Lithium chloride promotes the odontoblast differentiation of hair follicle neural crest cells by activating Wnt/β-catenin signaling
Abstract
The Wnt/β-catenin signalling pathway contributes to the maintenance of pluripotency and partial reprogramming of stem cells. Postnatal neural crest cells (NCCs) can differentiate into odontoblast-like cells due to their multi-potential property, but further endeavors need to be made to promote odontogenic differentiation of hair follicle neural crest cells (hfNCCs). This study investigated whether the Wnt pathway activator lithium chloride (LiCl) promotes odontoblast differentiation of hfNCCs. Change of proliferation, β-catenin and pluripotency markers of hfNCCs were examined after treatment with LiCl. An in vitro odontoblast differentiation model of hfNCCs was built using dental cell conditioned media (DC-CM). The effects of LiCl on odontoblast differentiation of hfNCCs showed that proliferation and expression of β-catenin in the cytosolic and nuclear compartments were increased in the LiCl-treated hfNCCs, and the pluripotency marks, Oct4, Klf4, Sox2 and Nanog, were more highly expressed in the LiCl-treated group than in the control group. The odontoblast markers such as DSP, DMP1 and Runx2, could be detected in hfNCCs induced by DC-CM, but in LiCl -treated group all three markers had stronger expression. Expression of β-catenin in the nuclear of LiCl-treated hfNCCs induced by DC-CM was higher than in the other groups. The data indicate that the Wnt pathway activator LiCl can promote proliferation and odontoblast differentiation of hfNCCs, and chemical approaches are of benefit in obtaining more desirable seed cell types for cell-based therapies.
Introduction
One of the significant goals of regenerative medicine is to repair wounds or to regenerate injured organs with seed cells from autologous tissues to minimize the immunological rejections. For tooth regeneration, most focus is on the mesenchymal stem cells from the tooth, such as dental pulp stem cells (DPSCs), stem cells from human exfoliated deciduous teeth (SHED), stem cells from apical papilla (SCAP) and others (Huang et al., 2009; Demarco et al., 2010; Ding and Chen, 2011; Mao and Prockop, 2012). However, there are challenges to the use of these cells in tooth regeneration; the sources and availability of these cells are limited, as they cannot be obtained from the edentulous jaw (Kim et al., 2010; Yildirim et al., 2011).
The mesenchymal cell types in tooth are derived from neural crest cells (NCCs) (Chai et al., 2000). The postnatal NCCs, which are multipotent cells, have been isolated from hair follicles (Sieber-Blum et al., 2004), dental pulp (D'Aquino et al., 2011), corneas (Yoshida et al., 2006), the inferior turbinate (Hauser et al., 2012) and the olfactory epithelium (Suzuki et al., 2013). These special adult stem cells have been the focus of tissue regeneration research because of their ability to differentiate into different types of cells and tissues. The morphogenesis of the hair follicle, as well as the tooth, initiates from the epithelial and NCC-derived mesenchymal interactions. The source and availability of hair follicle NCCs (hfNCCs) is unlimited. Therefore, hfNCCs have been used as seed cells in tooth regeneration research (Wu et al., 2009). However, promotion of odontoblast differentiation of hfNCCs is still needed.
The Wnt/β-catenin signalling pathway regulates many aspects of development, differentiation and disease (Clevers and Nusse, 2012). Interaction of the Wnt proteins with the frizzled (Fz) receptors and low-density lipoprotein-receptor-related protein 5/6 (LRP5/6) co-receptors results in the disruption of a complex that contains axin, adenomatous polyposis coli (APC) and GSK3β. In contrast to the classical Wnt/β-catenin signalling pathway, lithium chloride (LiCl) directly disrupts this complex by inhibiting GSK3β, leading to β-catenin stabilisation and nuclear translocation, thereby activating the Wnt/β-catenin signalling pathway (Klein and Melton, 1996; Lenox and Wang, 2003). The Wnt/β-catenin signalling pathway contributes to the maintenance of pluripotency in embryonic stem cells (ESCs) and the self-renewal of undifferentiated adult stem cells in many tissues (De Boer et al., 2004; Merrill, 2008; Lluis and Cosma, 2009). Treating ES cells with Wnt or inhibiting GSK-3β activity can stimulate ESC self-renewal and support pluripotency (Lluis et al., 2008). Thus, Wnt/β-catenin signalling activates a “reprogramming” factor in the cell nucleus that allows for a more effective conversion of the differentiated cell nucleus into a pluripotent cell. Activation of Wnt/β-catenin signalling may promote the dedifferentiation of adult stem cells.
