p21-activated kinase 7 is an oncogene in human osteosarcoma
Abstract
p21-activated kinase 7 (PAK7), also named as PAK5, is a member of Rac/Cdc42-associated Ser/Thr protein kinases. It is overexpressed in some types of cancer such as colorectal and pancreatic cancers. However, the expression status and biological function of PAK7 in osteosarcoma are still ambiguous. To evaluate the expression levels of PAK7 in osteosarcoma tissues and cell lines, immunohistochemistry was used. To investigate the role of PAK7 in cell proliferation, apoptosis and tumorigenicity in vitro and vivo, a recombinant lentivirus expressing PAK7 short hairpin RNA (Lv-shPAK7) was developed and transfected into Saos-2 cells. The silencing effect of PAK7 was confirmed by quantitative real-time PCR (qRT-PCR) and Western blot technique. PAK7 was overexpressed in osteosarcoma tissue and cell line. By knocking-down of PAK7, the proliferation and colony formation of Saos-2 cells were inhibited and apoptosis enhanced significantly. The in vivo tumorigenic ability in xenograft model of Saos-2 cells was also notably inhibited when PAK7 was knocked down. Our results imply that PAK7 promotes cell proliferation and tumorigenesis and may be an attractive candidate for the therapeutic target of osteosarcoma.
Introduction
Osteosarcoma (OS) is the most common primary malignant solid bone tumor in children and adolescents, which often originates in the metaphyses of long bones with a high tendency for local invasion and distant metastasis (Poletajew et al., 2011; Endo-Munoz et al., 2012; Friedrich et al., 2013; Rainusso et al., 2013). There has not been a drastic change in overall prognosis of osteosarcoma patients within the past decades despite multidisciplinary treatment (Hattinger et al., 2010; Ray-Coquard and Le Cesne, 2012; Yang and Zhang, 2013). The mechanisms of formation and development of osteosarcoma have been studied for a long time. Osteosarcoma is characterized by dysregulation of tumor suppressor genes and oncogenes, such as retinoblastoma tumor suppressor RB (Sosa-García et al., 2010), p53 (Lin et al., 2009; Rubio et al., 2013). P21 signaling is an important pathway that regulates the onset of osteosarcoma (Shen and Maki, 2010; Lossaint et al., 2011; Xu et al., 2013). PAKs (p21-activated kinases) are serine/threonine protein kinases that are stimulated by activated forms of the small GTPases, Cdc42 and Rac and bind to them (Martin et al., 2013). PAKs are divided into two groups, group I PAKs (PAK1–3) and group II PAKs (PAK4–6). They participate in various facets of events implicated in cell survival and apoptosis, such as modulating the organization of the actin cytoskeleton to control cell morphology and motility (Aslan et al., 2013; Itakura et al., 2013; Melzer et al., 2013). p21-activated kinase 7 (PAK7) is a distinctive member of PAKs, which activates JNK (Jun N-terminus kinase), but not p38 or ERK (extracellular signal-regulated kinase) (Pandey et al., 2002). Group I PAKs contains an autologous inhibitory sequence in the NH2-terminal regulatory domain and group II PAKs does not have the sequence which stands as a marked difference between the two groups. PAK7 cannot complement an STE20 mutation in Saccharomyces cerevisiae like PAK-Is GTPases do not regulate PAK7 kinase activity, although it interacts with Cdc42, which is mediated by the CRIB motif in the N-terminus, and which is constitutive and stronger than any other PAK (Belmonte et al., 2006). PAK7 is important in carcinogenesis as it is known to be overexpressed in some types of cancers, such as colorectal and pancreatic cancers (Eswaran et al., 2007; Giroux et al., 2009; Gu et al., 2013; Zhang et al., 2013). We have discovered that PAK7 is significantly positive in osteosarcoma tissue, but negative in osteoclastoma. PAK7 expression in human osteosarcoma cell lines and nude mouse model shows a correlation between PAK7 level and the biologic activity of osteosarcoma, which implies that PAK7 acts as molecular switch that regulates tumorigenesis and can be a potential therapeutic target in osteosarcoma.
