Biosystems). mRNA level of TRPC1 was quantified by RT-PCR with a Takara RNA PCR Kit (Takara, Dalian, China). The relative expression levels were subsequently calculated using the 2−ΔΔCt method (Schmittgen and Livak, 2008). For mRNA analysis, GAPDH level was used to normalize the mRNA level of TRPC1 and U6 RNA was for the expression of miR-135a.
Cell transfection and luciferase activity assay
Cells were cultured in six-well plates and transfected with a miR-135a mimic, miR-135a inhibitor or the negative control, si-control or si-TRPC1 at 70–80% confluence using Lipofectamine 2000 (Sigma, Shanghai, China) according to the manufacturer's instructions. Stable clones were acquired at 2 weeks after antibiotic selection. The miR-135a mimics or inhibitor and corresponding negative control, si-control, or si-TRPC1 were designed and synthesized by Genechem (Shanghai, China).
For the luciferase assays, the cells were transfected with the appropriate plasmids in 24-well plates and harvested for luciferase activity assays using the dual-luciferase reporter assay system (Promega, Madison, WI, USA) at 48 h after transfection (Martin et al., 1996). The relative luciferase activity was normalized to that of firefly luciferase. The transfection experiments were performed in triplicate for each plasmid construct.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). Statistical comparisons between pairs of groups were analyzed by a two-tailed Student's t-test. The statistical analysis was carried out using the SPSS 16.0 statistical software package (SPSS, Inc., Chicago, IL, USA). A P-value >0.05 was considered statistically significant.
Results
Schisandrin B attenuates the inspiratory and expiratory resistances in sensitized rats
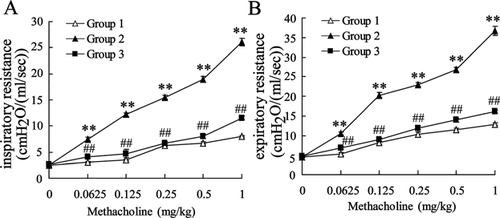
To investigate the effect of Schisandrin B on the pathology of asthma, rats were divided into the control group (Group 1), asthma group (Group 2), and Schisandrin B group (Group 3). Inspiratory and expiratory resistances were detected in rats of three groups. As shown in Figure 1, the inspiratory and expiratory resistances were both increased in Group 2 compared with that of Group 1. In addition, Schisandrin B reduced the inspiratory and expiratory resistances in sensitized rats. The findings indicated that Schisandrin B attenuated the pathology of asthma in rats.
Schisandrin B reverses the expression of miR-135a and TRPC1 in sensitized rats
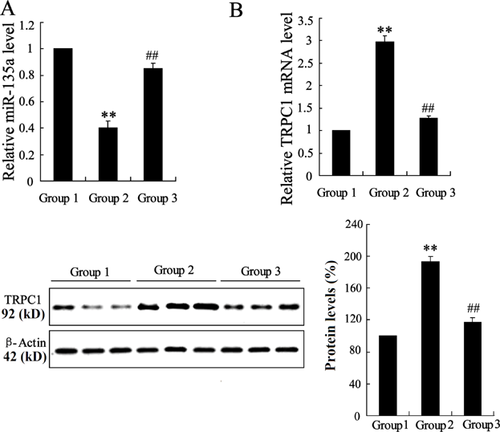
To ascertain the possible molecular mechanism of Schisandrin B attenuating asthma, the mRNA level of miR-135a and TRPC1 was examined in rats of three groups. Comparing the mRNA level of miR-135a and TRPC1 in Group 1 with that of Group 2, we found that the mRNA level of miR-135a was decreased and the expression of TRPC1 was enhanced in Group 2. While, Schisandrin B reversed the expression of miR-135a and TRPC1 in sensitized rats, as shown in Figure 2. The protein level of TRPC1 confirmed the dysregulation of TRPC1 in sensitized rats. The findings indicated that the dysregulation of miR-135a and TRPC1 in sensitized rats was related to the effect of Schisandrin B.