Differentiation of stem cells is restricted by “niches”, which play decisive roles in the final differentiation outcome of the stem cells (Ikeda et al., 2009; Kim et al., 2010). Currently, many have agreed that the microenvironment is important for tooth regeneration (Jiang et al., 2008). Both growth factors and extracellular matrix components in the dental microenvironment are thought to be important in this cellular commitment pathway. Tooth germ cell conditioned culture medium has been used to induce the differentiation of DPSCs into functional odontoblasts and form the regular-shaped dentin-pulp complex (Yu et al., 2006). The same strategy was successful when applied to hfNCCs (Wu et al., 2009).
We investigated whether the Wnt pathway activator (LiCl) could promote the odontogenic differentiation of hfNCCs. Our results demonstrated that the proliferation activity and the expression of the pluripotency marks Oct4, Klf4, Sox2 and Nanog were increased in the LiCl-treated hfNCCs, and the odontogenic differentiation of the LiCl-treated hfNCCs was enhanced. The data indicate that treating postnatal hfNCCs with LiCl may be helpful for cell-based therapies.
Materials and methods
Cell culture and the dental cell conditioned media (DC-CM)
All procedures were performed following the guidelines from the animal care committee of Nanjing Medical University. The hfNCCs and dental cells were isolated as described elsewhere, with some modifications (Sieber-Blum et al., 2004; Yu et al., 2006). The bulge explants were isolated from the hair follicles of 8 week old ICR mice and were incubated in 0.3 mg/mL type I collagenase at 37 °C for 30 min. The bulge explants were placed in 25 cm2 culture flasks containing DMEM/F12 supplemented with 10% fetal bovine serum (FBS) (Gibco Life Technologies, Grand Island, NY, USA). The tooth bud tissues were carefully isolated from 2 day postnatal ICR mice pups and were minced into pieces smaller than 1 mm3 in 0.01 M phosphate-buffered saline (PBS). The tissues were digested with 0.3 mg/mL type I collagenase (Sigma, St. Louis, MO, USA) for 1 h.
The cells were dissociated by gentle triturating, collected by centrifugation, and washed twice with DMEM/F12 (Gibco Life Technologies) containing 10% FBS, 0.3 mg/mL glutamine (Invitrogen, Carlsbad, CA), 100 U/mL penicillin G (Gibco Life Technologies), 100 mg/mL streptomycin and 2.5 mg/mL ascorbic acid. Single-cell suspensions were generated by filtration through a 70 mm strainer, and the cells were washed again with DMEM/F12 supplemented with 10% FBS, then placed into 75 cm2 culture flasks (Costar, Cambridge, MA) at 1 × 105 cells/mL; the cells were grown in an incubator with 5% carbon dioxide at 37 °C. The culture medium of the primary dental cell was changed every 24 h until the cells were fully confluent; the supernatant was then collected and filtered through a 0.22 μm Millipore strainer (Carrigtwohill Co., Cork, Ireland), followed by centrifugation at 2,000 g for 20 min. The supernatant was mixed with an equal volume of fresh DMEM/F12 supplemented with 10% FBS and was used as the DC-CM for hfNCC induction. The DMEM/F12 supplemented with 10% FBS was used as a control medium. This experiment was repeated 40 times to collect the medium for future experiments, and the average duration of culture was 7 days.