Materials and methods
Patients and tissue samples
A total of 85 paraffin-embedded tissues samples from patients with osteosarcoma enrolled for immunohistochemistry were collected. Another 33 tissue samples from cases of osteoclastoma were used as control. The samples were collected from the affiliated 6th People's Hospital of Shanghai Jiaotong University from January 2005 to January 2012. Clinical pathology of patients included age at diagnosis, gender, tumor sizes, Ennecking stage and pathological type. This study was performed under a protocol approved by the Ethic Committee of the 6th hospital with written informed consent of all patients for research.
Immunohistochemistry
PAK7 expression was examined in 85 paraffin-embedded osteosarcoma tissues and 33 cases of osteoclastoma by immunohistochemistry method (Analysis Kit was purchased from Maixin Com, Fuzhou, China). The tissue slides were first dehydrated by heating to remove the paraffin. The microwave antigen retrieval procedure is applied to block unspecialized reaction by H2O2 and non-specific serum. The sections were incubated with anti-PAK7 antibody (ab110069, 1:100) and goat anti-rabbit IgG-H&L (HRP) (ab6721, 1:100), acquired from Abcam (Cambridge, MA, USA). For the negative controls, the primary antibody was substituted with rabbit IgG. Immunohistochemical scoring was performed before the clinical response was known. The tissue sections were observed under a ZEISS AX10-Imager A1, and pictures were taken using AxioVision 4.7 microscopy software. Manual scoring of tissue sections for cytoplasmic staining was done semi-quantitatively. Positive particles showed brown color in the cytoplasm. The staining intensity was dominant in tumor cells and was scored as follows: 0 for staining <10%, 1 for 10–25%, 2 for 26–50%, 3 for 51–75%, and 4 for >75% of the examined cells.
Cell culture
The human osteosarcoma cell lines Saos-2 and human osteoblastic cell line hFOB1.19 were obtained from the American Type Culture Collection (ATCC). The cells were cultured in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 units of penicillin/streptomycin (Invitrogen, San Diego, USA) and maintained at 37°C in air plus 5% CO2 humidified atmosphere.
Construction of recombinant lentivirus and lentivirus infection
The cDNA sequence of PAK7 was obtained from GenBank (accession number NM_177990). The nucleotides 1934–1954 of the PAK7 mRNA targeted by PAK7 shRNA (5′-CTAGCCGGGATTACCACCATGACAATTTCAAGAGAATTGTCATGGTGGTAATCCCGTTTTTTGGAATTAAT-3′) was inserted into the plasmid pFH-L (Hollybio, Shanghai, China). Non-silencing shRNA (5′-CTAGCCCGGTTCTCCGAACGTGTCACGTATCTCGAGATACGTGACACGTTCGGAGAATTTTTTTAAT-3′) was used as control that does not target any genes in humans, mice or rats as determined by screening with NCBI RefSeq. The digestion analysis of restriction endonuclease confirms the recombinant vector and all inserted sequences were verified by DNA sequencing. Lentiviruses were developed by triple transfection of 80% confluent 293T cells with PAK7 (or control) shRNA-expressing vector and the virion-packaging elements (pVSVG-I and pCMV△R8.92) using Lipofactinmine 2000 (Invitrogen) and were harvested in serum-free medium after 3 days and filtered through a 0.45 µm filter (Millipore, Bedford, MA).
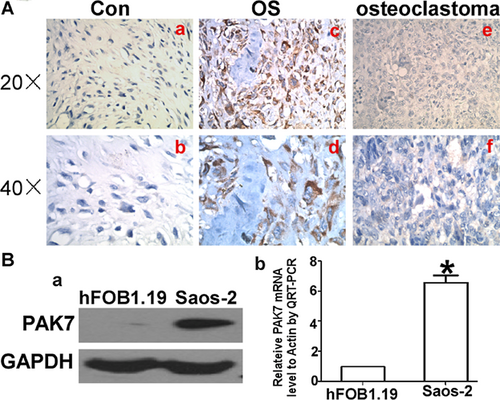
Transduction was done by planting Saos-2 cells at 5 × 104 cells/well in six-well plates along with shRNA encoded recombinant lentivirus against PAK7 (Lv-shPAK7) at a multiplicity of infection (MOI) of 60 in serum-free growth medium. When serum containing growth medium was added to the cells after 2 h, there was complete replacement of growth medium with recombinant lentivirus after 24 h. After 3 days of post-transfection, expression of reporter gene (GFP) was examined using fluorescent microscopy.
