Folate decorated magnetite nanoparticles: Synthesis and targeted therapy against ovarian cancer
Abstract
Doxorubicin (DOX) loaded folate-coated magnetic Fe3O4 nanoparticles (MNPs) were prepared by co-precipitation and emulsification cross-linking method and uniform NPs with an average particle size of 15 (bare) and 35 nm (FA-MNPs), with high encapsulation efficiencies (EE), were obtained. The release profiles of conjugated DOX showed that pH 6.0 provided suitable conditions for proper drug release. IC50 analysis of C30 and CP70 cell lines after 96 h (4 days) exposure to DOX-FA-MNPs showed that the most significant IC50 were at 5.4 ± 0.6 and 11.2 ± 2.7 mM DOX loaded onto the FA-MNPs. Thus CP70 cells are more resistant to lower concentrations of drug compared to C30 cells (P < 0.05). C30 and CP70 cells reached 91 and 81.8% apoptosis after administration of DOX-FA-MNPs (5 mg/mL) compared to 49.2 and 46.6% after administration of the free drug, respectively (P < 0.05). this study indicates that FA modified MNPs enhances the DOX-induced apoptosis in both of the human ovarian cancer cell lines with sharp decreases in the levels of bcl-2 and surviving, and increased expression of caspase-3.
Introduction
There is increasing interest in biocomposites containing magnetite (Fe3O4) nanoparticles (MNPs) dispersed in polymeric, glassy or ceramic matrices. Iron oxide nanoparticles are of great scientific interest in technological applications, such as high-density magnetic recording media, biosensors, ferrofluids, magnetic resonance imaging, and biomedicine. Biomedical applications require iron-oxide nanoparticles smaller than 20 nm and a narrow size distribution. The properties of these materials depend strongly on the particle size, the particle-matrix interactions and the disposal of the nanoparticles in the matrix (Moreno et al., 2002; Cornell and Schwertmann, 2003; Perez et al., 2003).
MNPs can be engineered with desired functionality. The techniques used for surface functionalisation comprise grafting of or coating with organic materials, including surfactants or polymers (Chandra et al., 2010; Khosroshahi and Ghazanfari, 2010). The biocompatible surface-coating not only stabilises the iron oxide nanoparticles, but provides accessible surface for the biomolecular conjugation through the well-developed bioconjugation chemistry for biomedical applications (Sahoo et al., 2001; Nayak et al., 2004). In addition, multiple grafting or coating of small molecules can provide multivalent systems that exhibit significantly enhanced efficacy towards the drugs and biomolecules (Mammen et al., 1998). Degradable polymeric nanoparticles are the most optimal choice for use as anticancer agents due to their unusual beneficial properties, most notably enhanced drug availability for prolonging the drug effects in tumour tissues (Fonseca et al., 2002). However, with respect to free drugs, when these have been loaded on nanoparticles, they decrease the permeability across the cell membrane (Müller and Kreuter, 1999).
Folic acid (FA) has recently emerged as a prominent targeting moiety, capable of interacting with cells expressing the folate receptor (FR) (Low and Kularatne, 2009). FR consists of a high affinity folate binding protein (FBP) attached to the membrane through a glycosylphosphatidyl-inositol anchor. It is overexpressed in ovarian carcinomas and other human tumours, but has little expression in normal tissues. This provides tumour cells with increased amounts of the FA, which is essential for DNA synthesis and seems to aid in aggressive tumour growth. In ovarian cancer patients, the overexpression of FR isoform a correlates with a higher histological grade and more advanced stage of the disease. Differential expression of FR in ovarian and other cancers makes it an attractive marker and target molecule for diagnosis and therapy of the disease (Low et al., 2008). Several folate-conjugated drugs have reached clinical evaluation. The site-specific delivery of drugs to the tumours using FR can be enhanced using high capacity carriers that can simultaneously incorporate multiple drug molecules into one particle and target them to the disease sites (Xia and Low, 2010).
Here, we demonstrate the successful synthesis of FA decorated magnetite nanoparticles. The drug delivery efficacy of FA-MNPs has been assessed against the human ovarian cancer cell lines, C30 and CP70.