The proliferation of the hfNCCs was examined using the MTT assay (Sigma) (MTT: 3-[4, 5-dimethyl-thiazolyl-2]-2, 5-diphenol tetrazolium biomide). Briefly, the hfNCCs were seeded into 96-well plates at 5 × 103 cells/well in complete medium (DMEM containing 10% FBS and 100 U/mL penicillin). After 1, 2, 4, 6, 8 or 10 days of culture, the cells were treated with 5 mg/mL MTT reagent (Sigma) and incubated for 4 h at 37 °C. The cells were washed twice with 0.01 M PBS, followed by treatment with dimethyl sulfoxide (Sigma). The absorbance at 490 nm was measured using an automatic enzyme-linked immunosorbent assay reader (ELx800, BioTek Instruments, USA).
Western blot analysis
Nuclear proteins were extracted from the hfNCCs after treatment with LiCl (Sigma, St. Louis, MO). Additionally, total protein was extracted from untreated hfNCCs, hfNCCs treated with LiCl for 24 h, hfNCCs treated with DC-CM for 7 days, and hfNCCs treated with LiCl for 24 h followed by treatment with DC-CM for 7 days. Ten micrograms of cell lysate was used for western blot analysis, and the lysate was resolved on a 10% SDS-PAGE gel, and then transferred to an Immobilon-P transfer membrane (Millipore Corporation, Billerica, MA, USA). The membranes were blocked with PBST (PBS with 0.05% Tween-20, 5% fat-free milk) for 30 min at room temperature and were then subsequently incubated with primary and secondary antibodies for 1 h each. The following primary antibodies were used: β-catenin at 1:500 (Santa Cruz, Biotechnology, INC, USA), Cyclin D1 at 1:500 (Bioworld, USA), Cyclin E1 at 1:500 (Bioworld, USA), Oct4 at 1:200 (Santa Cruz, Biotechnology, INC, USA), Klf4 at 1:700 (proteintech, USA), Nanog at 1:200 (Santa Cruz, Biotechnology, INC, USA), Sox2(Bioworld, USA), DSP at 1:500 (Abcam, England), DMP1 at 1:400 (sc-28596, R&D, USA), Runx2 at 1:500 (Boster, Wuhan, China), β-actin at 1:1000 (Boster, Wuhan, China) and Lamin B1 at 1:200 (Bioworld, USA). The following secondary antibodies were used: goat anti-mouse IgG at 1:10000 (Boster, Wuhan, China), goat anti-rabbit IgG at 1:10000 (Bioworld, USA) and rabbit anti-sheep IgG at 1:10000 (Bioworld, USA). The proteins bands were visualized using an enhanced chemiluminescence detection method WBKLS0100; Millipore Corporation.
Flow cytometry
The cells were harvested by trypsinization, collected by centrifugation at 1000 rpm for 5 min and washed in 2 mL of ice-cold PBS. The cells were fixed by resuspension in 2 mL ice-cold 75% ethanol and were incubated for 30 min at 4 °C. Finally, the cells were washed twice with PBS as previously mentioned, stained with 100 mg/mL propidium iodide (Sigma) at 48 °C for 30 min, and subjected to Elite ESP flow cytometry (Partec, Germany). One million cells were counted per sample, and the numbers of cells in the G1, S and G2 phases of the cell cycle were analyzed.
Comparative real-time PCR analysis
Total RNA was extracted from the hfNCCs, the hfNCCs treated with LiCl for 24 h, the hfNCCs treated with DC-CM for 7 days, the hfNCCs stimulated with LiCl for 24 h then treated with DC-CM for 7 days, and the DC-CM using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Reverse transcription of the total RNA was performed using the PrimeScriptR RT Reagent Kit (Perfect Real Time) (TaKaRa Code: DRR036A) (TaKaRa Holdings Inc., Kyoto, Japan). Real-time RT-PCR was performed with SYBRR Premix Ex TaqTM (Perfect Real Time) (TaKaRa Code: DRR042A) and ABI 7300 PCR system (Applied Biosystems) (Invitrogen, Carlsbad, CA, USA). Each reaction consisted of 100 ng of cDNA, 10 μL of 1 × SYBRR Premix Ex TaqTM and 0.2 μM (final concentration) of each primer. The reactions were performed in triplicate, and the experiments were repeated three times. Expression of the Oct4, Klf4, Nanog and Sox2 genes were measured, with β-actin gene being used as the reference gene. The PCR primers are shown in Supplementary Table S1. The amount of mRNA was normalized to the expression of the housekeeping gene β-actin, and the data were calculated using the 2-SS CT method.