Quantitative real-time PCR
Total cellular RNA was isolated from Saos-2 cells using Trizol Reagent and was amplified by QRT-PCR Kit (Invitrogen). The first strand of cDNA was synthesized using 1 μg of total RNA in the presence of M-MLV Reverse Transcriptase. The primer 5′-GGCGTCCTCTTGTGTCTTC-3′ as forward and 5′- GTACTGAGTCCTTCTGATTTGC-3′ as reverse were used for PAK7 detection. β-Actin was applied as the internal control and amplified with 5′-GTGGACATCCGCAAAGAC-3′ as forward and 5′- AAAGGGTGTAACGCAACTA-3′ as reverse as primers. QRT-PCR was conducted with BioRad Connet Real-Time PCR platform. Relative expression levels of PAK7 in non-transduced and transduced Saos-2 cells were determined using 2−ΔΔCt methods.
Western blot
Both non-transduced and transduced Saos-2 cells were lysed in 50 µL lysis buffer (100 mM Tris–HCl (pH 6.8), 10 mM EDTA, 4% SDS, 10% glycine) on ice for 15 min. By centrifugation at 12,000g for 30 min at 4°C, the lysates were clarified and the supernatants collected for the determination of the protein concentration by the bicinchoninic acid (BCA) method. Equal amounts (30 µg protein) of lysate were run on 12% SDS–PAGE. After electrophoresis, protein blots were transferred onto PVDF membrane using an electro-blotting apparatus and the membrane incubated overnight with anti-PAK7 antibody (Sigma-Aldrich, K3265, dilution 1:100) by blocking with 5% nonfat milk in TBST solution. After three washes with TBST solution, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz, SC-2005, dilution 1:5,000) at room temperature for 2 h. The signals of detected proteins were visualized on ECL plus Western blotting detection system (Amersham) using GAPDH protein levels as control.
MTT assay
MTT assay was used to follow proliferation of Lv-shPAK7 transduced Saos-2 cells. Both the non-transduced and transduced Saos-2 cells (2 × 103/well) were placed in 96-well plates. Each batch was treated with MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) at a final concentration of 5 mg/mL for 4 h for 5 days repeated once every 24 h. The culture medium was removed and each well had with 100 µl of acidic isopropanol (10% SDS, 5% isopropanol and 0.01 M HCl) added. Absorbance was measured on a microplate reader at 595 nm by spectrometry. Assays were done in triplicate for each group and result was taken as the average of 3.
Colony formation assay
After the infection of lentivirus for 3 days, a total of 200 Saos-2 cells were seeded in 6-well plates, and the medium was changed at 3-day intervals. After 11 days of culturing, the colonies formed were washed with PBS and fixed in 4% paraformaldehyde at 37°C for 30 min, after which the colonies were stained with Giemsa for 15 min, washed with water and air-dried. Colonies were counted under a light microscopy. This experiment also was performed in triplicate.
Flow cytometric analysis of cell cycle and apoptosis
Saos-2 cells, both non-transduced and transduced, were collected after 4 days of infection and divided into two parts; one was stained with both AnnexinV-APC (eBioscience, San Diego, USA) and PI to detect apoptosis ratio, and the other was fixed with 70% ice-cold ethanol to detect DNA content staining with PI. An FACS flow cytometer (Cell Lab Quanta Beckman Coulter) was used to filter the suspension through a 50-mm nylon mesh and cells, which was repeated for three times.
TUNEL analysis of cell apoptosis
This assay was performed using the In Situ Cell Death Detection kit (Roche). Apoptotic cells were also visualized by the terminal deoxytransferase-mediated dUTP-biotin nick end labeling (TUNEL) method. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature, and made permeable with 25 µg/mL of proteinase K for 30 min at 37°C before being incubated with a 1:9 mixture of enzyme solution and labeling solution at 37°C for 2 h. They were washed three times with PBST for 5 min and incubated with peroxidase at 37°C for 30 min. Subsequently, the slides were incubated with 50 µL DAB substrate at room temperature for 10 min. After being washed three times with 1 × TBS, the cells were stained with hematoxylin. The TUNEL-positive cells showed orange-brown nuclei under light ZEISS AX10-Imager A1 microscope.
