Overexpression of vasostatin-1 protects hypoxia/reoxygenation injuries in cardiomyocytes–endothelial cells transwell co-culture system
Abstract
Vasostatin-1 (VS-1) plays important roles in myocardial ischemia/reperfusion injury. We have explored the protective effects of VS-1 on cardiomyocytes using cardiomyocyte–endothelial cells Transwell Co-culture System. Cardiomyocytes and rat aortic endothelial cells (RAECs) were prepared from ventricles and thoraco-abdominal aorta of Sprague–Dawley rats. The experiment used cardiomyocytes alone culture group (C) and cardiomyocytes-RAECs co-culture group (T), each with three subgroups: C-Ad-Null, C-Ad-VS-1, C-Hb (Ad-VS-1 + NO scavenger Hb), or T-Ad-Null, T-Ad-VS-1 transfection, T-Hb. After 48 h incubation, all groups were treated with hypoxia for 60 min and then reoxygenated for 120 min. We also investigated endothelial cells-mediated cardiomyocytes protection. RAECs were treated with hypoxia for 30 min and reoxygenated with normal cardiomyocytes for 120 min. The cardiomyocytes apoptosis rate, aspartate aminotransferase (AST) and creatine kinase isozyme MB (CK-MB) were recorded. As expected, cardiomyocytes apoptosis, AST and CK-MB were significantly increased in the T-Ad-Null group than in the C-Ad-Null group. VS transfection significantly reduced these levels. However, apoptosis, AST and CK-MB levels were increased again after Hb treatment, returning to the similar level of the C-Ad-null group in the C-Hb group, but still significantly lower in the T-Hb group compared with the T-Ad-null group. RAEC injury caused cardiomyocyte injury, and VS-1 transfection of the RAEC decreased apoptosis and the levels of AST and CK-MB. The findings suggest that VS-1 exerts protective effects on the cardiomyocytes directly or indirectly by cardiomyocyte–endothelial cells interaction.
Introduction
Myocardial ischemia/reperfusion is a prevalent cause of myocardial damage, cardiac arrhythmia (del Monte et al., 2004), myocardial infarction (Vakeva et al., 1998), ventricular contractile dysfunction (Wildhirt et al., 1999), even heart failure and death (Yellon and Hausenloy, 2007). Although the mechanisms underlying the reflex are not entirely understood, oxygen free radicals, calcium overload, inflammation, cell apoptosis, and vascular endothelial dysfunction are involved (Moens et al., 2005; Turer and Hill, 2010). Therefore, strategies that can reverse the above response mechanism may effectively aid the restoration of cardiac function.
Vasostatin-1 (VS-1) is a recently identified regulatory peptides derived from the N-terminal domain of chromogranin A (CGA), which is not only secreted by granules of several endocrine and neuronal cells (Tota et al., 2007), but is produced by human myocardium (Cerra et al., 2008). There is strong evidence that VS-1, which is highly expressed in cardiovascular stress region, can cause vasodilation, enhance innate immunity, promote cell adhesion and inhibit the production of inflammatory cytokines to keep the balance of cardiovascular homeostasis (Brekke et al., 2002; Blois et al., 2006; Cerra et al., 2006; Helle, 2010). Cappello et al. (2007) and Pagliaro et al. (2007) report that, VS-1 with the concentration of more than 33 nmol/L can produce an ischemic preconditioning-like anti-myocardial ischemia reperfusion injury (MIRI) effects on myocardial cells. VS-1 may reduce myocardial injuries indirectly by inducing a Ca2+-independent/PI3-K-dependent NO release (Gallo et al., 2007; Cerra et al., 2008), or inhibiting P38MAPK phosphorylation via a pertussis toxin sensitive, presumably Gαi coupled mechanism in endothelial cells (Blois et al., 2006), that is, an endothelial-dependent manner. However, by constructing the VS-1 recombinant adenovirus and establishing the myocardial hypoxia/reoxygenation (H/R) cell model, preliminary experiments demonstrated that VS-1 inhibits the production of oxygen free radicals, inflammation and adhesion-related factors, expression of apoptosis-related genes, but upregulates the levels of endothelial nitrous oxide synthase (eNOS) and NO in myocardial cells in the absence of endothelial cells, that is, an endothelial-independent manner (Yu et al., 2011).