Materials and methods
Chemicals
Cell culture reagents were from Life Technologies, Inc. (Grand Island, NY, USA). Doxorubicin and FA were purchased from Sigma–Aldrich Chemical Co. (St. Louis. Missouri, USA). RPMI-1640 medium and all the additives were purchased from GIBCO Co. (Grand Island, NY, USA). The ovarian cancer cell lines, CP70 and C30, were purchased from Pasteur Institute, Tehran, Iran. All other chemicals were obtained through standard suppliers.
Synthesis of folate loaded magnetite NPs
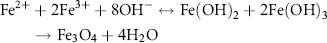
FA was activated with EDC (3–4 equiv.) in double-distilled water (pH 7.4) by stirring it for 5 min in the dark and then allowing it to react with the solution of iron chlorides. Pressurised air was supplied to the above solution to oxidise Fe2+ to Fe3+ for the formation of magnetite (Fe3O4) (24–26). Change in the colour of solution from dark brown to black due to the precipitation of Fe3O4 indicated the formation of bare and FA-MNPs (Qi et al., 2004). TMAOH (tetramethyl ammonium hydroxide) was used as the surfactant in preparing the bare MNPs to maintain an aqueous solution of bare NPs in colloidal suspension. The supernatant was discarded and the resulting precipitate was collected with a strong magnet and rinsed thrice with distilled water to remove excess NH4OH. FA-MNPs were purified using PD-10 desalting columns, thoroughly dialysed against double-distilled water (MWCO 3.5 kDa) and lyophilised.
Characterisation of synthesised NPs
Size and surface morphology of the synthesised NPs were characterised with the help of Transmission Electron Microscope (TEM; H-7600, Hitachi High-Technologies Corporation, Tokyo, Japan). A dynamic light-scattering spectrometer (DLS-7000AL, Otsuka Electronics, Japan) was used to determine the average diameters of the bare and the coated NPs. Magnetisation measurements were carried out at room temperature using a vibrating sample magnetometer (VSM, Oxford Instruments, UK), with the magnetic field rage of −1 to +1 T. The presence of FA-coating onto the surface of MNPs was studied by wavelength-dependent data of transmittance, obtained for the powdered samples of bare and FA-MNPs pressed into KBr pellets. The experiment was carried out using a FTIR Spectrophotometer (Model 8300, Shimadzu Corporation, Tokyo, Japan) at 4,000–400 cm−1. The crystallographic state of bare and HP-SPIO NPs was determined by XRD (JDX -8030).
Doxorubucin conjugation to the FA–MNPs
For drug conjugation to modified MNPs, 10, 50 and 100 µg of FA coated MNPs were first sonicated with 0.1–5.0 mg/mL DOX solution for 0.5 h and stirred overnight at room temperature in the dark before being centrifuged at 18,000g for 1 h. The DOX concentration of the samples was measured using a standard DOX concentration curve obtained by UV–Vis spectrophotometer at 233 nm.
Loading efficiency of incorporated drug
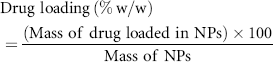

Cancer cell culture
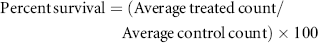
Cell viability and apoptosis analyses
Viability was determined by staining cancer cells with 1% methylene blue in 70% ethanol, followed by three washes in de-ionised water. Data obtained were used to calculate the IC50 and IC90 values for each cell line given DOX-FA-MNPs. For all experiments, 3 × 106 cells from each cell line were plated into 150 mm tissue culture dishes 24 h prior to nano-composite treatment. Cells were either untreated or treated with DOX-FA-MNPs at the corresponding IC50 level for 24–96 h at 37°C. Plates were washed thrice with PBS and incubated in 10 mL of fresh medium for an additional 48 h before being collected by sedimenting at 2,000g for 5 min.