Tumorigenesis assay
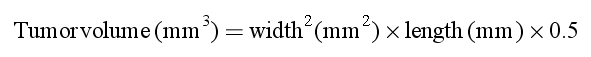 (1)
(1)At the end of the experiment, the mice were killed, and their tumors collected and immediately weighed. PAK7 gene expression of xenograft tumor samples were determined by using quantitative real-time PCR and Western blot.
Statistical analysis
Data were expressed as mean ± standard deviations (SD). Statistical analysis was performed using Student's t-test. Difference was considered to be statistically significant when the P < 0.05.
Results
PAK7 overexpression in osteosarcoma tissue and cell line
IHC was used to detect PAK7 expression both in osteosarcoma and osteoclastoma tissues, and to investigate the expression of PAK7 in osteosarcoma. In the negative control (Figure 1A-a and A-b), there were strong brown granula in the osteosarcoma tissue (Figure 1A-c and A-d), but infrequent pallide-flavens particle in osteoclastoma (Figure 1A-e and A-f). Statistical analyses are shown in Tables 1 and 2. Expressional difference between osteosarcoma cell line and human osteoblastic cell line was clarified at both mRNA and protein level (Figure 1Ba and Bb).
| PAK7 expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | N | − | + | ++ | +++ | ++ ++ | Positive rate (%) | X2 | P-value |
| Osteoclastoma | 28 | 28 | 0 | 0 | 0 | 0 | 0 | ||
| Osteosarcoma | 85 | 24 | 21 | 27 | 10 | 3 | 71.8 | 49.467 | <0.000 |
| PAK7 expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | ||||||||
| Clinical character | N | + | ++ | +++ | ++++ | Positive rate (%) | X2 | P-value | |
| Gender | 0.706 | 0.401 | |||||||
| Male | 58 | 18 | 14 | 17 | 7 | 2 | 69.0 | ||
| Female | 27 | 6 | 7 | 10 | 3 | 1 | 77.8 | ||
| Age/years | 0.005 | 0.946 | |||||||
| ≤18 | 43 | 12 | 8 | 15 | 6 | 2 | 72.1 | ||
| >18 | 42 | 12 | 13 | 12 | 4 | 1 | 71.4 | ||
| Tumor location | 1.261 | 0.532 | |||||||
| Axial | 3 | 0 | 1 | 1 | 1 | 100 | |||
| Upper limb | 4 | 1 | 0 | 2 | 1 | 0 | 75 | ||
| Lower limb | 78 | 23 | 20 | 25 | 8 | 2 | 70.5 | ||
| Tumor size (cm) | 0.216 | 0.642 | |||||||
| <10 | 57 | 17 | 12 | 20 | 6 | 2 | 70.2 | ||
| ≥10 | 28 | 7 | 9 | 7 | 4 | 1 | 75.0 | ||
| Enneking stage | 4.182 | 0.041 | |||||||
| II | 70 | 23 | 19 | 20 | 5 | 3 | 67.1 | ||
| III | 15 | 1 | 2 | 7 | 5 | 0 | 93.3 | ||
| Pathological type | 1.627 | 0.202 | |||||||
| Conventional type | 55 | 13 | 13 | 19 | 8 | 2 | 76.4 | ||
| Non-convention type | 30 | 11 | 8 | 8 | 2 | 1 | 63.3 | ||
| Local recurrence | 3.680 | 0.055 | |||||||
| Yes | 14 | 1 | 2 | 7 | 2 | 2 | 92.9 | ||
| No | 71 | 23 | 19 | 20 | 8 | 1 | 67.6 | ||
| Tumor cell necrosis rate (%) | 7.041 | 0.008 | |||||||
| <90 | 56 | 10 | 20 | 15 | 8 | 3 | 82.1 | ||
| ≥90 | 29 | 13 | 2 | 12 | 2 | 0 | 55.2 | ||
| Lung metastasis | 23.003 | 0.000 | |||||||
| Yes | 49 | 4 | 19 | 19 | 5 | 2 | 91.8 | ||
| No | 36 | 20 | 2 | 8 | 5 | 1 | 44.4 | ||
shRNA inhibited the expression of PAK7 in osteosarcoma cell
When compared to benign bone tumor, PAK7 expression was markedly higher in osteosarcoma tissue and cell line, which implies that PAK7 overexpression is important in osteosarcoma tumorigenesis. We designed the shRNA-PAK7 to explore its function. The transfection ratio is almost 100%. For protein level (Figure 2A), this inhibitory ratio was 7.9% according to the result of WB gray analysis (Figure 2C), whereas regarding the mRNA level, shRNA-PAK7 was about one third that of the control group (P < 0.001, Figure 2B).
