Inhibition of Clinical Multidrug-Resistant Pseudomonas aeruginosa Biofilms by Cinnamaldehyde and Eugenol From Essential Oils: In Vitro and In Silico Analysis
Corresponding Author
Asma Benaissa
Laboratory of Applied Microbiology in Food, Biomedical, and Environment (LAMAABE), Department of Biology, Faculty of Sciences of Nature, Life, Earth, and Universe, Abou Bekr Belkaïd University of Tlemcen, Tlemcen, Algeria
Correspondence: Asma Benaissa ([email protected]) | Alfred Ngenge Tamfu ([email protected])
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Writing - original draft (equal), Writing - review & editing (equal)
Search for more papers by this authorWafaa Bouali
Laboratory Antifungal, Antibiotic, Physico-chemical, Synthesis and Biological Activity, Department of Biology, Faculty of Natural Sciences and Life, Sciences of the Earth and the Universe, University Abou Bekr Belkaid Tlemcen, Tlemcen, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorCorresponding Author
Alfred Ngenge Tamfu
Department of Chemical Engineering, School of Chemical Engineering and Mineral Industries, University of Ngaoundere, Ngaoundere, Cameroon
Food Quality Control and Analysis Program, Ula Ali Kocman Vocational School, Mugla Sitki Kocman University, Mugla, Turkey
Correspondence: Asma Benaissa ([email protected]) | Alfred Ngenge Tamfu ([email protected])
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Resources (equal), Software (equal), Validation (equal), Writing - original draft (lead), Writing - review & editing (lead)
Search for more papers by this authorBousselham Ammara
Microbiology Laboratory, University Hospital Center of Tlemcen, Tlemcen, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorSelcuk Kucukaydin
Department of Medical Services and Techniques, Koycegiz Vocational School of Health Services, Mugla Sitki Kocman University, Mugla, Turkey
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorNawel Latti
Laboratory of Applied Microbiology in Food, Biomedical, and Environment (LAMAABE), Department of Biology, Faculty of Sciences of Nature, Life, Earth, and Universe, Abou Bekr Belkaïd University of Tlemcen, Tlemcen, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorAbdelmounaim Khadir
Laboratory of Applied Microbiology in Food, Biomedical, and Environment (LAMAABE), Department of Biology, Faculty of Sciences of Nature, Life, Earth, and Universe, Abou Bekr Belkaïd University of Tlemcen, Tlemcen, Algeria
Department of Biology, Oran University, Oran, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Resources (equal), Supervision (equal), Validation (equal)
Search for more papers by this authorMourad Bendahou
Laboratory of Applied Microbiology in Food, Biomedical, and Environment (LAMAABE), Department of Biology, Faculty of Sciences of Nature, Life, Earth, and Universe, Abou Bekr Belkaïd University of Tlemcen, Tlemcen, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Supervision (equal), Validation (equal)
Search for more papers by this authorEl Hassane Anouar
Department of Chemistry, College of Sciences and Humanities in Al-Kharj, Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Resources (equal), Software (lead), Validation (equal), Writing - original draft (equal), Writing - review & editing (equal)
Search for more papers by this authorOzgur Ceylan
Food Quality Control and Analysis Program, Ula Ali Kocman Vocational School, Mugla Sitki Kocman University, Mugla, Turkey
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Resources (equal), Supervision (lead), Visualization (equal)
Search for more papers by this authorCorresponding Author
Asma Benaissa
Laboratory of Applied Microbiology in Food, Biomedical, and Environment (LAMAABE), Department of Biology, Faculty of Sciences of Nature, Life, Earth, and Universe, Abou Bekr Belkaïd University of Tlemcen, Tlemcen, Algeria
Correspondence: Asma Benaissa ([email protected]) | Alfred Ngenge Tamfu ([email protected])
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Writing - original draft (equal), Writing - review & editing (equal)
Search for more papers by this authorWafaa Bouali
Laboratory Antifungal, Antibiotic, Physico-chemical, Synthesis and Biological Activity, Department of Biology, Faculty of Natural Sciences and Life, Sciences of the Earth and the Universe, University Abou Bekr Belkaid Tlemcen, Tlemcen, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorCorresponding Author
Alfred Ngenge Tamfu
Department of Chemical Engineering, School of Chemical Engineering and Mineral Industries, University of Ngaoundere, Ngaoundere, Cameroon
Food Quality Control and Analysis Program, Ula Ali Kocman Vocational School, Mugla Sitki Kocman University, Mugla, Turkey
Correspondence: Asma Benaissa ([email protected]) | Alfred Ngenge Tamfu ([email protected])
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Resources (equal), Software (equal), Validation (equal), Writing - original draft (lead), Writing - review & editing (lead)
Search for more papers by this authorBousselham Ammara
Microbiology Laboratory, University Hospital Center of Tlemcen, Tlemcen, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorSelcuk Kucukaydin
Department of Medical Services and Techniques, Koycegiz Vocational School of Health Services, Mugla Sitki Kocman University, Mugla, Turkey
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorNawel Latti
Laboratory of Applied Microbiology in Food, Biomedical, and Environment (LAMAABE), Department of Biology, Faculty of Sciences of Nature, Life, Earth, and Universe, Abou Bekr Belkaïd University of Tlemcen, Tlemcen, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal)
Search for more papers by this authorAbdelmounaim Khadir
Laboratory of Applied Microbiology in Food, Biomedical, and Environment (LAMAABE), Department of Biology, Faculty of Sciences of Nature, Life, Earth, and Universe, Abou Bekr Belkaïd University of Tlemcen, Tlemcen, Algeria
Department of Biology, Oran University, Oran, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Resources (equal), Supervision (equal), Validation (equal)
Search for more papers by this authorMourad Bendahou
Laboratory of Applied Microbiology in Food, Biomedical, and Environment (LAMAABE), Department of Biology, Faculty of Sciences of Nature, Life, Earth, and Universe, Abou Bekr Belkaïd University of Tlemcen, Tlemcen, Algeria
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Supervision (equal), Validation (equal)
Search for more papers by this authorEl Hassane Anouar
Department of Chemistry, College of Sciences and Humanities in Al-Kharj, Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Resources (equal), Software (lead), Validation (equal), Writing - original draft (equal), Writing - review & editing (equal)
Search for more papers by this authorOzgur Ceylan
Food Quality Control and Analysis Program, Ula Ali Kocman Vocational School, Mugla Sitki Kocman University, Mugla, Turkey
Contribution: Conceptualization (equal), Data curation (equal), Formal analysis (equal), Resources (equal), Supervision (lead), Visualization (equal)
Search for more papers by this authorFunding: This work is supported by the General Directorate for Scientific Research and Technological Development (DGRSDT), Algeria.