The structure and function of cardiac endothelial cells and myocardial cells are linked closely in human. Thus, we hypothesize that VS-1 may reduce myocardial cells injury by a co-protection mechanism, not only acting on cardiomyocytes, but also on endothelial cells. To test these assumptions, we have used a myocardial cell-endothelial cell Transwell Co-culture System, which imitates myocardial tissue microenvironment in vivo and may reflect whether there is additional protection of VS-1 when co-cultured.
Materials and methods
Construction of VS-1 recombinant adenovirus
Human VS-1 gene was amplified by PCR from pcDNACgA1–58 (donated by Xinzhi Shan of the Cardiovascular Research Institute of Guangdong Provincial People's Hospital) using the following primers: VS-1-F (5′-GGGGTACCATGCGCTCCG CCGCTGTCCT-3′) and VS-1-R (5′-CGAGATCTCTGCTGATGTGCCCTCTCCTTGGCGCCTTGGAGAGCGAGGTCTTGGAGCTCCTTCA GTAAATTCTGATGTCT-3′). The PCR procedure was 95°C for 5 min, 25 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 30 s. The amplified VS-1 fragment was ligated into pAd-Track under CAG cassette (Invitrogen, Carlsbad, USA), forming shuttle vectors pAdTrack-VS-1. After sequence confirmation, the shuttle vector plasmid (500 g) was linearized by Pme I (New England Biolabs, Beverly, USA) and co-transformed with the adenoviral genome vector, pAdEasy-1 (100 g, Invitrogen) into Escherichia coli BJ5183. The successful recombinant was selected by Kanamycin resistance and confirmed by Pac I (New England Biolabs) digestion. The recombinant vector Ad-VS-1 was finally linearized and transfected into QBI-293 cells using Lipofectamin™2000 reagent (Invitrogen) to pack into viral particles.
Cell preparation and transfection
The animal experiments were approved by local ethics committee. Ventricular myocytes of 1-day old neonatal Sprague–Dawley rat (150–180 g, Shanghai Institutes of Biological Science, China) were cultured by routine methods. Briefly, cardiomyocytes were prepared from ventricles and cultured in 60 mm dishes at 1 × 105 cells/cm2 in Dulbecco modified Eagle medium (DMEM) supplemented with 20% fetal bovine serum. After 24 h incubation at 37°C and air with 5% CO2, cell were grown to ∼70% confluency, followed by incubation for another 4–5 h after being given new medium. RAECs were prepared from the thoraco-abdominal aorta and cultured in DMEM/Ham's F12 medium containing 20% fetal bovine serum (Gibco, Carlsbad, USA), 100 pg/mL endothelial cell growth supplement (ECGS, Gibco), 100 µg/mL heparin, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C in an atmosphere of air with 5% CO2. When the RAECs showed typical cobblestone appearance, they were purified by differential digestion and sub-cultured in DMEM/F12 medium containing 10% calf serum and 2% ECGS at 37°C. Cardiomyocytes and RAECs were transfected at 100 MOI with empty pAdTrack vector, Ad-VS-1, or Ad-VS-1 + NO scavenger bovine hemoglobin (Hb) for 48 h before co-culture. Western blotting analysis was used to determinate VS-1 expression.