PAK7 modulated the proliferation, apoptosis, and cell cycle of human osteosarcoma cells
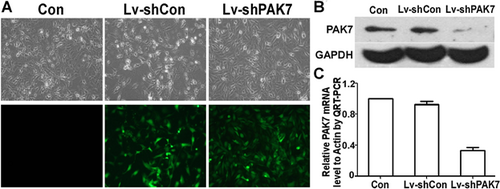
We investigated whether inhibiting PAK7 expression could modulate cell survival and activity. MTT assays were used investigate the role of PAK7 on Saos-2 cell proliferation. About 1 × 104 Saos-2, Lv-shCon, and Lv-shPAK7-transfected cells were seeded into 96-well plates and grown for 5 days. Cell proliferation was markedly attenuated in the Lv-shPAK7 cell compared to the vector control (P < 0.01, Figure 3A). The reproduction curve of Lv-shPAK7 group was flattened considerably, which was investigated, and found growth curve decreased considerably due to apoptosis ratio being >20% higher for Lv-shPAK7-transfected group, that is, significantly greater than both Lv-shCon and control groups. TUNEL data supported this view. Scatter profiles and the percentage of cells in the various phases of the cell cycle are shown in Figure 3B, but there were no statistical differences among the three groups.
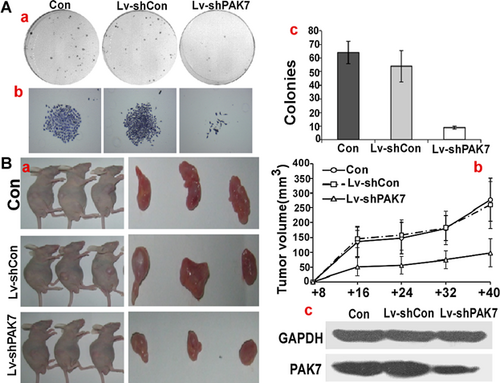
PAK7 modulated the tumorigenicity of human osteosarcoma cells in vitro and in vivo
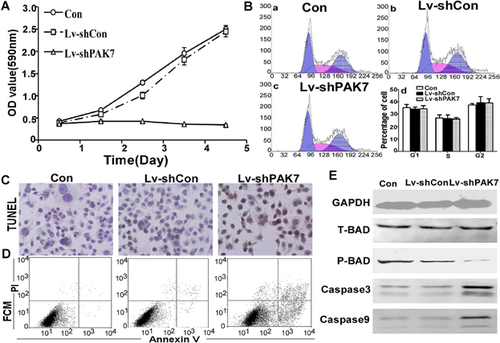
To measure the colony formation ability of Lv-shPAK7 transfected cells, soft agar assay was used. The average number of colonies in con and Lv-shCon was 64 ± 5/plate and 57 ± 7/plate, whereas the number of colonies in Lv-shPAK7 was just 10 ± 2/plate (P < 0.05). There was a significant reduction in both the rate and size of colony formation because of the expression of PAK7 (Figure 4A). For direct evaluation of the role of PAK7 in tumor formation in vivo, 2 × 106 con, Lv-shCon, and Lv-shPAK7 cells were injected into nude mice, each group consisting of three mice. A tumor was first detected on day 10 post-injection in control group. The tumor volume was measured every 4 days until 40 days, when the mice were killed. A significantly smaller tumor size was seen in the group of mice injected with Lv-shPAK7 cells (Figure 4B-a and B-b), showing that tumor formation was suppressed by PAK7 downregulation (Figure 4Bc).