For the MTT assay, C30 and CP70 cells were plated in 96-well plates and exposed to uncoated MNPs, FA-MNPs and DOX-FA-MNPs at 50 µg/mL for 24–96 h. After this culture period the medium replaced with fresh medium. Cellular apoptosis was detected by Annexin-V FLUOS staining and studied by flow cytometry.
Immunoblot analysis
C30 and CP70 cells were lysed in a buffer containing 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.2% (v/v) Nonidet P40 and protease inhibitor cocktail in ice for a period of 10 minutes. Cell debris was pelleted by centrifugation, and the total protein of the soluble extracts was determined by Bradford assay. Total soluble protein extract (30 µg) for each sample was resolved by SDS PAGE (12% gels). After electrophoresis, the caspase-3, survivin, bcl-2 and bax proteins were transferred to nitrocellulose, and the blot was blocked for 1 h at room temperature with a solution of 5% dried milk in PBST (0.1% (v/v) Tween 20 in PBS). The blot was incubated overnight at 4°C with either of the anti-bcl-2 monoclonal antibody, anti-caspase-3, anti-bax and anti–survivin polyclonal antibodies to measure the levels of protein expression. Primary antibodies were detected using an HRP conjugated secondary antibody and enhanced chemiluminescence.
Statistical analyses
Results are presented as mean ± SD. The two-way ANOVA and the Student's t-test were used to compare data from the different treatment groups. Statistical significance was accepted at P < 0.05.
Results
Nanoparticle characterisation
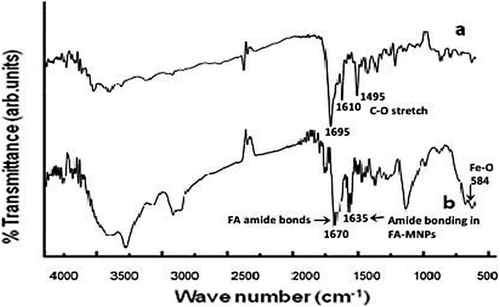
FT-IR analysis
According to the the MNPs spectrum by FTIR spectrometer (Figure 1), the 1,695 cm−1 region of the FA spectrum (a) represents amide bonding in the FA molecules, whereas the corresponding 1,670 and 1,635 cm−1 region of spectrum (b) shows amide bonding in the FA-MNP nanocomposite. The 584 cm−1 region of the FA-MNP spectrum represents the Fe–O group.
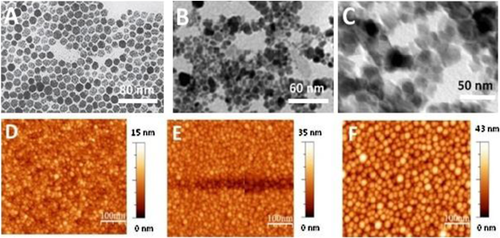
TEM and AFM analyses
Synthesised FA-MNPs characterised by transmission electron microscopy (TEM) and atomic force microscopy (AFM) gave final diameters of 15, 35 and 43 nm for bare, FA-MNPs and DOX-FA-MNPs, respectively (Figures 2A–2F).
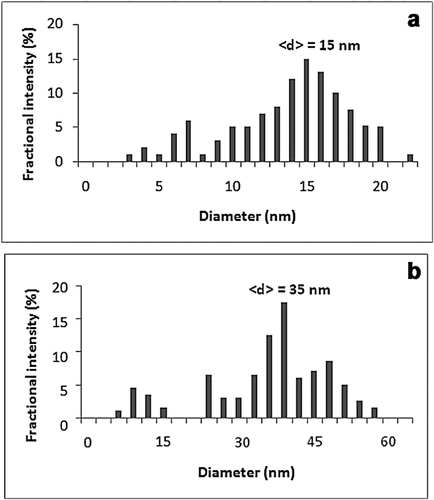
Dynamic light scattering (DLS analysis)
Size distribution of the bare and FA-MNPs was investigated in an aqueous solution by dynamic light scattering (DLS) spectrometry. the mean diameter of the FA-MNPs was ∼35 nm (Figures 3A and 3B). The size distribution of the bare MNPs was also determined at ∼15 nm, which confirms the findings of the TEM and AFM analyses.