Immunofluorescence
The cells were grown and treated on glass cover slips (Marienfield No. 1; 22 × 22 mm) and were fixed by immersion in ice-cold 4% paraformaldehyde in 0.25 M HEPES (pH 7.4) for 30 min at 4 °C. The cells were then washed three times in cold PBS and permeabilized in PBS containing 0.5% Triton X-100 for 20 min at room temperature. The cells were preincubated with 10% normal rabbit serum for 30 min to block any nonspecific staining. They were incubated overnight at 4 °C with primary antibodies against nestin (Bioss, Beijing, China), p75 (Bioss, Beijing, China), Sox10 (SANTA CRUZ BIOTECHNOLOGY, INC, USA), vimentin (Boster, Wuhan, China), Ck8 (Bioworld, USA), β-catenin (Santa Cruz, Biotechnology, INC, USA), DSP (Abcam, England) or DMP1 (sc-28596, R&D, USA); all of the primary antibodies were diluted 1:50 in PBS. The cells were washed three times for 5 min each in PBS and were incubated with the appropriate FITC- (green) and TRITC- (red) conjugated secondary antibody at a dilution of 1:50 for 2 h at room temperature and were then washed in PBS containing 0.1% Tween-20. DAPI was used to label the nuclei (c1002; Beyotime Institute of Biotechnology, Haimen, China) at a dilution of 1:1000 for 1 min, and the cells were washed in PBS three times. In the negative group, PBS was used in place of the primary antibody. The fluorescence images were obtained using a fluorescence microscope (Leica Microsystems, Mannheim, Germany).
Statistical analysis
The data were expressed as the mean ± standard deviation. T-test and ANOVA were used to analyze the differences between the groups. P < 0.05 was considered statistically significant. The SPSS 17.0 software package was used as the data processing tool.
Results
Biological characteristics of hfNCCs in vitro
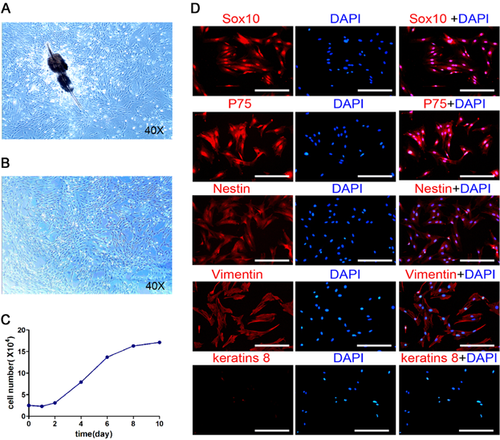
Bulge explants were isolated from the hair follicles of ICR mice. The hfNCCs could be seen migrating out of the bulge explants within 3 days of culture. These fibroblast-like cells showed a typical spindle-shape (Fig. 1A). The third-passage cells with a high proliferation rate were used for the following experiments (Fig. 1B). MTT assay showed that the hfNCCs began to enter the logarithmic growth phase after 2 days of culture, growth plateauing after 8 days (Fig. 1C). To validate the relationship between the hfNCCs and the neural crest derived cells, we examined the expression of several cell phenotype markers; hfNCCs expressed Sox10, nestin, p75 and vimentin, but not express keratinized protein 8 (CK8) (Fig. 1D). These results suggest that hfNCCs possessed the characteristics of neural crest cells.