ABSTRACT
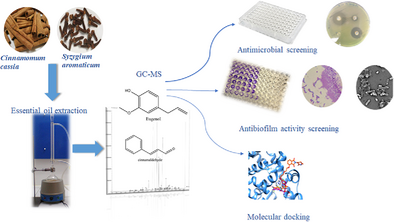
Pseudomonas aeruginosa causes nosocomial infections and chronic diseases. Cinnamomum cassia and Syzygium aromaticum are used natural antimicrobials. Essential oil (EO) from C. cassia (CCEO) and S. aromaticum (CEO) was characterized using GC-MS analysis. Eugenol (82.31%), eugenol acetate (10.57%), and β-caryophyllene (3.41%) were major constituents in CEO while cinnamaldehyde (88.18%), cinnamyl acetate (2.85%) and 2-methoxy cinnamaldehyde (1.77%) were main components in CCEO. The EOs and major constituents exhibited good antimicrobial activity against clinical strains of P. aeruginosa. Cinnamaldehyde exhibited the best antimicrobial effect with minimal inhibitory concentration (MIC) as low as 0.031% ± 0.07% (v/v) and inhibition zones reaching 30 ± 0.5 mm diameter. Test samples showed antibiofilm activities against two culture types and seven clinical strains of P. aeruginosa at concentrations of 2MIC to MIC/4. CCEO and its major constituent cinnamaldehyde were more active, compared to CEO and its major constituent eugenol. Scanning electron microscopy images showed untreated colonies with well-developed biofilms while there was significant reduction of biofilms with distorted architecture and cell shrinkage upon treatment with test samples. In silico studies indicated great interactions between the major compounds, eugenol and cinnamaldehyde, with the receptor proteins of P. aeruginosa revealed by negative binding energies. Eugenol and cinnamaldehyde exhibited appreciable druglikeness.
Graphical Abstract
Essential oils from Cinnamomum cassia and Syzygium aromaticum are abundantly rich in cinnaldehyde and eugenol respectively. The essential oils of both plants and their major compounds, cinnaldehyde and eugenol displayed very good antibiofilm activity against clinical strains of P. aeruginosa which was confirmed by SEM images. Molecular docking indicated good binding affinity of cinnaldehyde and eugenol with LasR protein as well as good druglikness. This shows that the essential oils and their major components can be used to treat infections caused by P. aeruginosa and relief resistant infections resulting from biofilms.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| cbdv202402693-sup-0001-SuppMat.pdf315.1 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. N. Tamfu, G. Kocak, O. Ceylan, F. Citak, V. Bütün, and H. Çiçek, “Synthesis of Cross-Linked Diazaborine-Based Polymeric Microparticles With Antiquorum Sensing, Anti-Swarming, Antimicrobial, and Antibiofilm Properties,” Journal of Applied Polymer Science 140 (2023): e53631.
- 2O. Ceylan, A. N. Tamfu, Y. I. Dogaç, and M. Teke, “Antibiofilm and Anti-Quorum Sensing Activities of Polyethylene Imine Coated Magnetite and Nickel Ferrite Nanoparticles,” 3 Biotech 10, no. 12 (2020): 513.
- 3Y. İ. Doğaç, A. N. Tamfu, S. Bozkurt, M. Kayhan, M. Teke, and O. Ceylan, “Inhibition of Biofilm, Quorum-Sensing, and Swarming Motility in Pathogenic Bacteria by Magnetite, Manganese Ferrite, and Nickel Ferrite Nanoparticles,” Biotechnology and Applied Biochemistry 71, no. 2 (2024): 356–371.
- 4A. N. Tamfu, O. Ceylan, G. Cârâc, E. Talla, and R. M. Dinica, “Antibiofilm and Anti-Quorum Sensing Potential of Cycloartane-Type Triterpene Acids From Cameroonian Grassland Propolis: Phenolic Profile and Antioxidant Activity of Crude Extract,” Molecules 27 (2022): 4872.
- 5R. Belas, “Biofilms, Flagella, and Mechanosensing of Surfaces by Bacteria,” Trends in Microbiology 22, no. 9 (2014): 517–527.
- 6S. M. Huszczynski, J. S. Lam, and C. M. Khursigara, “The Role of Pseudomonas aeruginosa Lipopolysaccharide in Bacterial Pathogenesis and Physiology,” Pathogens 9, no. 1 (2019): 6.
