Erythropoietin-enhanced endothelial progenitor cell recruitment in peripheral blood and renal vessels during experimental acute kidney injury in rats
Abstract
Beneficial effects of erythropoietin (EPO) have been reported in acute kidney injury (AKI) when administered prior to induction of AKI. We studied the effects of EPO administration on renal function shortly after ischemic AKI. For this purpose, rats were subjected to renal ischemia for 30 min and EPO was administered at a concentration of 500 U/kg either i.v. as a single shot directly after ischemia or with an additional i.p. dose until 3 days after surgery. The results were compared with AKI rats without EPO application and a sham-operated group. Renal function was assessed by measurement of serum biochemical markers, histological grading, and using an isolated perfused kidney (IPK) model. Furthermore, we performed flow cytometry to analyze the concentration of endothelial progenitor cells (EPCs) in the peripheral blood and renal vessels. Following EPO application, there was only a statistically non-significant tendency of serum creatinine and urea to improve, particularly after daily EPO application. Renal vascular resistance and the renal perfusion rate were not significantly altered. In the histological analysis, acute tubular necrosis was only marginally ameliorated following EPO administration. In summary, we could not demonstrate a significant improvement in renal function when EPO was applied after AKI. Interestingly, however, EPO treatment resulted in a highly significant increase in CD133- and CD34-positive EPC both in the peripheral blood and renal vessels.
Abbreviations
-
- AKI
-
- acute kidney injury
-
- EPC
-
- endothelial progenitor cell
-
- EPO
-
- erythropoietin
-
- FCS
-
- fetal calf serum
-
- HIF
-
- hypoxia-inducible factor
-
- IPK
-
- isolated perfused kidney
Introduction
Acute kidney injury (AKI) is a common, serious condition, especially in critically ill patients, and is independently associated with high mortality and prolonged hospitalization. Its underlying pathophysiological mechanisms are complex. In particular, ischemia-reperfusion leads to acute tubular necrosis and loss of renal function (Jang and Rabb, 2009). Until now, the different treatment modes with, for example, vasoactive substances have shown limited success (Wu et al., 2011). Based on animal and some limited preliminary human studies, the therapeutic use of erythropoietin (EPO) appears promising, at least for those at higher risk for AKI (Song et al., 2009).
EPO is a 30.4 kD glycoprotein and class I cytokine, which is mainly produced by peritubular fibroblasts of the renal cortex and outer medulla. Transcription of the EPO gene is regulated by hypoxia-inducible factor (HIF). With decreased oxygen delivery, the von Hippel–Lindau tumor suppressor protein decreases HIF proteolytic activity, thereby increasing EPO production and blood cell maturation.
In addition, EPO is also synthesized locally by many tissues, especially in response to metabolic stress and may directly reduce apoptosis (Sirén et al., 2001), oxidative stress, and inflammation. Although hematopoiesis is mediated via the EPO receptor homodimer, tissue protection is interferred via a heterocomplex composed of the EPO receptor and CD131, the β common receptor, through activation of mainly JAK/STAT5 and PI3K (phosphatidyl-inositol-3 kinase) signaling (Brines et al., 2008). The relevance of EPO for regeneration or protection against ischemia has been analyzed in different organ systems such as heart (Calvillo et al., 2003), liver (Yilmaz et al., 2004; Sepodes et al., 2006), vasculature (Nakano et al., 2007), and brain (Ratilal et al., 2014). The discovery of EPO receptors in non-hematopoietic organs like the kidney (Westenfelder et al., 1999) or in blood vessel endothelium (Anagnastou et al., 1994) is in agreement with the pleiotropic effects of EPO.
A mechanism by which EPO may improve regeneration processes is its ability to increase the mobilization of endothelial progenitor cells (EPCs) from bone marrow (Heeschen et al., 2003). These cells were first described by Asahara et al. (1997) and express three characteristic surface markers: CD34, CD133, and vascular endothelial growth factor receptor-2. Progenitor cells with this phenotype can be detected mostly in the bone marrow and in very small amounts in the peripheral blood (Kaushal et al., 2001). Their potential to migrate to regions of neovascularization and differentiate into CD133-negative mature endothelial cells has been described previously (Gehling et al., 2000; Peichev et al., 2000).