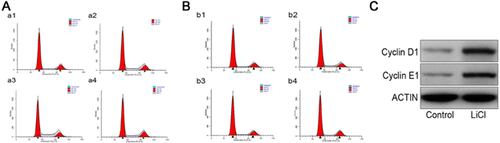
LiCl promotes the proliferation of hfNCCs
Proliferation of hfNCCs can be promoted by different concentrations of LiCl, due to the activation of the Wnt/β-catenin signalling pathway (Li et al., 2011 Tang et al., 2011). To determine the optimal concentration of LiCl and the optimal induction time, we followed changes in the cell cycle using FCM (Fig. 2A and B). The percentage of cells in S phase was larger when the hfNCCs were cultured in 20 mM LiCl for 24 h compared to the other groups, which suggests that the Wnt/β-catenin signalling pathway is activated efficiently under these conditions (Supplementary Table S2, S3, S4 and S5). We also detected Cyclin D1 and Cyclin E1 expression, which are essential for DNA synthesis and intimately involved in cell cycle regulation. Western blot analysis indicated that the expression of Cyclin D1 and Cyclin E1 increased in the hfNCCs after treatment with LiCl (Fig. 2C), suggesting that the promotion of the hfNCC proliferation by LiCl may be due to the upregulation of cell cycle proteins.
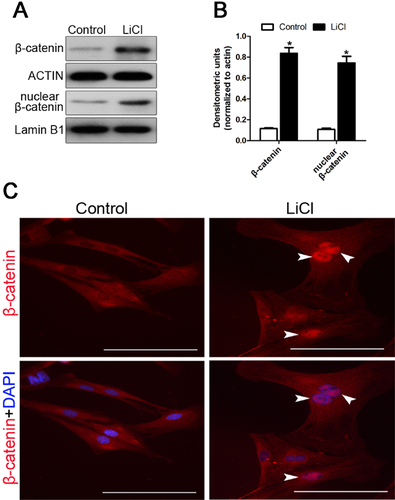
LiCl enhances the expression of β-catenin in hfNCCs
Activation of the Wnt/β-catenin signalling pathway has become a strategic goal in tooth regeneration due to its role in tooth development, and upregulation of β-catenin can activate the Wnt/β-catenin signalling pathway. LiCl can inhibit GSK3β expression, which leads to β-catenin stabilization and nuclear translocation, thus activating the Wnt/β-catenin signalling pathway (Lenox and Wang, 2003). Our results show that both the nuclear and cytoplasmic expression of β-catenin in hfNCCs are enhanced after treatment with 20 mM LiCl for 24 h (Fig. 3A, B and C), which suggests that the Wnt/β-catenin signalling pathway has been activated.
LiCl up-regulates the expression of pluripotency marks in hfNCCs
Upregulation of pluripotency genes, such as Oct4, Klf4, Nanog and Sox2, can promote the transition of the cells into a reprogrammed state, which prolongs cell survival and maintains self-renewal. Real-time RT-PCR analysis showed that Oct4, Klf4, Nanog and Sox2 expression in hfNCCs was stimulated by treatment with LiCl compared to the control group (Fig. 4A), and western blot analysis also showed that the protein level of the Oct4, Klf4, Nanog and Sox2 was higher in LiCl-treated hfNCCs compared to the control group (Fig. 4B). These results suggest that hfNCCs acquires a stronger pluripotency after treatment with LiCl, perhaps due to the activation of the Wnt/β-catenin pathway, leading to the partial reprogramming of the hfNCCs.

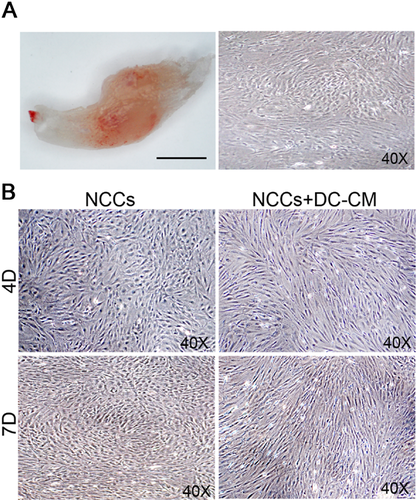
Effect of DC-CM on the odontoblast differentiation of hfNCCs in vitro
Dental cells were carefully isolated from the lower incisor pulps of 2 day postnatal ICR mice. After 7 days in culture, the cells had a polygonal shape (Fig. 5A). Dental cell conditioned medium (DC-CM) contains growth factors and extracellular matrix components from the dental microenvironment. To investigate whether hfNCCs can undergo odontoblast differentiation, we cultured the hfNCCs in DC-CM for 7 days. After 4 days, some of the spindle-shaped hfNCCs began to exhibit long single cellular processes, and after 7 days, the cells had a long, spindle shape and a tightly packed arrangement. However, these morphological changes were not observed in the hfNCCs that were not cultured in DC-CM (Fig. 5B). Moreover, the results of the IMF and western blot analyses showed that odontoblast markers, such as DSP and DMP1, were upregulated in the hfNCCs that were cultured in DC-CM (Fig. 6A–C). These results suggest that DC-CM is used to induce hfNCCs differentiation into functional odontoblasts.