- 7K. Brindhadevi, F. LewisOscar, E. Mylonakis, S. Shanmugam, T. N. Verma, and A. Pugazhendhi, “Biofilm and Quorum Sensing Mediated Pathogenicity in Pseudomonas aeruginosa,” Process Biochemistry 96 (2020): 49–57.
- 8R. Vasudevan, “Biofilms: Microbial Cities of Scientific Significance,” Journal of Microbiology & Experimentation 1, no. 3 (2014): 00014.
10.15406/jmen.2014.01.00014 Google Scholar
- 9W. Yin, Y. Wang, and J. He, “Biofilms: The Microbial “Protective Clothing” in Extreme Environments,” International Journal of Molecular Sciences 20, no. 14 (2019): 3423.
- 10A. Kumar, A. Alam, M. Rani, N. Z. Ehtesham, and S. E. Hasnain, “Biofilms: Survival and Defense Strategy for Pathogens,” International Journal of Medical Microbiology 307, no. 8 (2017): 481–489.
- 11B. Prakash, B. Veeregowda, and G. Krishnappa, “Biofilms: A Survival Strategy of Bacteria,” Current Science 85, no. 9 (2003): 1299–1307.
- 12E. Roilides, M. Simitsopoulou, A. Katragkou, and T. J. Walsh, “ How Biofilms Evade Host Defenses,” in Microbial Biofilms, eds. M. A. Ghannoum, M. R. Parsek, M. Whiteley, and P. K. Mukherjee (Washington DC: ASM Press, 2015), 287–300.
10.1128/9781555817466.ch14 Google Scholar
- 13A. N. Tamfu, O. Ceylan, G. C. Fru, M. Ozturk, M. E. Duru, and F. Shaheen, “Antibiofilm, Antiquorum Sensing and Antioxidant Activity of Secondary Metabolites From Seeds of Annona senegalensis, Persoon,” Microbial Pathogenesis 144 (2020): 104191.
- 14P. Shree, C. S. Singh, K. K. Sodhi, J. N. Surya, and D. K. Singh, “Biofilms: Understanding the Structure and Contribution Towards Bacterial Resistance in Antibiotics,” Medicine in Microecology 16 (2023): 100084.
10.1016/j.medmic.2023.100084 Google Scholar
- 15R. M. Donlan, “Role of Biofilms in Antimicrobial Resistance,” ASAIO Journal 46, no. 6 (2000): S47–S52.
- 16A. N. Tamfu, A. M. Munvera, A. V. D. Botezatu, et al., “Synthesis of Benzoyl Esters of β-Amyrin and Lupeol and Evaluation of Their Antibiofilm and Antidiabetic Activities,” Results in Chemistry 4 (2022): 100322.
10.1016/j.rechem.2022.100322 Google Scholar
- 17T. Larsen and N. E. Fiehn, “Dental Biofilm Infections—An Update,” Apmis 125, no. 4 (2017): 376–384.
- 18O. Ciofu, O. T. Tolker-Nielsen, P. Ø. Jensen, H. Wang, and N. Høiby, “Antimicrobial Resistance, respiratory Tract Infections and Role of Biofilms in Lung Infections in Cystic Fibrosis Patients,” Advanced Drug Delivery Reviews 85 (2015): 7–23.
- 19J. H. Fastenberg, W. D. Hsueh, A. Mustafa, N. A. Akbar, and W. M. Abuzeid, “Biofilms in Chronic Rhinosinusitis: Pathophysiology and Therapeutic Strategies,” World Journal of Otorhinolaryngology-Head and Neck Surgery 2, no. 4 (2016): 219–229.
- 20P. Homøe, T. Bjarnsholt, M. Wessman, H. C. F. Sørensen, and H. K. Johansen, “Morphological Evidence of Biofilm Formation in Greenlanders With Chronic Suppurative Otitis Media,” European Archives of Oto-Rhino-Laryngology 266 (2009): 1533–1538.
- 21S. Macfarlane and J. Dillon, “Microbial Biofilms in the Human Gastrointestinal Tract,” Journal of Applied Microbiology 102, no. 5 (2007): 1187–1196.
- 22J.-P. Motta, J. L. Wallace, A. G. Buret, C. Deraison, and N. Vergnolle, “Gastrointestinal Biofilms in Health and Disease,” Nature Reviews Gastroenterology & Hepatology 18, no. 5 (2021): 314–334.
- 23S. Veerachamy, T. Yarlagadda, G. Manivasagam, and P. K. D. V. Yarlagadda, “Bacterial Adherence and Biofilm Formation on Medical Implants: A Review,” Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 228, no. 10 (2014): 1083–1099.
- 24D. Costa, K. Johani, D. S. Melo, et al., “Biofilm Contamination of High-Touched Surfaces in Intensive Care Units: Epidemiology and Potential Impacts,” Letters in Applied Microbiology 68, no. 4 (2019): 269–276.
- 25P. S. Stewart and J. W. Costerton, “Antibiotic Resistance of Bacteria in Biofilms,” Lancet 358, no. 9276 (2001): 135–138.
- 26I. Olsen, “Biofilm-Specific Antibiotic Tolerance and Resistance,” European Journal of Clinical Microbiology & Infectious Diseases 34 (2015): 877–886.
- 27S. Singh, S. Datta, K. B. Narayanan, and K. N. Rajnish, “Bacterial Exo-Polysaccharides in Biofilms: Role in Antimicrobial Resistance and Treatments,” Journal of Genetic Engineering and Biotechnology 19 (2021): 140.