In contrast to previous AKI studies where EPO was mostly administered simultaneously with or prior to induced ischemia, in this study, we aimed to analyze the functional and structural changes in hypoxic-ischemic injured rat kidneys when EPO was applied after the injury. Furthermore, we evaluated the relation between EPO and circulating EPCs in peripheral blood.
Materials and methods
Drugs
EPO (Erypo FS 2000, Ortho Biotech Janssen-Cilag, Neuss, Germany) was provided as a solution containing 2,000 U/mL. Dose-dependent responses were not examined in our model. In previous studies, EPO was used in doses varying from 100 to 5000 U/kg (Patel et al., 2004; Sharples et al., 2004; Vesey et al., 2004; Lee et al., 2009). We choose a rather low dose, which we expected to ensure the protective effect of EPO without evoking negative effects such as hypertension and thrombosis.
Heparin sodium 25000 (Ratiopharm, Ulm, Germany) was used during the preparation of the IPK and thiopental (Trapanal®, Altana, Wesel, Germany) for anesthesia.
Experimental animals
The experimental studies were performed in accordance with German law and approved by the State Agency for Agriculture, Environment, and Rural Areas (LLUR), Germany (No: V312-72241.122-4.) Male adult Sprague–Dawley rats weighing 260–480 g obtained from Charles River Laboratories (Kißlegg, Germany) were housed in a temperature-controlled environment with a 12-h light/dark circle and had free access to food and water. The animals were randomly allocated into five groups: (i) single-shot EPO-group, in which rats were subjected to renal ischemia for 30 min and then administrated EPO (500 U/kg, i.v.) directly after this period; (ii) EPO-group, in which the animals received EPO on post-surgery days 1 until 3 (500 U/kg i.p./d) in addition to the single dose as described in (i); (iii) ischemia-group, in which rats were subjected to renal ischemia for 30 min without EPO application; (iv) sham-operated group, in which the animals underwent an identical surgical procedure without renal occlusion; and (v) control group with non-operated rats, which did not receive EPO.
Rats were anesthetized with Thiopental (60 mL/kg, i.p.) and body temperature was maintained at 37°C during surgery. The left jugular vein was cannulated for the administration of EPO as required. A midline abdominal incision was followed by bilateral renal occlusion for 30 min using non-traumatic vascular clamps. Occlusion was verified visually by observing blanching of the entire kidney surface. The abdominal incision was sutured and the animals were then allowed to move freely under the conditions described above. The day of renal ischemia was considered day 0. Urine was drawn directly out of the bladder and a catheter was placed into the right ureter for later urine collections.
Preparation of the isolated perfused rat kidney
We used the standardized operating procedure according to Pagel et al. (1991). The perfusate was delivered into the renal vasculature with a speed pump adjusted to maintain a constant mean arterial pressure (100 mmHg). The effluate was collected and recirculated. A gas mixture of O2:CO2 (95:5) was continuously delivered to ensure that the perfusate was well oxygenated throughout the study, maintaining a constant pH of 7.4. Urine was collected through a cannula inserted in the ureter.
Perfusate components
We used Krebs–Henseleit bicarbonate buffer (pH 7.4) as the basic medium and added human erythrocytes (hct 5%) and bovine serum albumin (10 g/L). To maintain stable organ function by eliminating metabolites and replacing used substrates and volume, the perfusate was dialyzed continuously against a 25-fold excess of a protein- and cell-rich solution.