Schisandrin B reverses the expression of miR-135a and TRPC1 in PDGF-BB-induced ASMCs
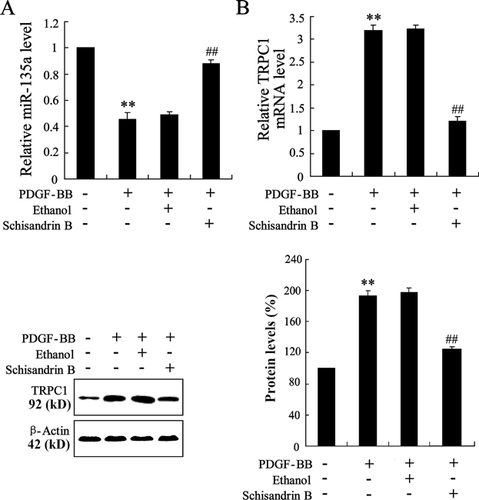
ASMCs were separated from the rats and PDGF-BB was used to induce the proliferation of ASMCs. Then, we investigated the effect of Schisandrin B on the expression of miR-135a and TRPC1 in PDGF-BB-induced ASMCs. As shown in Figure 3A, lower expression of miR-135a was observed in PDGF-BB-induced ASMCs than that in controls. In addition, the mRNA and protein levels of TRPC1 were both enhanced in PDGF-BB-induced ASMCs (Figure 3B). When PDGF-BB-induced ASMCs were exposed to Schisandrin B, the mRNA level of miR-135a was increased and the expression of TRPC1 was decreased. The findings indicated that Schisandrin B reversed the expression of miR-135a and TRPC1 in PDGF-BB-induced ASMCs.
Si-TRPC1 reverses the increasing proliferation of ASMCs induced by miR-135a inhibitor
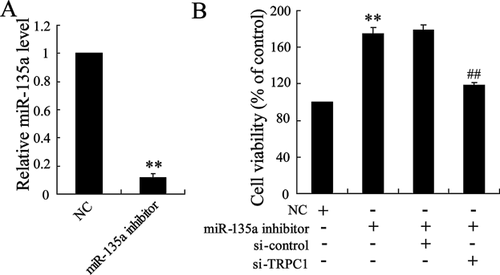
To investigate the effect of miR-135a on the proliferation of ASMCs, miR-135a inhibitor was used to induce miR-135a knockdown in ASMCs (Figure 4A). As shown in Figure 4B, miR-135a inhibitor highly enhanced the cell viability of ASMCs. In addition, si-TRPC1 abrogated the increasing proliferation of ASMCs induced by miR-135a inhibitor.
MiR-135a regulates the expression of TRPC1 in the A10 cells
To investigate the effect of miR-135a on the expression of TRPC1 in vitro, the A10 cells were utilized which were derived from the thoracic aorta of embryonic rat and have been used extensively as models of VSMCs. A10 cells were transfected with miR-135a mimic or miR-135a inhibitor and pGL3-luciferase reporter vector containing 3′-UTR of TRPC1. As shown in Figure 5A, miR-135a mimic significantly decreased the activity of 3′-UTR of TRPC1, as well as the mRNA and protein level of TRPC1. Transient co-transfection with miR-135a inhibitor and luciferase expression plasmids in A10 cells resulted in significant raise of TRPC1 at transcript and translational levels (Figure 5B).
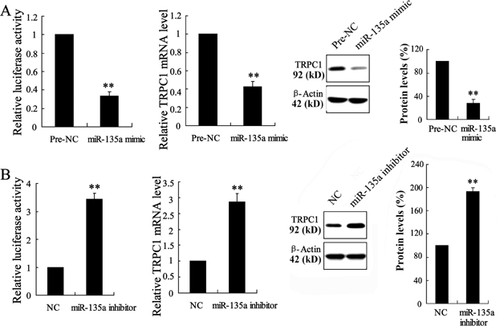
The effect of Schisandrin B and miR-135a inhibitor in the proliferation of PDGF-BB-induced ASMCs
To investigate the effect of Schisandrin B on the proliferation of PDGF-BB-induced ASMCs, ASMCs were treated with PDGF-BB, or PDGF-BB and ethanol, or PDGF-BB and Schisandrin B, or PDGF-BB and Schisandrin B and NC, or PDGF-BB and Schisandrin B, and miR-135a inhibitor. PDGF-BB induced the proliferation of ASMCs, as shown in Figure 6. Schisandrin B reversed the proliferation of PDGF-BB-induced ASMCs. While, miR-135a inhibitor abrogated the effect of Schisandrin B on the proliferation of PDGF-BB-induced ASMCs (Figure 6).