- 28I. Alav, J. M. Sutton, and K. M. Rahman, “Role of Bacterial Efflux Pumps in Biofilm Formation,” Journal of Antimicrobial Chemotherapy 73, no. 8 (2018): 2003–2020.
- 29D. Sharma, L. Misba, and A. U. Khan, “Antibiotics Versus Biofilm: An Emerging Battleground in Microbial Communities,” Antimicrobial Resistance & Infection Control 8 (2019): 1–10.
- 30J. Masák, A. Čejková, O. Schreiberová, and T. Řezanka, “Pseudomonas Biofilms: Possibilities of Their Control,” FEMS Microbiology Ecology 89, no. 1 (2014): 1–14.
- 31G. K. Wijesinghe, S. B. Feiria, F. C. Maia, et al., “In-vitro Antibacterial and Antibiofilm Activity of Cinnamomum verum Leaf Oil Against Pseudomonas aeruginosa, Staphylococcus aureus and Klebsiella pneumoniae,” Anais Da Academia Brasileira De Ciências 93 (2021): e20201507.
- 32N. Sathe, P. Beech, L. Croft, Suphioglu, A. Kapat, and E. Athan, “Pseudomonas aeruginosa: Infections and Novel Approaches to Treatment “Knowing the Enemy” the Threat of Pseudomonas aeruginosa and Exploring Novel Approaches to Treatment,” Infectious Medicine 2, no. 3 (2023): 178–194.
10.1016/j.imj.2023.05.003 Google Scholar
- 33C. Liao, X. Huang, Q. Wang, D. Yao, and W. Lu, “Virulence Factors of Pseudomonas aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance,” Frontiers in Cellular and Infection Microbiology 12 (2022): 926758.
- 34H. Lund-Palau, A. R. Turnbull, A. Bush, et al., “Pseudomonas aeruginosa Infection in Cystic Fibrosis: Pathophysiological Mechanisms and Therapeutic Approaches,” Expert Review of Respiratory Medicine 10, no. 6 (2016): 685–697.
- 35C. Watters, K. DeLeon, U. Trivedi, et al., “Pseudomonas aeruginosa Biofilms Perturb Wound Resolution and Antibiotic Tolerance in Diabetic Mice,” Medical Microbiology and Immunology 202 (2013): 131–141.
- 36D. Badal, A. V. Jayarani, M. A. Kollaran, A. Kumar, and V. Singh, “Pseudomonas aeruginosa Biofilm Formation on Endotracheal Tubes Requires Multiple Two-component Systems,” Journal of Medical Microbiology 69, no. 6 (2020): 906–919.
- 37S. Malhotra, D. Hayes Jr., and D. J. Wozniak, “Cystic Fibrosis and Pseudomonas aeruginosa: The Host-Microbe Interface,” Clinical Microbiology Reviews 32, no. 3 (2019): e00138-18.
- 38M. T. T. Thi, D. Wibowo, and B. H. Rehm, “Pseudomonas aeruginosa Biofilms,” International Journal of Molecular Sciences 21, no. 22 (2020): 8671.
- 39B. Gökalsın, D. Berber, and N. C. Sesal, “ Pseudomonas aeruginosa Quorum Sensing and Biofilm Inhibition,” in Quorum Sensing, ed. G. Tommonaro (Amsterdam, the Netherlands: Elsevier, 2019), 227–256.
- 40S. Boudiba, A. N. Tamfu, K. Hanini, et al., “Synthesis of a New Diarylhydrazone Derivative and an Evaluation of Its in Vitro Biofilm Inhibition and Quorum Sensing Disruption Along With a Molecular Docking Study,” Journal of Chemical Research 47, no. 4 (2023): 17475198231184603.
- 41A. N. Tamfu, W. Boukhedena, S. Boudiba, S. Deghboudj, and O. Ceylan, “Synthesis and Evaluation of Inhibitory Potentials of Microbial Biofilms and Quorum-Sensing by 3-(1,3-Dithian-2-ylidene) Pentane-2,4-dione and Ethyl-2-cyano-2-(1,3-dithian-2-ylidene) Acetate,” Pharmacia 69 (2022): 973–980.
- 42R. M. Talla, A. N. Tamfu, B. N. K. Wakeu, et al., “Evaluation of Anti-Quorum Sensing and Antibiofilm Effects of Secondary Metabolites From Gambeya lacourtiana (De Wild) Aubr. & Pellegr Against Selected Pathogens,” BMC Complementary Medicine and Therapies 23, no. 1 (2023): 300.
- 43E. Silva, J. A. C. Teixeira, M. O. Pereira, C. M. R. Rocha, and A. M. Sousa, “Evolving Biofilm Inhibition and Eradication in Clinical Settings Through Plant-Based Antibiofilm Agents,” Phytomedicine 119 (2023): 154973.
- 44H. N. Ikome, A. N. Tamfu, J. P. Abdou, et al., “Disruption of Biofilm Formation and Quorum Sensing in Pathogenic Bacteria by Compounds From Zanthoxylum Gilletti (De Wild) P.G. Waterman,” Applied Biochemistry and Biotechnology 195, no. 10 (2023): 6113–6131.
- 45M. Angane, S. Swift, K. Huang, C. A. Butts, and S. Y. Quek, “Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry,” Foods 11, no. 3 (2022): 464.