XRD analysis
Figure 4A illustrates the powder XRD analysis of the crystallographic structure and physical properties of the bare and FA-MNPs, which show identical characteristic diffraction peaks at 2θ = 28°, 33.5°, 42.0°, 51.4°, 54.0° and 58.8°, corresponding respectively to the reflection plane indices of (220), (311), (400), (422), (511) and (440). However, no peaks corresponding to γ-Fe2O3 and α-Fe2O3, like 210, 213 etc., were found, suggesting the purity of MNPss. The XRD pattern of FA-MNPs depicted the same peaks at the same position (Figure 4A). The inset in the figure shows the selected-area electron diffraction (SAED) patterns of the FA-MNPs, which shows that the surface modification and conjugation of the MNPs to the FA did not cause phase change.
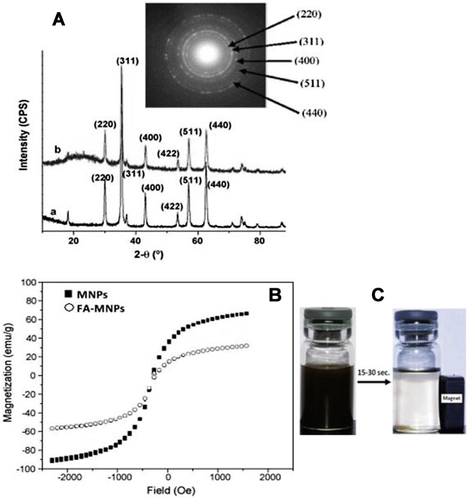
Magnetisation analysis
The formation of magnetic beads in FA-MNPs was confirmed by a change in the amount of saturation magnetisation. The samples analysed by VSM show superparamagnetic behavior. Figure 4B shows typical hysteresis curves of bare and FA-MNPs. The hysteresis loop had negligible coercivity at room temperature, and the saturation magnetisation (Ms) value at 1.0 T (after subtracting the diamagnetic background) was 65–70 emu/g at room temperature, which is lower than the bulk value of 90 emu g−1. From the magnetisation values, it can be calculated that 100% w/w FA-MNPs had in fact only 15% w/w FA coating on their surface.
Figure 4C shows the photographs of FA-MNPs dispersed in solution (left). The application of an external magnetic field (Mext) to the container attracted NPs towards it and their attachment to the wall of the container in close proximity to the magnet, with the dispersion becoming clear (right-side photograph). Removal of Mext and shaking led to the complete recovery of the dispersion, confirming that the prepared FA-MNPs were sensitive to Mext and retained a superparamagnetic property even after FA-coating.
Doxorubicin loading onto the FA-coated MNPs
Figure 5a and b give the UV–Vis spectra of the bare MNPs, FA-MNPs and DOX-FA-MNPs. To assess the conjugation capacity of DOX-modified NPs, the drug at 0.5–3.0 mg/mL concentrations were added to the FA-MNPs and incubated for 30 min, and then stirred overnight at room temperature in the dark. A DOX concentration curve (233 nm) was constructed from standard solutions. DOX loading of modified MNPs at an initial drug concentration of 2.5 mg/mL, was nearly saturating (Figure 5c).
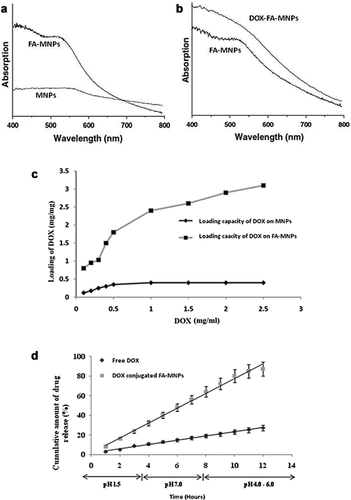
DOX release profile
Figure 5d shows the DOX release profiles of the free and the conjugated drug over the pH range of 1.5–7.0. At pH 7, a small amount of the drug release was observed after 48 h incubation. This is a desirable characteristic as pH 7.4 is an undesirable pH for the proper release of the drugs from the nanoconjugated drug carrier. This also prevents the premature release of the drugs before the nanoconjugates reach the cancer cells. pH 6.0 provided desirable conditions for proper drug release (Figure 5d). The first 10 h-represent the period of initial rapid release, followed by a steady-state. This pH-dependent drug release is favorable for the chemotherapeutic process as it can significantly reduce the pre-term drug release at the body pH level (pH 7.4) and maximises the amount of drug reaching the target tumour cells once the DOX-FA-MNPs internalise and enter the tumour by endocytosis (pH 4.5–6.5).