Transwell co-culture
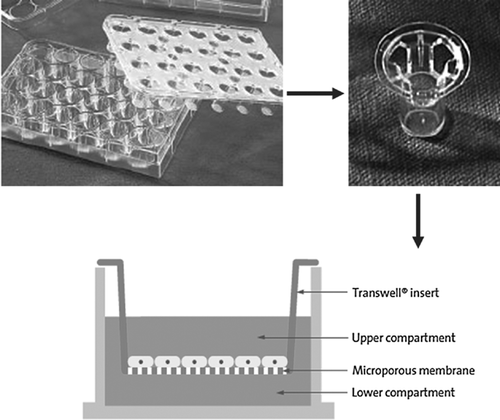
The in vitro assay procedure to co-culture endothelial cells and cardiomyocytes in the Transwell filter was essentially the same as in the previous reports (Akedo et al., 1986; Li and Zhu, 1999; Mason et al., 1999; Huang et al., 2009; Zhang et al., 2011). Assays were done in a 24-well transwell permeable support [polyester; thickness, 10 µm; pore size, 0.4 µm; pore density, 1 × 108 pores/cm2 (Corning Company); Figure 1]. The experiment was divided into two main groups and then six subgroups based on the above three different treatments (see Cell Preparation and Transfection Section). Cardiomyocytes alone culture group: this was cardiomyocytes seeded in the upper compartment and only medium was seeded in the lower compartment; three subgroups were C-Ad-Null, C-Ad-VS-1, C-Hb. In the cardiomyocytes-RAECs culture group, cardiomyocytes were seeded in the upper compartment and RAECs were seeded in the lower compartment; the three subgroups were T-Ad-Null, T-Ad-VS-1, T-Hb. After 48 h co-culture, all the groups made hypoxic for 60 min using Genbox (Biomerieux) (95% N2 and 5% CO2) and reoxygenated for 120 min (70% N2, 25% O2, and 5% CO2) to simulate the H/R process.
Endothelial cell-mediated cardiomyocyte protection
To determine whether endothelial cells can mediate cardiomyocytes protection, the RAECs in the three different treatments groups, Ad-Null, Ad-VS-1, Ad-VS-1 + Hb, were placed in the lower compartment under hypoxia for 30 min, and normal cardiomyocytes were added in the upper compartment followed by reoxygenation for 120 min collectively to simulate the H/R process.
Cardiomyocyte apoptosis
Apoptosis was estimated by staining cells with a combination of fluoresceinated (FITC) annexin V and propidium iodide (PI) (Yang et al., 2011). Briefly, for annexin V-PI analysis, cells were resuspended in 500 µl of binding buffer (140 mM NaCl, 2.5 mM CaCl2, 10 Mm HEPES; pH 7.4). Then 5 µl of annexin V-FITC (Biovision, CA, USA) and 10 µl of PI-PBS solution (50 mg/mL, PI, Sigma, St Louis, MO, USA) were added to 1 × 105 cells and incubated for 15 min at room temperature in darkness. Data were acquired within 1 h by flow cytometry to avoid cell damage and diffusion of PI through the cell membrane. Results were analyzed using Cellquest software.
Analysis of AST and CK-MB in cardiomyocytes
Measurements of aspartate aminotransferase (AST) and creatine kinase isozyme MB (CK-MB) from cell supernatants were analyzed with full-automated biochemical analyzer (Beckman, USA).
Statistical analysis
Statistical analysis used SPSS for Windows, version 13.0. Parametric data were statistically analyzed by the Student–Newman–Keuls (SNK) or one-way analysis of variance (ANOVA) followed by post hoc tests where appropriate. Data are expressed as mean ± standard deviation (SD). A significant difference was defined as P < 0.05.