- 46J. Yammine, N.-E. Chihib, A. Gharsallaoui, E. Dumas, A. Ismail, and L. Karam, “Essential Oils and Their Active Components Applied as: Free, Encapsulated and in Hurdle Technology to Fight Microbial Contaminations. A Review.” Heliyon 8, no. 12 (2022): e12472.
- 47T. Alfred Ngenge, S. Kucukaydin, O. Ceylan, and M. E. Duru, “Evaluation of Enzyme Inhibition and Anti-Quorum Sensing Potentials of Melaleuca alternifolia and Citrus sinensis Essential Oils,” Natural Products Communications 16 (2021): 1934578X211044565.
- 48S. Boudiba, A. N. Tamfu, B. Berka, et al., “Anti-quorum Sensing and Antioxidant Activity of Essential Oils Extracted From Juniperus Species, Growing Spontaneously in Tebessa Region (East of Algeria),” Natural Products Communications 16 (2021): 1934578X211024039.
- 49D. F. Firmino, T. T. A. Cavalcante, G. A. Gomes, et al., “Antibacterial and Antibiofilm Activities of Cinnamomum Sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities,” Scientific World Journal 2018, no. 1 (2018): 7405736.
- 50Y. Zhang, Y. Wang, X. Zhu, P. Cao, S. Wei, and Y. Lu, “Antibacterial and Antibiofilm Activities of Eugenol From Essential Oil of Syzygium aromaticum (L.) Merr. & LM Perry (Clove) Leaf Against Periodontal Pathogen Porphyromonas gingivalis,” Microbial Pathogenesis 113 (2017): 396–402.
- 51Q. Luo, S.-M. Wang, Q. Lu, J. Luo, and Y.-X. Cheng, “Identification of Compounds From the Water Soluble Extract of Cinnamomum cassia Barks and Their Inhibitory Effects Against High-Glucose-Induced Mesangial Cells,” Molecules 18, no. 9 (2013): 10930–10943.
- 52C. Zhang, L. Fan, S. Fan, et al., “Cinnamomum cassia Presl: A Review of Its Traditional Uses, Phytochemistry, Pharmacology and Toxicology,” Molecules 24, no. 19 (2019): 3473.
- 53R. Rosato, E. Napoli, G. Granata, et al., “Study of the Chemical Profile and Anti-fungal Activity Against Candida auris of Cinnamomum cassia Essential Oil and of Its Nano-formulations Based on Polycaprolactone,” Plants 12, no. 2 (2023): 358.
- 54P. Danthu, R. Simanjuntak, F. Fawbush, et al., “The Clove Tree and Its Products (Clove Bud, Clove Oil, Eugenol): Prosperous Today but What of Tomorrow's Restrictions?” Fruits 75, no. 5 (2020): 224–242.
- 55G.-Q. Zheng, P. M. Kenney, and L. K. Lam, “Sesquiterpenes From Clove (Eugenia caryophyllata) as Potential Anticarcinogenic Agents,” Journal of Natural Products 55, no. 7 (1992): 999–1003.
- 56W. G. Anderson, R. S. McKinley, and M. Colavecchia, “The Use of Clove Oil as an Anesthetic for Rainbow Trout and Its Effects on Swimming Performance,” North American Journal of Fisheries Management 17, no. 2 (1997): 301–307.
10.1577/1548-8675(1997)017<0301:TUOCOA>2.3.CO;2 Google Scholar
- 57E. Pinto, L. Vale-Silva, C. Cavaleiro, and L. Salgueiro, “Antifungal Activity of the Clove Essential Oil From Syzygium aromaticum on Candida, Aspergillus and Dermatophyte Species,” Journal of Medical Microbiology 58, no. 11 (2009): 1454–1462.
- 58H. A. A. Nauertz Aboubakr, N. T. Luong, et al., “In Vitro Antiviral Activity of Clove and Ginger Aqueous Extracts Against Feline Calicivirus, a Surrogate for Human Norovirus,” Journal of Food Protection 79, no. 6 (2016): 1001–1012.
- 59K. Chaieb, K. H. Hajlaoui, T. Zmantar, et al., “The Chemical Composition and Biological Activity of Clove Essential Oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A Short Review,” Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 21, no. 6 (2007): 501–506.
- 60A. T. Zari, T. A. Zari, and K. R. Hakeem, “Anticancer Properties of Eugenol: A Review,” Molecules 26, no. 23 (2021): 7407.
- 61G. E.-S. Batiha, L. M. Alkazmi, L. G. Wasef, A. M. Beshbishy, E. H. Nadwa, and E. K. Rashwan, “Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities,” Biomolecules 10, no. 2 (2020): 202.
- 62N. Hiwandika, S. E. Sudrajat, and I. Rahayu, “Antibacterial and Antifungal Activity of Clove Extract (Syzygium aromaticum),” Eureka Herba Indonesia 2, no. 2 (2021): 86–94.
- 63M. S. A. Khan and I. Ahmad, “Biofilm Inhibition by Cymbopogon Citratus and Syzygium aromaticum Essential Oils in the Strains of Candida albicans,” Journal of Ethnopharmacology 140, no. 2 (2012): 416–423.
- 64M. Kačániová, L. Galovičov, B. Borotová, et al., “Chemical Composition, in Vitro and in Situ Antimicrobial and Antibiofilm Activities of Syzygium aromaticum (Clove) Essential Oil,” Plants 10, no. 10 (2021): 2185.
- 65A. Marchese, R. Barbieri, E. Coppo, et al., “Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint,” Critical Reviews in Microbiology 43, no. 6 (2017): 668–689.