Discussion
Normally, PAK7 showed restricted tissue-specific expression patterns that were both found to be highly expressed in the brain by virtue of PAK7 located on chromosome 20p12 and belong to the group II family of PAK serine/threonine kinases family (Pandey et al., 2002; Li and Minden, 2003), which activates mitogen-activated protein kinase (MAPK) signaling pathways and interacts with the small GTPase Cdc42 and Rac1, which are known to regulate diverse intracellular processes through their interaction with downstream effector proteins(Coste et al., 2012). Though many studies indicate that PAK7 is oncogenic when overexpressed, it is still controversial as to what PAKs does in tumorigenesis (Giroux et al., 2009; Chan and Manser, 2012; Eswaran et al., 2012; He and Baldwin, 2013). Zhang et al. (2013) found the differences of miRs, protein kinase, and methylation modification, among 3 non-small cell lung cancer (NSCLC) cell lines, which have different sensitivities to gemcitabine, in order to predict who would benefit from gemcitabine-based therapy, and to improve the effect of clinical therapy on NSCLC.
PAK7 is a novel potential biomarkers for prediction of gemcitabine sensitivity and putative targets to overcome gemcitabine resistance in NSCLC patients. Gu et al. (2013) also showed that PAK7 expression was upregulated in different gastric cancer cell lines and gastric cancer tissues. Gong et al. (2009) indicated PAK7 expression was increased with CRC progression, invasive and metastatic, which is negative correlation with OS. Based on these findings, we investigated whether PAK7 is overexpressed in OS patients, and if it correlated with OS tumorigenesis. From the immunohistochemistry in 85 cases of osteosarcoma tissues and 33 cases of osteoclastoma as control, we found that, in contrast to Figure 1A-e and A-f where the PAK7 immunoactivity was absent, its expression was significantly upregulated in OS tissue (Figure 1A-c and A-d). Statistical analysis pointed to a remarkable PAK7 increase in OS (P < 0.0001; Table 1). Table 2 shows the correlation of the PAK7 expression with clinical and pathological data. Tumors were seen with high PAK7 expression which had more advanced Ennecking stage (P = 0.019). However, PAK7 expression was not associated with age (P = 0.740), gender (P = 0.551), or histological subgroups (P = 0.151). This data suggests that PAK7 expression might be functionally important in tumor progression in osteosarcoma as the PAKs are important in cell motility and activate survival signaling pathways. In particular, mitochondrial localization of PAK7 is vital to its effects on apoptosis and survival (Wells and Jones, 2010). To identify the role of PAK7 in OS cell growth progression, we assumed that fluctuations of PAK7 protein levels influence either apoptosis or arresting of cell cycle and constructed the shRNA-plasmid serving as a research platform in vitro and vivo for exploring its function. There was a reduction of OS cell growth, clone forming and xenograft tumor with the downregulation of PAK7. As soon as PAK7 was knocked down by shRNA, apoptosis increased dramatically with cell cycle stable. PAK7 prevents apoptosis induced by camptothecin and C2-ceramide by phosphorylating BAD at Ser-112 in a protein kinase A-independent manner and prevents the localization of BAD to mitochondria (Cotteret et al., 2003). BAD is a pro-apoptotic member of the BCL-2 family that translocates between the cytosol and mitochondrial membrane-based partners, such as BCL-2 or BCL-xL. This complex formation inhibits a critical step in the activation of the downstream caspase protease cascade, and thus the ability of BCL-2 and BCL-xL to block the release of cytochrome c from mitochondria. The repression of PAK7 expression triggered the apoptotic cascade that leads to apoptosis through the inhibition of BAD phosphorylation for sure.
In conclusion, osteosarcoma has a poor prognosis, and PAK7 is important in osteosarcoma carcinogenesis. Overexpression of PAK7 only can be found in OS tissue, and does not correlate with histological classification. Repression of PAK7 activates the apoptosis pathway by influencing BAD phosphorylation.
Acknowledgment and funding
This work was supported by the National Natural Science Foundation of China (No. 81172548 and No. 81172105).