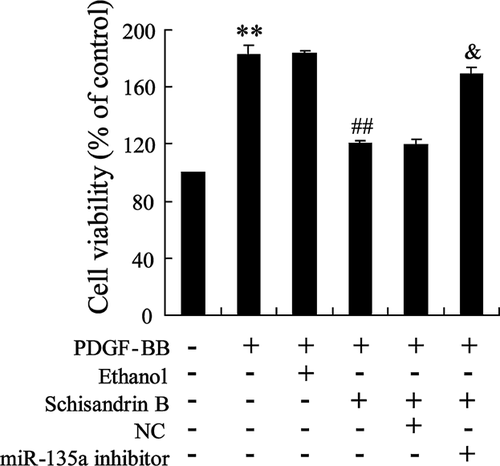
Discussion
In the present study, we have demonstrated that Schisandrin B attenuated the inspiratory and expiratory resistances in sensitized rats. Schisandrin B upregulated the expression of miR-135a and decreased the expression of TRPC1 in sensitized rats. In addition, Schisandrin B reversed the expression of miR-135a and TRPC1 in PDGF-BB-induced ASMCs. Si-TRPC1 abrogated the increasing proliferation of ASMCs induced by miR-135a inhibitor. We also found that miR-135a regulated the expression of TRPC1 in the A10 cells. These results suggested that targeting miR-135a may be a good approach to inhibit the proliferation of ASMCs.
Asthma is a chronic disorder of the airways and an increase in airway smooth muscle mass has been reported in asthmatic patients (Chang et al., 2012). In spite of the improvements in diagnosis and treatment, asthma is one of the most prevalent chronic respiratory disorders. In the present study, we showed that Schizandrin B significantly inhibited the inspiratory and expiratory resistances in sensitized rats, which indicated the effective function of Schizandrin B on asthmatic pathophysiology. A previous study also found the anti-asthmatic effect of schizandrin on OVA-induced airway inflammation in a murine asthma model (Lee et al., 2010). In addition to the anti-inflammatory property, antiproliferative effects of Schizandrin B were reported in human cancer cells. Here, we observed that Schizandrin B suppressed the proliferation of ASMCs induced by PDGF-BB.
To elucidate the molecular mechanism involving in the antiproliferative effects of Schizandrin B, we detected the expression of miR-135a and TRPC1 in asthmatic rats and PDGF-BB-induced ASMCs both treated with Schizandrin B. We found the higher mRNA level of miR-135a and lower expression of TRPC1 in these rats and ASMCs. These data indicated that miR-135a and TRPC1 might contribute to the antiproliferative effects of Schizandrin B.
Members of the TRP family may be the molecular counterparts of some store-operated Ca2+ channels, which play important roles in ASMCs contraction and proliferation (Smani et al., 2015). The TRPC1 channel has been implicated in regulation of cell fate and motility. TRPC1 knockdown was reported to inhibit the proliferation of pulmonary artery smooth muscle cells in culture (Sweeney et al., 2002). Also, TRPC1 is increased in the hyperplasia after arterial injury in vivo (Kumar et al., 2006). Roberto et al. found that the expression of TRPC1 was upregulated in proliferating ASMCs as well as in store-operated Ca2+ entry (Berra-Romani et al., 2008). Our findings indicated that TRPC1 knockdown contributed to the proliferation of ASMCs.
There are several reports concerning the regulation of TRPC1 by miRNAs. MiR-135a was reported to promote renal fibrosis in diabetic nephropathy by regulating TRPC1. In this study, we also found that TRPC1 was downregulated by miR-135a in A10 cells. Mounting evidence indicated the processes under the control of miR-135a, containing hypertension, bone and muscle development, epithelial ovarian cancer and endometriosis, and breast cancer (Li et al., 2008; Petracco et al., 2011; Chen et al., 2012). MiR-135a could inhibit cell proliferation by targeting bmi1 in pancreatic ductal adenocarcinoma (Dang et al., 2014). In human malignant melanoma cells, miR-135 post-transcriptionally regulates FOXO1 expression and promotes cell proliferation (Ren et al., 2015). In this study, we found that miR-135a inhibitor promoted proliferation of PDGF-BB-induced ASMCs, which reversed the effect of Schisandrin B.
In summary, we proposed that Schisandrin B inhibited the proliferation of airway smooth muscle cells via miR-135a suppressing the expression of TRPC1. These findings provide new insights into the role of Schisandrin B and miR-135a in asthma and support the development of a novel therapeutic strategy.
Acknowledgments and funding
This work was supported by a grant from the National Natural Science Foundation of China (No. U1304801).
Conflict of interest
The authors have no actual or potential conflicts of interest to declare.