- 66 European Pharmacopoeia, Determination of Essential Oils in Herbal Drugs (France: Council of Europe Strasbourg Cedex, 2008): 251–256.
- 67T. Alfred Ngenge, S. Kucukaydin, O. Ceylan, and M. E. Duru, “Evaluation of Enzyme Inhibition and Anti-Quorum Sensing Potentials of Melaleuca alternifolia and Citrus sinensis Essential Oils,” Natural Product Communications 16, no. 9 (2021): 1934578X211044565.
- 68U. Edwards, T. Rogall, H. Blöcker, M. Emde, and E. C. Böttger, “Isolation and Direct Complete Nucleotide Determination of Entire Genes. Characterization of a Gene Coding for 16S Ribosomal RNA,” Nucleic Acids Research 17, no. 19 (1989): 7843–7853.
- 69 European Committee on Antimicrobial Susceptibility Testing, Comité de L'antibiogramme de la Société Française De Microbiologie (CA-SFM). Recommandations 2020. (Paris: Société Française De Microbiologie, 2020).
- 70M. Larif, M. Ouhssine, A. Soulaymani, and A. Elmidaoui, “Potential Effluent Oil Mills and Antibacterial Activity Polyphenols Against Some Pathogenic Strains,” Research on Chemical Intermediates 41 (2015): 1213–1225.
- 71A. Benaissa, K. Abdelmounaim, F. Benbelaid, et al., “Inhibitory Effect of Essential Oils Obtained From Five Algerian Plants Against Pseudomonas aeruginosa, Including Carbapenem-Resistant Strains,” Natural Product Sciences 29, no. 4 (2023): 225–234.
- 72I. Wiegand, K. Hilpert, and R. E. Hancock, “Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances,” Nature Protocols 3, no. 2 (2008): 163–175.
- 73A. Benaissa, A. Khadir, A. N. Tamfu, et al., “Biofilm Disruption and Virulence Attenuation Effects of Essential Oil from Endemic Algerian Cistus munbyi (Cistaceae) against Clinical Strains of Pseudomonas aeruginosa,” Natural Product Communications 19, no. 4 (2024): 1934578X241245234.
- 74G. A. O'Toole, “Microtiter Dish Biofilm Formation Assay,” JoVE (Journal of Visualized Experiments) no. 47 (2011): e2437.
- 75F. Malek, “Evaluation of a Non-submerged Cultivation Assay Combined to ESEM Imaging for Analysis of Biofilms Formed by Dairy-Associated Sporeforming Bacteria,” African Journal of Microbiology Research 10, no. 32 (2016): 1263–1273.
- 76S. D. Sarker, L. Nahar, and Y. Kumarasamy, “Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the in Vitro Antibacterial Screening of Phytochemicals,” Methods 42, no. 4 (2007): 321–324.
- 77S. K. Bose, M. Chauhan, N. Dhingra, S. Chhibber, and K. Harjai, “Terpinen-4-ol Attenuates Quorum Sensing Regulated Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa,” Future Microbiology 15, no. 2 (2020): 127–142.
- 78N. T. Metiefeng, A. N. Tamfu, M. Fotsing Tagatsing, et al., “In Vitro and In Silico Evaluation of Anticholinesterase and Antidiabetic Effects of Furanolabdanes and Other Constituents From Graptophyllum pictum (Linn.) Griffith,” Molecule 28 (2023): 4802, https://doi.org/10.3390/molecules28124802.
- 79R. T. Feunaing, A. N. Tamfu, A. J. Y. Gbaweng, et al., “In Vitro and Molecular Docking Evaluation of the Anticholinesterase and Antidiabetic Effects of Compounds From Terminalia macroptera Guill. & Perr. (Combretaceae),” Molecules 29 (2024): 2456, https://doi.org/10.3390/molecules29112456.
- 80B. Wang, J. S. Xu, C. X. Wang, et al., “Antimicrobial Susceptibility of Neisseria gonorrhoeae Isolated in Jiangsu Province, China, With a Focus on Fluoroquinolone Resistance,” Journal of Medical Microbiology 55, no. 9 (2006): 1251–1255, https://doi.org/10.1099/jmm.0.46401-0.
- 81P. Pachori , R. Gothalwal, and P. Gandhi, “Emergence of Antibiotic Resistance Pseudomonas aeruginosa in Intensive Care Unit; a Critical Review,” Genes & Diseases 6, no. 2 (2019): 109–119.
- 82Z. Pang, R. Raudonis, R. Raudonis, B. R. Glick, T.-J. Lin, and z. Cheng, “Antibiotic Resistance in Pseudomonas aeruginosa: Mechanisms and Alternative Therapeutic Strategies,” Biotechnology Advances 37, no. 1 (2019): 177–192.
- 83F. Al-Wrafy, E. Brzozowska, S. Górska, and A. Gamian, “Pathogenic Factors of Pseudomonas aeruginosa—The Role of Biofilm in Pathogenicity and as a Target for Phage Therapy,” Advances in Hygiene and Experimental Medicine 71 (2017): 78–91.
- 84S. U. Pratiwi, E. Lagendijk, T. Hertiani, S. de Weert, and C. van den Hondel, “Antimicrobial Effects of Indonesian Medicinal Plants Extracts on Planktonic and Biofilm Growth of Pseudomonas aeruginosa and Staphylococcus aureus,” International Journal of Pharmacy and Pharmaceutical Sciences 7 (2015): 183–191.