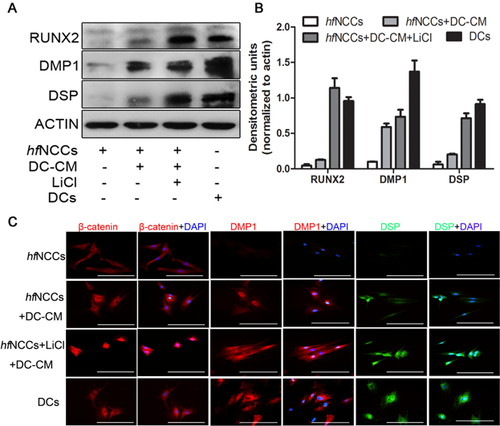
LiCl improves the odontoblast differentiation of hfNCCs
LiCl can activate the Wnt/β-catenin signal pathway, leading to the partial reprogramming of hfNCCs. Therefore, we investigated whether the odontogenic differentiation of the hfNCCs could be improved by the activation of the Wnt/β-catenin signalling pathway. Our results indicated that the nuclear expression level of β-catenin in hfNCCs was enhanced in the LiCl-treated hfNCCs cultured in DC-CM, and the expression of the odontoblast markers RUNX2, DSP and DMP1 was higher in the LiCl-treated hfNCCs than in the hfNCCs only cultured in DC-CM (Fig. 6A, B and C). These results suggested that the odontogenic differentiation of hfNCCs is improved by an activated Wnt/β-catenin signalling pathway.
Discussion
The odontogenic process can be initiated in stem cells, such as bone-marrow-derived cells, adipose-derived mesenchymal stem cells (ADSCs) and neural crest cells (NCCs) when the proper odontogenic signals are provided (Wu et al., 2009; Ferro et al., 2011; Miranda et al., 2012). Most maxillofacial tissues, such as bone and teeth, are derived from the neural crest (Chai et al., 2000). NCCs, a type of multi-potent adult cells that originate from the embryonic neural crest, reside in the bulge of hair follicles (Sieber-Blum et al., 2004). NCCs possess characteristics that combine the advantages of embryonic and adult stem cells (Yu et al., 2006 Sieber-Blum, 2010). Similar to embryonic stem cells, NCCs have a high degree of innate plasticity and a high level of purity, and can be expanded in vitro (Wong et al., 2006). Similar to adult stem cells, NCCs are readily accessible by minimal invasive procedures. In this study, hfNCCs were successfully isolated from the outer root sheaths of hair follicles from the mouse whisker pad. Consistent with a previous study, hfNCCs, like fibroblast cells, showed a typical spindle-shaped (Fig. 1B) and a high proliferation rate (Fig. 1C) (Sieber-Blum et al., 2004). hfNCCs expressed Sox10, nestin and p75, which are markers of neural crest-derived cells, but did not express CK8, a marker of epithelial cells (Fig. 1D). These results suggest that the cells isolated from the outer root sheaths of hair follicles are mostly derived from the neural crest.