Results
Effect of RAECs-cardiomyocytes co-culture on cardiomyocytes apoptosis
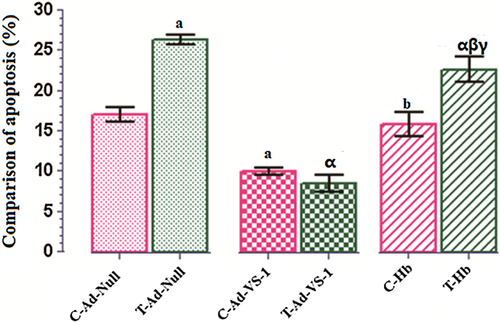
H/R-induced cardiomyocytes injury was taken as the amount of apoptosis. A significant increase in apoptosis in T-Ad-Null group compared with C-Ad-Null group was seen (P < 0.05). Cardiomyocytes transfected with Ad-VS-1 at 100 MOI significantly overexpressed VS-1 [data shown previously (Yu et al., 2011)]. After VS-1 transfection, cardiomyocyte apoptosis was significantly reduced, that is, no significant difference between T-Ad-VS-1 and C-Ad-VS-1 groups, and even lower level occurred in T-Ad-VS-1. However, Hb treatment, a NO scavenger, reversed the above myocardial protective effects of VS-1. In the C-Hb group, cardiomyocyte apoptosis was increased again to the level of the C-Ad-Null group. In the T-Hb group, although cardiomyocyte apoptosis was also increased, it was still significantly lower than in the T-Ad-Null group (Figure 2).
Effects of RAEC-cardiomyocyte co-culture on myocardial enzymes
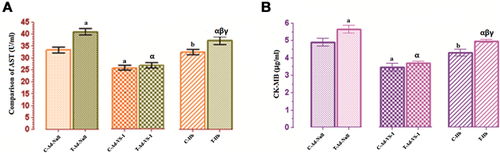
AST and CK-MB, cardiac cell-specific enzymes, levels are elevated upon the damage of myocardial cells (Francis et al., 2012; Prabodh et al., 2012). As expected, the levels of AST (Figure 3A) and CK-MB (Figure 3B) in T-Ad-null group were significantly increased compared with the C-Ad-null group (P < 0.05). VS-1 gene transfection could downregulate the expression of AST and CK-MB in C-Ad-VS-1 and T-Ad-VS-1 groups, which was reversed after the administration of Hb. Similarly, myocardial protection of VS-1 was completely inhibited by Hb in the C-Hb group, but partially suppressed by Hb in the T-Hb group.
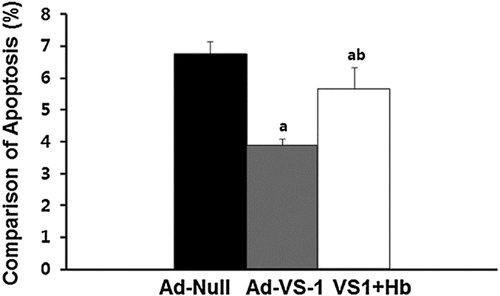
RAECs-mediated cardiomyocytes apoptosis
Myocardial apoptosis in groups of endothelial cell-mediated cardiomyocytes treated with H/R (Figure 4) was measured at the end of the experiment. After stimulation of H/R, apoptosis in cardiomyocytes in the Ad-null group was 6.8 ± 0.2%. This was reduced to 3.9 ± 0.12% in the Ad-VS-1 group, which was significantly less than in the Ad-null group (P < 0.05). However, after treatment with Hb, apoptosis rate was 5.7 ± 0.39%, which showed a significant difference with the above two groups (P < 0.05; Figure 4).
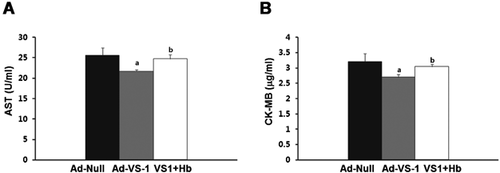
RAEC-mediated myocardial enzymes changes
AST (Figure 5A) and CK-MB (Figure 5B) were highly expressed in T-Ad-null group. VS-1 gene transfection significantly decreased their expression. But Hb eliminated the above protective effects of VS-1 on the levels of AST and CK-MB, leading to their high levels (Figure 5).