- 85P. E. Budri, N. C. C. Silva, E. C. R. Bonsaglia, et al., “Effect of Essential Oils of Syzygium aromaticum and Cinnamomum zeylanicum and Their Major Components on Biofilm Production in Staphylococcus aureus Strains Isolated From Milk of Cows With Mastitis,” Journal of Dairy Science 98, no. 9 (2015): 5899–5904.
- 86L. V. Costa, J. M. A. R. Moreira, I. D. G. Menezes, V. Dutra, and A. D. B. P. F. de Almeida, “Antibiotic Resistance Profiles and Activity of Clove Essential Oil (Syzygium aromaticum) Against Pseudomonas aeruginosa Isolated of Canine Otitis,” Veterinary World 15, no. 10 (2022): 2499.
- 87A. Prashar, I. C. Locke, and C. S. Evans, “Cytotoxicity of Clove (Syzygium aromaticum) Oil and Its Major Components to human Skin Cells,” Cell Proliferation 39, no. 4 (2006): 241–248.
- 88Q. Shen, F. Chen, and J. Luo, “Comparison Studies on Chemical Constituents of Essential Oil From Ramulus Cinnamomi and Cortex Cinnamomi by GC-MS,” Zhong Yao Cai = Zhongyaocai = Journal of Chinese Medicinal Materials 25, no. 4 (2002): 257–258.
- 89R. Wang, R. Wang, and B. Yang, “Extraction of Essential Oils From Five Cinnamon Leaves and Identification of Their Volatile Compound Compositions,” Innovative Food Science & Emerging Technologies 10, no. 2 (2009): 289–292.
- 90Y. Xu, B. Q. Cheng, J. K. Ding, Z. Yu, Z. H. Chen, and J. N. Zeng, “Investigation on Cinnamon Resource, Growth and Yield of Oil in Guangxi and Yunnan,” Tropical Agricultural Science & Technology 27, no. 3 (2004): 4–7.
- 91J.-W. Huang, “Study on Chemical Components of Essential Oils in Cinnamomum cassia Presl From Different Growth Years by GC-MS,” Chinese Journal of Pharmaceutical Analysis 25, no. 3 (2005): 288–291.
- 92Y. Qin, J. Zhu, Z. Zhang, M. Zhang, and M. Qin, “Annual Variation Laws of Main Chemical Compositions and Oil Yielding Rate in Branches and Leaves of Cinnamomum cassia,” Nonwood Forest Research 24 (2006): 9–13.
- 93M. Elgayyar, F. A. Draughon, D. A. Golden, and J. R. Mount, “Antimicrobial Activity of Essential Oils From Plants Against Selected Pathogenic and Saprophytic Microorganisms,” Journal of Food Protection 64, no. 7 (2001): 1019–1024.
- 94L. S. Ooi, Y. Li, S.-L. Kam, H. Wang, E. Y. L. Wong, and V. E. C. Ooi, “Antimicrobial Activities of Cinnamon Oil and Cinnamaldehyde From the Chinese Medicinal Herb Cinnamomum cassia Blume,” American Journal of Chinese Medicine 34, no. 3 (2006): 511–522.
- 95D. Liang, B. Feng, N. Li, et al., “Preparation, Characterization, and Biological Activity of Cinnamomum cassia Essential Oil Nano-emulsion,” Ultrasonics Sonochemistry 86 (2022): 106009.
- 96P. Rathinam, H. Vijay Kumar, and P. Viswanathan, “Eugenol Exhibits Anti-virulence Properties by Competitively Binding to Quorum Sensing Receptors,” Biofouling 33, no. 8 (2017): 624–639.
- 97L. A. S. Pereira, H. H. D. A. Martins, L. A. do Vale, et al., “Sanitizing Cinnamaldehyde Solutions Against Pseudomonas aeruginosa Biofilms Formed on Stainless Steel Surfaces,” Brazilian Journal of Food Technology 22 (2019): e2018144.
- 98M. Mahboobi, F. Shahcheraghi, and M. M. Feizabadi, “Bactericidal Effects of Essential Oils From Clove, Lavender and Geranium on Multi-drug Resistant Isolates of Pseudomonas aeruginosa,” Iranian Journal of Biotechnology 4, no. 2 (2006): 137–140.
- 99S. Oulkheir, M. Aghrouch, F. E. Mourabit, et al., “Antibacterial Activity of Essential Oils Extracts From Cinnamon, Thyme, Clove and Geranium Against a Gram Negative and Gram Positive Pathogenic Bacteria,” Journal of Diseases and Medicinal Plants 3 (2017): 1–5.
- 100T. Rasamiravaka, Q. Labtani, P. Duez, and M. El Jaziri, “The Formation of Biofilms by Pseudomonas aeruginosa: A Review of the Natural and Synthetic Compounds Interfering With Control Mechanisms,” BioMed Research International 2015 (2015): 759348.
- 101S. Ghosh, U. Sett, A. Pal, et al., “Antibiofilm Potential of Nanonized Eugenol Against Pseudomonas aeruginosa,” Journal of Applied Microbiology 135, no. 1 (2024): lxad305.
- 102A. A. Doyle and J. C. Stephens, “A Review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents,” Fitoterapia 139 (2019): 104405.
- 103M. M. M. de Oliveira, D. F. Brugnera, J. A. D. Nascimento, N. N. Batista, and R. H. Piccoli, “Cinnamon Essential Oil and Cinnamaldehyde in the Control of Bacterial Biofilms Formed on Stainless Steel Surfaces,” European Food Research and Technology 234 (2012): 821–832.