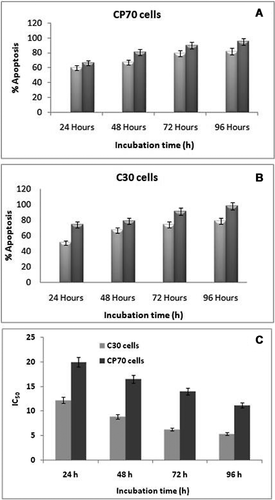
Cytotoxicity and synergistic effect of DOX-FA-MNPs on cancer cells
For cytotoxicity analysis, C30 and CP70 cells were treated separately with DOX, MNPs, FA-MNPs and DOX-FA-MNPs for 24 h. MTT analysis shows that 96 and 90% cell death was seen in the C30 and CP70 cells after the administration of DOX-FA-MNPs for 96 h, respectively (Figures 6A and 6B).
Apoptotic cell death was analysed by staining the cells with Annexin V-FLUOS staining kit and analysed by flow cytometry. A dose-dependent induction of early or late apoptotic cell death occurred in both cell lines (Figure 7A). Compared to CP70 cells, C30 cells were 91% apoptotic after DOX loaded FA-MNPs (5 mg/mL) (P < 0.05; Figure 7A), whereas 87.1% of the CP70 cells were apoptotic under same conditions (P < 0.05). This indicates that FA modified MNPs enhance DOX-induced apoptosis in both ovarian cancer cell lines.
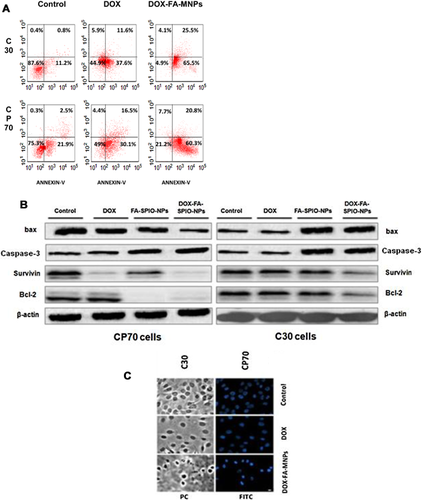
Western blot analysis for gene expression
Expression of bax, bcl-2, Caspase-3 and survivin proteins was followed in both cell lines treated with DOX alone, bare MNPs, FA-coated MNPs and DOX- FA-MNPs, respectively. Levels of bcl-2 and survivin proteins decreased sharply, while caspase-3 and bax were upregulated in the cultures treated with DOX-FA-MNPs cells (Figures 7B). Figure 7C shows by the Annexin-FITC staining procedure activation of the apoptotic process in cancer cells after nanoparticle-based DOX delivery in vitro.
Discussion
MNPs have a proven candidacy for their biocompatibility and wide applications in medicine (Artursson et al., 2004). This study has demonstrated the possibility of targeted delivery of DOX to the cancer cells via FA-MNPs. Achieving this goal required controlled modification of MNPs with multiple chemical functionalities. Indeed, each MNP carried at least several hundred carboxylic, amino, and hydroxyl groups that could simultaneously participate in chemical reactions and obstruct their direction and yield. Therefore, we were able to synthesise FA-MNPs. All reactions were carried out in mild conditions in aqueous media. Another task was the optimisation of the number of folate groups linked to a MNP. As the amount of folate moieties became too large, the MNPs swelled, which decreased drug-loading capacity and dispersion stability, and inhibited cell uptake. Hence, an optimal number of folate molecules were conjugated to MNPs to maintain good loading, stability and cell uptake. Such optimised FA-MNPs were shown to carry DOX to targeted cell populations expressing the FR. It was also important that drug loading did not affect the ability of targeted MNPs to interact with the receptors.