Renal parameters
First jugular blood samples for the measurement of serum urea, creatinine, and hematocrit levels were taken prior to surgery and 4 h after EPO application and on days 1, 3, 5, and 7. Perfusion flow rate [mL/(min × g kidney)] was derived from the rotational speed pump. Renal vascular resistance according to Ohm's law [mmHg/[mL/(min × g kidney)], urine flow rate [µL/(min × g kidney)], glomerular filtration rate [GFR, assessed with inulin, (µL/(min × g kidney)], fractional sodium and water reabsorption rate, and albumin excretion rate were determined from the IPK.
Immunohistochemistry
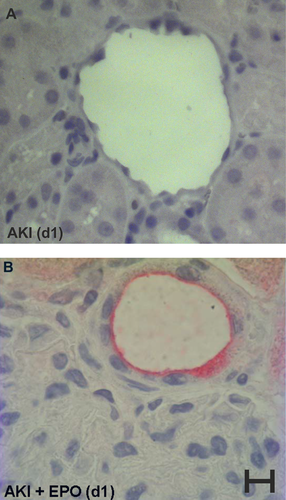
On the first postoperative day, the left, unperfused kidney was decapsulated, divided into four equal sections, and fixed in formalin (Roti®-Histofix). Kidney blocks were dehydrated and embedded in paraffin (Sherwood Medical, Mahwah, NJ, USA), cut in fine (3 μm) sections, and mounted on glass slides. Sections were then dewaxed and rehydrated. The primary antibody (CD133 rabbit polyclonal IgG, Selleck Chemicals) diluted in fetal calf serum (FCS) 1:50 was added. The slides were incubated overnight at 4°C and washed with buffer before the secondary antibody (polyclonal rabbit anti-mouse immunoglobulin from the EnViso Kit G2, Dako) was added. After rinsing, an enzyme enhancer [AP Enzyme (ENHANCER) from EnVision Kit G2, Dako] was supplied followed by another rinse and counterstaining with liquid-permanent-red (from the EnVision Kit G2, Dako). The prepared samples were stained with hematoxylin and eosin according to Mayer (1903).
Histological evaluation
Deparaffinized kidney sections were counterstained with hematoxylin and eosin and viewed under the light microscope (Axioplan 2, Carl Zeiss, Oberkochen, Germany) with 40× objective. Data were calculated for 10 tubules and 100 tubules per specimen (n = 3).
Flow cytometry
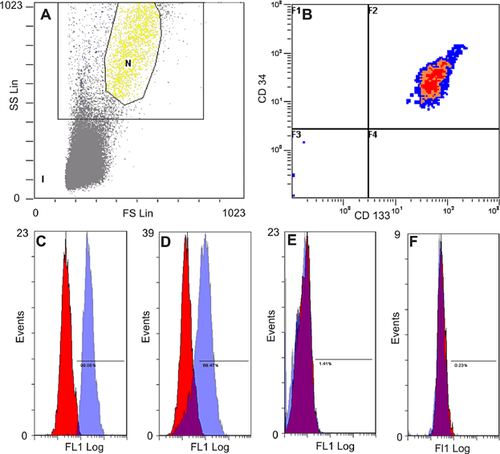
For every single probe, 1 × 106 cells from the peripheral blood were incubated with 10 µL of the primary CD133-antibody (CD133 rabbit polyclonal IgG, Santa Cruz) for 15 min at 4°C. To remove excess antibody, the material was centrifuged at 4,000 rpm for 2 min, the supernatant was removed, and the cells were rinsed in buffer. Subsequently, 10 µL of the secondary antibody (FITC-conjugated goat anti-rabbit IgG, Jackson Immuno Research) and the CD34-antibody (Cy-7 conjugated CD34, SC-7324 Santa Cruz) was added, the solution was resuspended, incubated at room temperature for 20 min, and rinsed with buffer again. Finally, the suspensions were rediluted up to 500 μL, and 100 μL of each suspension was used for the measurements. To quantify EPCs, the number of CD34/CD133 double-positive cells within the monocyte cell population was counted.