- 104M. Lang, A. Montjarret, E. Duteil, and G. Bedoux, “Cinnamomum cassia and Syzygium aromaticum Essential Oils Reduce the Colonization of Salmonella Typhimurium in an in Vivo Infection Model Using Caenorhabditis elegans,” Molecules 26, no. 18 (2021): 5598.
- 105N. L. Kavanaugh and K. Ribbeck, “Selected Antimicrobial Essential Oils Eradicate Pseudomonas Spp. And Staphylococcus aureus Biofilms,” Applied and Environmental Microbiology 78, no. 11 (2012): 4057–4061.
- 106Y.-G. Kim, J.-H. Lee, S.-I. Kim, K.-H. Baek, and J. Lee, “Cinnamon Bark Oil and Its Components Inhibit Biofilm Formation and Toxin Production,” International Journal of Food Microbiology 195 (2015): 30–39.
- 107C. Niu and E. Gilbert, “Colorimetric Method for Identifying Plant Essential Oil Components That Affect Biofilm Formation and Structure,” Applied and Environmental Microbiology 70, no. 12 (2004): 6951–6956.
- 108S. H. Topa, S. Subramoni, E. A. Palombo, P. Kingshott, S. A. Rice, and L. L. Blackall, “Cinnamaldehyde Disrupts Biofilm Formation and Swarming Motility of Pseudomonas aeruginosa,” Microbiology 164, no. 9 (2018): 1087–1097.
- 109P. K. Sagar, P. Sharma, and R. Singh, “Antibacterial Efficacy of Different Combinations of Clove, Eucalyptus, Ginger, and Selected Antibiotics Against Clinical Isolates of Pseudomonas aeruginosa,” AYU (An International Quarterly Journal of Research in Ayurveda) 41, no. 2 (2020): 123–129.
10.4103/ayu.AYU_101_19 Google Scholar
- 110F. M. Husain, I. Ahmad, M. Asif, and Q. Tahseen, “Influence of Clove Oil on Certain Quorum-Sensing-Regulated Functions and Biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila,” Journal of Biosciences 38, no. 5 (2013): 835–844.
- 111L. Zhou, H. Zheng, Y. Tang, W. Yu, and Q. Gong, “Eugenol Inhibits Quorum Sensing at Sub-Inhibitory Concentrations,” Biotechnology Letters 35 (2013): 631–637.
- 112D. Lahiri, M. Nag, B. Dutta, et al., “Antibiofilm and Anti-Quorum Sensing Activities of Eugenol and Linalool From Ocimum tenuiflorum Against Pseudomonas aeruginosa Biofilm,” Journal of Applied Microbiology 131, no. 6 (2021): 2821–2837.
- 113M. Kalia, V. K. Yadav, P. K. Singh, et al., “Effect of Cinnamon Oil on Quorum Sensing-Controlled Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa,” PLoS One 10, no. 8 (2015): e0135495.
- 114S. H. Al-Mijalli, N. El Hachlafi, E. M. Abdallah, et al., “Exploring the Antibacterial Mechanisms of Chemically Characterized Essential Oils From Leaves and Buds of Syzygium aromaticum (L.) Merr. Et Perry Against Staphylococcus aureus and Pseudomonas aeruginosa,” Industrial Crops and Products 205 (2023): 117561.
- 115S. Farisa Banu, D. Rubini, S. Rakshitaa, et al., “Antivirulent Properties of Underexplored Cinnamomum tamala Essential Oil and Its Synergistic Effects With DNase Against Pseudomonas aeruginosa Biofilms—An In Vitro Study,” Frontiers in Microbiology 8 (2017): 1144.
- 116C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeney, “Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings,” Advanced Drug Delivery Reviews 46, no. 1-3 (2001): 3–26.
- 117D. Lagorce, D. Douguet, M. A. Miteva, and B. O. Villoutreix, “Computational Analysis of Calculated Physicochemical and ADMET Properties of Protein-Protein Interaction Inhibitors,” Scientific Reports 7 (2017): 4627, https://doi.org/10.1038/srep46277.
- 118N. T. Issa, H. Wathieu, A. Ojo, S. W. Byers, and S. Dakshanamurthy, “Drug Metabolism in Preclinical Drug Development: A Survey of the Discovery Process, Toxicology, and Computational Tools,” Current Drug Metabolism 18 (2017): 556–565.
- 119C. F. Thorn, E. Aklillu, T. E. Klein, and R. B. Altman, “PharmGKB Summary: Very Important Pharmacogene Information for CYP1A2,” Pharmacogenetics and Genomics 22 (2012): 73–77.
- 120Durán-Iturbide, B. I. Díaz-Eufracio, and J. L. Medina-Franco, “In Silico ADME/Tox Profiling of Natural Products: A Focus on BIOFACQUIM,” ACS Omega 5, no. 26 (2020): 16076–16084.
- 121S. Pathania and P. K. Singh, “Analyzing FDA-approved Drugs for Compliance of Pharmacokinetic Principles: Should There be a Critical Screening Parameter in Drug Designing Protocols?” Expert Opinion on Drug Metabolism & Toxicology 17, no. 4 (2021): 351–354.
- 122C. J. Ononamadu and A. Ibrahim, “Molecular Docking and Prediction of ADME/Drug-Likeness Properties of Potentially Active Antidiabetic Compounds Isolated From Aqueous-Methanol Extracts of Gymnema sylvestre and Combretum micranthum,” BioTechnologia 102, no. 1 (2021): 85–99.