Discussion
Kuramochi et al. (2004) found that mixed culture of endothelial cells and myocardial cells inhibited oxygen free radical-induced apoptosis in myocardial cells, and helped to coordinate and couple the synchronization between systolic and diastolic myocardial cells. In addition, myocardial cells provided a feedback to secrete cytokines (such as vascular endothelial growth factor, VEGF) that affected the function of endothelial cells. In examining the protection of endothelial cells on myocardial cells, we have shown that, compared with the cardiomyocytes alone culture group, cardiomyocyte injury was more serious in the presence of endothelial cells with higher levels pf apoptosis, AST, and CK-MB, which indicates that damage products (including oxygen free radicals, inflammatory cytokines, and lysosomal degradation pathway) may be exchanged between endothelial cells and myocardial cells, resulting in the injury being superimposed. In contrast, cardiomyocyte injury was significantly decreased by VS-1 transfection in the presence of endothelial cells, which suggests that VS-1 may not only provide the direct protective effects on the cardiomyocytes, but induce the endothelial cells to synthetize anti-inflammatory cytokines, leading to protective superimposed effects. This also might partially explain why apoptosis of cardiomyocytes in T-Ad-VS-1 was even lower than in the C-Ad-VS-1 group.
VS-1 may ameliorate myocardial injury by PI3-K-dependent NO release from endothelial cells (Cappello et al., 2007; Fornero et al., 2012). VS-1 can directly induce NO release in myocardial cells to avoid injury (Yu et al., 2011). Thus, we also used the NO scavenger Hb group to first explore whether NO also plays an important role in the anti-MIRI process of VS-1-mediated myocardial cell-RAEC co-culture. Hb significantly suppressed the protective effects of VS-1 for cardiomyocytes, leading to a further increase in apoptosis, AST and CK-MB compared with T-Ad-VS-1 group. However, their levels were still significantly lower than those in the T-Ad-Null group, suggesting that other pathways are involved in VS-1-mediated protection. We found that, compared with the cardiomyocytes alone culture, cardiomyocyte injury was more serious in Hb co-culture groups. We speculate that the inhibition of the NO from endothelial cells may lead to additional injury to the cardiomyocytes.
To confirm our speculation, we found that H/R induced endothelial cells injury could injure cardiomyocytes that were not hypoxic. Apoptosis of cardiomyocytes was significantly reduced in Ad-VS-1 group, which implied that VS-1 in endothelial cells could protect them. VS-1 inhibits TNF-α-induced vessel permeability and VEGF-induced endothelial cells proliferation, together with migration and matrix invasion, ultimately attenuating cardiomyocyte injury (Veschini et al., 2011). VS-1 can mediate PI3-K-dependent NO release from endothelial cells and eNOS phosphorylation. NO inhibitor can reverse the protective effects of VS-1 in endothelial cells, causing more injury to the cardiomyocytes. These findings also show that VS-1 can induce the endothelial cells to secrete protective metabolite to indirectly exert protective effects on the cardiomyocytes.
In conclusion, Transwell co-culture has helped follow the role of VS-1 gene therapy in myocardial protection. Compared with in vivo studies, the co-culture system not only allows free exchanges of metabolites and cytokines, such as endothelium-derived active substances (nitric oxide NO, endothelin ET), inflammatory factors, and oxygen free radicals between cells, but can detect single-cell necrosis/apoptosis/metabolism independently. We not only found myocardial protection with the VS-1 gene the co-culture H/R model, but investigated VS-1 gene transfected endothelial cell-mediated myocardial protection mechanisms, findings that suggest that VS-1 exerts its protective effects both directly or indirectly through cardiomyocyte–endothelial cell interaction.
Conflict of Interest
None.
Acknowledgments
This study was supported by Shanghai Municipal Health Bureau (20114267) and National Natural Science Foundation of China (81300094).