Statistical analysis
All values are expressed as mean ± SEM. Statistical analysis was carried out using “Sigma Plot 10.0” (Systat Software). Student's t-test and two-way ANOVAs were performed for statistical evaluation. P-values less than 0.05 were considered statistically significant.
Results
Hematocrit levels after EPO application
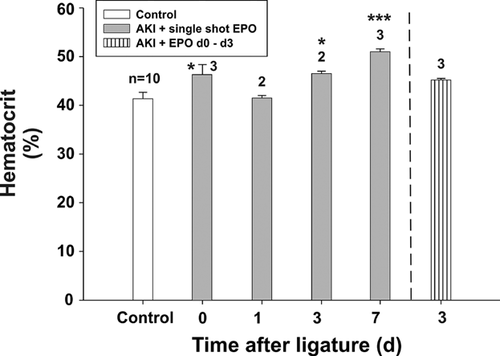
The average hematocrit in the control group on day 0 was 42.1 ± 2.02% (Figure 1). The hematocrit increased after single-shot EPO application (group 1) on day 0 (P = 0.11), day 1 (P = 0.03), and day 3 (P = 0.06); but in comparison to the control group, this increase was not always significant. On day 7, the hematocrit achieved its peak (51.0 ± 1.0 %) in group 1. Compared to the control, this increase was significant (P = 0.0027). In group 2 [single-shot EPO + EPO from days 0 to 3], no significance could be detected compared to the control group. We assumed that our EPO dose was only slightly hematologically effective.
Renal parameters after daily EPO application
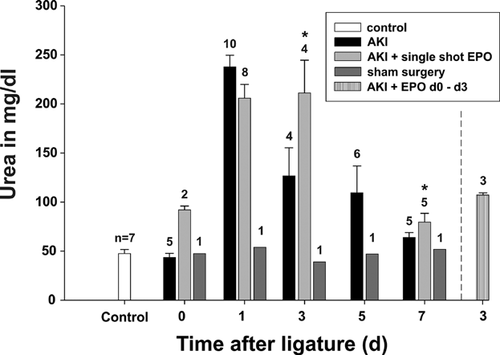
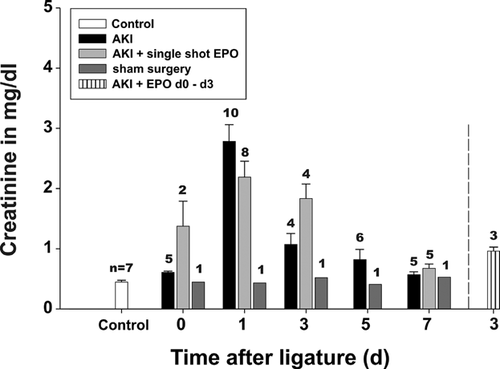
Generally, during AKI, the renal parameters urea (Figure 2) and creatinine (Figure 3) reached maximal levels on day 1 in the AKI groups in comparison to non-operated rats (control) as well as sham-operated animals. These values remained elevated until day 3. After a single EPO application (group 1), a moderate but not significant improvement in renal parameters was only detectable on day 1, whereas later, on days 3 and 7, the levels of urea and creatinine were found to be enhanced even in comparison to group 3 [AKI without EPO]. Thus, AKI seemed to be prolonged after a single EPO application. Daily EPO application (group 2) decreased the level of urea from 126.8 ± 57.0 mg/dL to 107.7 ± 3.79 mg/dL (P = 0.6) and creatinine from 1.07 ± 0.36 to 0.99 ± 0.96 mg/dL (P = 0.73) on day 3. However, the decrease was not statistically significant.
Effects of EPO on histological parameters
For histological analysis, the following parameters were investigated: number of tubules with brush border, number of tubules with a wide lumen, number of tubules with intraluminal cell detritus, the number of tubules and the number of mononuclear cells per visual field (Figure 4). All samples were taken on day 1, as positive effects of EPO, if any, on renal parameters were observed at that time. Group 2 [single-shot EPO + EPO from day 0 to day 3] was not analyzed anymore. Non-operated animals served as a control.