Many studies now are focused on the role of some small molecules in tissue regeneration because they are easily accessible, safe and economical (Wei et al., 2011). As a prominent representative of these small molecules, LiCl, which has a well characterized biological action, can activate the Wnt/β-catenin signalling pathway by inhibiting GSK-3β, leading to β-catenin stabilization and nuclear translocation (Lenox and Wang, 2003, Kugimiya et al., 2007 Silva et al., 2010). Accumulation of β-catenin in the nucleus can activate the transcription factor lymphoid enhancer binding factor1/T-cell-specific factor (Lef/Tcf) and transcriptional coactivators, which regulate the expression of several canonical Wnt target genes, such as c-Myc and Cyclin D1. These transcription factors are involved in the regulation of cell cycle progression and differentiation (Moon et al., 2004 Tamura et al., 2010). The duration of β-catenin stabilization in the nucleus can influence cell differentiation and proliferation. β-catenin can also promote mitotic activity of osteoprogenitor cells in cranial sutures by upregulating Cyclin D1, a key regulator of cell cycle entry (Liu et al., 2007). In our study, Cyclin D1 expression was enhanced in hfNCCs after treatment with LiCl (Fig. 2C). Interestingly, we also found that the expression of Cyclin E1, another key regulator of the cell cycle, was significantly upregulated in hfNCCs. These results suggest that Cyclin E1 may be another target of β-catenin, but it needs deeper investigation.
The Wnt/β-catenin signalling pathway has been considered an essential trigger for development and a switch for the cell-fate path. LiCl can stimulate β-catenin stabilization in the nucleus and enhance cell fusion-mediated reprogramming (Lluis et al., 2008). Fibroblasts cultured in conditioned medium containing Wnt3a upregulated their expression of Oct4, Sox2 and Klf4 approximately 20-fold higher than the non-treated cells, an increase due to the reprogramming of these cells (Marson et al., 2008). In our study, the expression levels of Oct4, Sox2, Klf4 and Nanog are upregulated in hfNCCs after treatment with 20 mM LiCl for 24 h, suggesting that hfNCCs undergo partial reprogramming and become more sensitive to the differentiation signal. DC-CM contains growth factors and extracellular matrix components from the dental microenvironment. Once the odontogenic differentiation signal is acquired from the DC-CM, the hfNCCs can undergo odontoblast-like differentiation. Therefore, we collected the conditioned medium from dental cells culture to induce the hfNCCs to undergo odontoblast differentiation.
Tooth and hair follicle morphogenesis consists of sequential and reciprocal interactions between the neural crest-derived mesenchymal cells and the epithelium, which regulate its morphogenesis and differentiation (Wang et al., 2011). We speculate that hfNCCs has a greater ability to differentiate into odontoblasts when they have already undergone partial reprogramming and dedifferentiation. The molecular signals that regulate the fate of adult stem cells are permanently expressed in the epithelial structure and the surrounding mesenchyme of the apical bud, whose function is similar to that of the prenatal tooth bud (Fujimori et al., 2010). In our results, hfNCCs expressed osteogenic and odontogenic markers at low levels, which suggests that the hfNCCs inherently possess the ability to differentiate into odontoblasts. DSP and DMP1 are expressed in non-mineralized tissues (Prasad et al., 2011). Both the IMF and western blot analyses show that the odontogenic markers DSP, DMP1 and RUNX2 are upregulated in hfNCCs after culture in DC-CM. We found that DC-CM provides a suitable growth environment and odontoblast differentiation signal for the hfNCCs. Moreover, these odontoblast markers are more strongly expressed in the LiCl-treated hfNCCs than in the hfNCCs that are only cultured in DC-CM (Fig. 6A, B and C). These findings suggest that odontoblast differentiation of hfNCCs is enhanced by LiCl treatment.
Conclusions
LiCl can activate canonical Wnt signalling and promote the proliferation and reprogramming of hfNCCs; as such, these cells acquired a stronger pluripotency and are more sensitive to the odontogenic differentiation signal. Thus, hfNCCs treated with LiCl may be good candidate seed cells for tooth regeneration.
Acknowledgements and funding
This work was supported by the National Natural Science Foundation of China grant (81070810) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (2014-37).