In vitro experiments show that FA-MNPs had good drug delivery and hence showed an improved anti-tumour effect. This suggests that the FA-MNPs displays folate specificity and elevated activity through a specific mechanism involving cell surface FR. While previous successes in folate-mediated targeting of drugs have been encouraging (Fernández-Urrusuno et al., 1999; Artursson et al., 2004; Qi et al., 2004), demonstration of a targeted delivery of MNPs-based therapeutic is a major step towards the practical use of this distinct and novel delivery system for cancer therapy.
Apoptosis can be promoted in cancer cells either by activating proteins upstream of the apoptotic signaling pathway or inhibiting anti-apoptotic factors. Overexpression of survivin is found in almost all human cancers. The presence or overexpression of survivin leads to resistance to apoptosis, which is associated with increased malignancy (Karen et al., 1997; Mohammadi-Samani et al., 2013). Previous in vitro work has shown that inhibition of survivin restores or enhances chemoreagent cytotoxicity (Gupta and Gupta, 2005).
Our results show a marked decrease in the expression of bcl-2 and survivin in both of the C30 and CP70 human ovarian cancer cell lines after DOX-FA-MNPs treatment (Figures 7A and 7B), with bax (a pro-apoptotic factor) protein being upregulated. Overexpression of bax accelerates cell death (Saifuddin and Dinara, 2012), while overexpression of bcl-2 represses bax function. Thus the ratio of bcl-2/bax can be a critical factor for estimating the cell's capacity for undergoing apoptosis (Schwertmann and Cornell, 2007). Although, bcl-2 and survivin are both apoptosis inhibitors, they work through different pathways in its regulation, which are called the mitochondrial and cytoplasmic pathways, respectively (Wang et al., 2008). These results have made it clear that the modified nanoparticulate system of drug delivery can attack the intrinsic pathway in cancer cells. Apoptosis and tumourigenesis are regulated by caspase-3-regulated gene products. Caspase-3 involves extrinsic death receptor signaling pathway of apoptosis, and suppression of caspase-3 promotes tumour necrosis factor (TNF)-induced apoptosis (Adams and Cory, 1998; Wang et al., 2003; Kirkin et al., 2004; Ling et al., 2004).
We found that DOX-FA-MNPs decreases expression of caspase-3 in both cell lines. We can assume that caspase-3 inhibited expression is related to the bcl-2 pathway. The nanoparticulate drug delivery system affects both the intrinsic (mitochondrial) and extrinsic (cytoplasmic) pathways by changing the expression of their regulatory factors, such as bax and bcl-2, and the transcription factors, NF-κB and caspase-3 (Pandey et al., 2007).
Drug loading is affected by particle size. Smaller particles have larger total surface areas. Therefore, most of the drug associated would be at or near the particle surface, leading to fast drug release. However, larger particles have large cores, which allow more drug to be encapsulated and slowly diffuse out (Pandey et al., 2007). Our results show that DOX-FA-MNPs have better drug delivery properties and release performance compared to bare nanoparticles.
Conclusions
This study demonstrates the possibility of delivering FR-MNPs with their therapeutic cargo to the cancer cells in vitro. An optimal number of folate molecules were conjugated to MNPs to maintain good anti-cancer drug loading, stability and cellular uptake. Such optimised FA-MNPs can carry their therapeutic cargo to targeted cell populations expressing FR. Finally, superior anti-tumour efficacy of DOX to the C30 and CP70 human ovarian cancer cells was seen using these FR-MNPs, along with decreased cellular toxicity. Thus the fundamental possibility for targeted delivery of the MNPs-based anti-cancer therapeutics has been established and opens a new prospect for clinical development of nanoformulations.