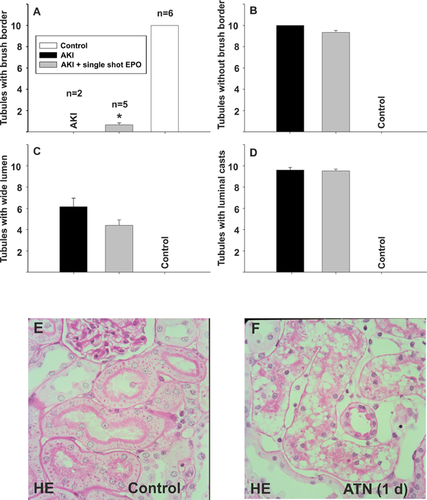
In the AKI group without EPO application, we did not find any tubules with brush borders (Figures 4A and 4B) and 6.15 ± 3.60 tubules with a wide lumen (Figure 4C). In contrast, after a single EPO application, the histological features of renal injury were remarkably and, to some extent, significantly altered. After single EPO application, 0.66 ± 1.3342 tubules with brush borders were detectable (Figures 4A and 4B) (P = 0.031) and the number of tubules with a wide lumen decreased to 4.4 ± 3.63 (Figure 4C) (P = 0.07). The number of tubules with cell detritus (Figure 4D) was not improved significantly after single EPO application (9.52 ± 1.13 with EPO vs. 9.60 ± 1.10 without EPO (P = 0.79) and the overall number of tubules per visual field was not significantly different among the groups analyzed (not shown).
Regarding the number of mononuclear cells, we could not detect a decrease in the single-shot EPO group compared to the AKI group without EPO (57.27 ± 10.89 vs. 56 ± 13.43). In the control group, we found significant less mononuclear cells (28.56 ± 10.56).
No significant improved renal function after EPO application in the ex vivo condition
On day 0, single EPO application improved the PFR to 18.2 ± 1.6 [mL/min × g kidney], which was similar to the value in the control group with 18.8 ± 0.9 [mL/min × g kidney] (Figure 5A). The PFR was generally enhanced from 0 to 7 days after single EPO application in comparison to the AKI group without EPO, although differences were not statistically significant (day 7, P = 0.087).
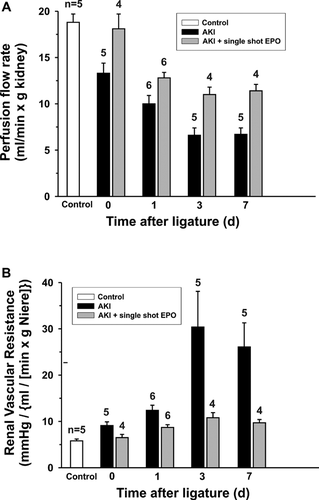
Renal vascular resistance (Figure 5B) was also improved after single EPO application throughout the experiment. A massive increase in renal vascular resistance in AKI animals on day 3 to a value of 30.4 ± 7.7 mmHg/(mL/[min × g kidney]) was reduced to 10.8 ± 1.1 mmHg/(mL/[min × g kidney]) after EPO application. The amelioration of renal vascular resistance after single EPO application was obvious, although not statistically significant (P = 0.32).
Analysis of the values for fractionated albumin clearance, albumin excretion rate, fractionated sodium reabsorption, water reabsorption, urine flow rate, and glomerular filtration rate was neither significantly nor consistently improved after single EPO application (Table 1).
| Albumin excretion rate (SEM) | Fractionated albumin clearance (SEM) | Fractionated water reabsorption (SEM) | Fractionated sodium reabsorption (SEM) | |
|---|---|---|---|---|
| 0-day control | 57.25 ± 16.74 | 9.26 ± 2.24 | 82.78 ± 3.56 | 85.12 ± 3.27 |
| 0-day EPO | 77.86 ± 8.12 | 243.72 ± 126.85 | 66.43 ± 14.58 | 69.33 ± 13.93 |
| 1-day control | 74.22 ± 24.68 | 302.29 ± 82.75 | 48.79 ± 6.21 | 52.34 ± 7.49 |
| 1-day EPO | 68.30 ± 31.01 | 508.98 ± 139.04 | 21.12 ± 20.93 | 22.86 ± 21.53 |
| 3-day control | 32.57 ± 6.21 | 21.82 ± 6.60 | 67.26 ± 9.19 | 70.94 ± 9.22 |
| 3-day EPO | 72.31 ± 19.41 | 381.990 ± 92.51 | 35.02 ± 13.81 | 39.62 ± 12.30 |
| 7-day control | 34,80 ± 8,08 | — | 81.72 ± 2.07 | 84.13 ± 1.89 |
| 7-day EPO | 49.00 ± 5.56 | — | 88.75 ± 3.37 | 90.90 ± 3.01 |
| Glomerular filtration rate (SEM) | Perfusion flow rate (SEM) | Renal vascular resistance (SEM) | Urine flow rate (SEM) | |
| 0-day control | 23.97 ± 10.25 | 13.33 ± 2.68 | 9.14 ± 1.76 | 5.11 ± 3.51 |
| 0-day Epo | 5.11 ± 3.51 | 18.06 ± 4.14 | 6.51 ± 1.73 | 23.97 ± 10.25 |
| 1-day control | 2.31 ± 0.63 | 10.70 ± 1.92 | 11.86 ± 1.92 | 1.67 ± 0.65 |
| 1-day Epo | 1.67 ± 0.65 | 12.86 ± 1.31 | 8.63 ± 1.07 | 2.31 ± 0.63 |
| 3-day control | 30.37 ± 24.17 | 6.67 ± 1.48 | 29.85 ± 15.72 | 2.03 ± 0.79 |
| 3-day Epo | 2.03 ± 0.79 | 10.95 ± 2.03 | 10.63 ± 2.62 | 30.37 ± 24.17 |
| 7-day control | 14.56 ± 4.65 | 6.67 ± 1.53 | 25.94 ± 11.47 | 11.39 ± 4.93 |
| 7-day Epo | 11.39 ± 4.93 | 11.41 ± 1.86 | 9.70 ± 1.87 | 14.56 ± 4.65 |
EPO increased the number of CD133-positive cells in peripheral blood and renal vessels
As demonstrated in Figure 6, EPO mobilized EPC out of the bone marrow into the peripheral blood. One day after single EPO application, 99.08% of the cells in peripheral blood were positive for CD133 (Figure 6C) and on day 3 after EPO application, 68.47% of these cells (Figure 6D) were still positive. In our immunohistological staining, we additionally found a significant increase in CD133 positive vessels on day 1 in AKI kidneys after single EPO administration when compared to AKI kidneys without EPO application (7.64 ± 4.38 cells vs. 1.42 ± 1.74 cells, P = 0.0000007) (Figure 7). In the AKI group without EPO application, and in the sham surgery group, the proportion of CD133 positive cells was less than 2%.
Discussion
In previous AKI studies, EPO was mostly administered simultaneously with or prior to induced ischemia. Kiris et al. (2008), for example, treated rats with an EPO dose of 1,000 U/kg 5 min before induced ischemic AKI. They found a significant decrease in the level of biomarkers of oxidative stress and in glomerular and tubular necrosis in the EPO group in comparison to the AKI group without EPO. As administering EPO prior to ischemic trauma is not practicable in most cases in the clinical routine, our intention was to establish a clinic-oriented model for the use of EPO as a positive mediator of regeneration in AKI. For this reason, we did not use EPO to prevent AKI, but to treat an already existent AKI (group 1). In addition, we included one group also receiving EPO on days 1–3 after surgery (group 2). We used the hematocrit as an indicator for the hematological effects of EPO. As expected, we found a higher hematocrit after EPO administration in comparison to the control group. On day 7, this difference was significant for group 1. It is worth mentioning, that hemodynamic improvements due to the correction of anemia can have positive effects on renal function by themselves. But the tissue-protective action of EPO is not only the result of the correction of anemia-related tissue hypoxia.
We characterized the kidney function by blood parameters, urine volume, functional parameters through our isolated perfused kidney (IPK) and histological evaluation. After a single EPO administration, we found at best a statistically non-significant tendency for improvement in renal parameters on day 1. Our data could not prove significant functional amelioration of the kidney by EPO administration after AKI. Contradictory results were described by Lee et al. (2009). The study of Lee et al. (2009) supports a daily EPO application after AKI. They administered an EPO dose of 100 U/kg from day 4 to day 10 to rats after cisplatin-induced AKI and showed an acceleration of renal function recovery. However, in the randomized, placebo-controlled, double-blind clinical trial of Endre et al. (2010), they could not show an improvement in renal function after EPO application in AKI.
In our histological evaluation, EPO-treated AKI rats exhibited a significantly higher number of tubules with brush border compared to AKI rats without EPO application (P = 0.031), although the number of unimpaired tubules was still low compared to control animals. Furthermore, although EPO reduced the number of tubules with a wide lumen, this effect was not statistically significant and EPO had no significant effects on tubular luminal casts. In terms of inflammation, EPO administration did not reduce the amount of mononuclear cells during AKI.
Some authors questioned the positive long-term effect of EPO on kidney function. Gobe et al. (2014) described that an increased progression of kidney fibrosis when EPO was administered for AKI treatment. The adverse effects, including hypertension, vascular thrombosis, and eventually death, related to abruptly increased hematocrit and thrombocyte activation could be due to the relatively high dosing. Although there have been no in vivo studies to determine the optimal renoprotective dose of EPO during AKI, in vitro studies suggest supratherapeutic doses (200–400 U/kg) (Vesey et al., 2004). Establishing a minimum effective dose for EPO treatment in future studies will allow beneficial effects to outweigh potential adverse effects in the setting of AKI, as well as other conditions of ischemic organ injury. EPO derivatives without hematopoietic and thrombopoietic effects, such as asialoerythropoietin and carbamylated EPO, could represent an alternative approach and need further investigation.
However, we did show a significantly increased number of CD133- and CD34-positive cells in peripheral blood and, furthermore, a significant increase in CD133-positive vessels of injured rat kidneys after EPO application. As shown in prior studies, EPCs could be involved in the regeneration of renal vessels (Ward et al., 2011). It has also been demonstrated in a mouse spinal cord injury model that cells isolated from peripheral blood using CD133 antibodies are able to enhance angiogenesis and functional recovery (Kamei et al., 2013). It might, thus, be speculated that EPO application results in beneficial pleiotropic effects during ischemic kidney injury at the level of the vascular system by increasing the number of CD133-positive cells in peripheral blood and injured renal vessels. Hypoxic conditions among others can be considered an indicator for the expansion of CD133+ cells.
Conclusions
There is evidence that EPO administration at or near the time of AKI improves recovery acutely, although the long-term effects are not clear. We could not demonstrate a significant improvement in renal function when EPO was applied after AKI, indicating that it is necessary to apply EPO before or exactly at the time of AKI to gain positive effects. However, although still speculative, our results suggest that EPO leads to tissue-protective effects in AKI as deduced from histology, probably at the level of the vascular system by increasing the number of CD133+ cells in peripheral blood and renal vessels. On the other hand, the dose-dependent hematological effects, especially, the abrupt hematocrit increase and the stimulation of platelet adherence to injured endothelium could moderate these beneficial effects on damaged renal vascular tissue.
Acknowledgments and funding
The authors would like to thank Alexandra Tiedtke and Urszula Frackowski for their excellent technical assistance.
Conflict of interest
The authors declare that they have no competing interests.




