Fortunefuroic Acid J from Keteleeria Hainanensis and its Dual Inhibitory Effects on ATP-Citrate Lyase and Acetyl-CoA Carboxylase.
Ze-Yu Zhao
Department of Natural Medicine, School of Pharmacy, Fudan University, Shanghai, 201203 P. R. China
Institute of Natural Medicine and Health Products, School of Pharmaceutical Sciences, Zhejiang Provincial Key Laboratory of Plant Evolutionary Ecology and Conservation, Taizhou University, Zhejiang, 318000 PR China
Search for more papers by this authorYi Zang
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Science, Shanghai, 201203 PR China
Search for more papers by this authorJia Li
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Science, Shanghai, 201203 PR China
Search for more papers by this authorYeun-Mun Choo
Chemistry Department, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
Search for more papers by this authorCorresponding Author
Juan Xiong
Department of Natural Medicine, School of Pharmacy, Fudan University, Shanghai, 201203 P. R. China
Search for more papers by this authorCorresponding Author
Jin-Feng Hu
Department of Natural Medicine, School of Pharmacy, Fudan University, Shanghai, 201203 P. R. China
Institute of Natural Medicine and Health Products, School of Pharmaceutical Sciences, Zhejiang Provincial Key Laboratory of Plant Evolutionary Ecology and Conservation, Taizhou University, Zhejiang, 318000 PR China
Search for more papers by this authorZe-Yu Zhao
Department of Natural Medicine, School of Pharmacy, Fudan University, Shanghai, 201203 P. R. China
Institute of Natural Medicine and Health Products, School of Pharmaceutical Sciences, Zhejiang Provincial Key Laboratory of Plant Evolutionary Ecology and Conservation, Taizhou University, Zhejiang, 318000 PR China
Search for more papers by this authorYi Zang
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Science, Shanghai, 201203 PR China
Search for more papers by this authorJia Li
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Science, Shanghai, 201203 PR China
Search for more papers by this authorYeun-Mun Choo
Chemistry Department, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
Search for more papers by this authorCorresponding Author
Juan Xiong
Department of Natural Medicine, School of Pharmacy, Fudan University, Shanghai, 201203 P. R. China
Search for more papers by this authorCorresponding Author
Jin-Feng Hu
Department of Natural Medicine, School of Pharmacy, Fudan University, Shanghai, 201203 P. R. China
Institute of Natural Medicine and Health Products, School of Pharmaceutical Sciences, Zhejiang Provincial Key Laboratory of Plant Evolutionary Ecology and Conservation, Taizhou University, Zhejiang, 318000 PR China
Search for more papers by this authorAbstract
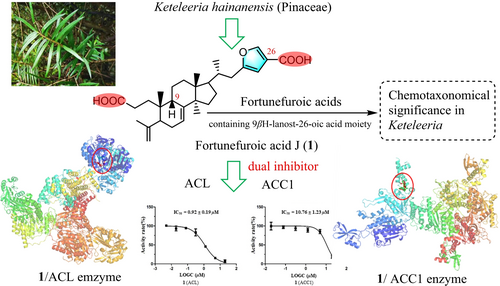
A previously undescribed triterpenoid (fortunefuroic acid J, 1) was isolated from the endangered conifer Keteleeria hainanensis, along with 20 other known terpenoids. Compound 1 is characterized by an unusual 3,4-seco-9βH-lanost-3-oic acid motif, featuring a rare furoic acid moiety in its lateral chain. The structure elucidation of this compound was achieved through a combination of spectroscopic and computational methods. The C-15 epimers of 15-methoxypinusolidic acid (15R-8 and 15S-9) were successfully separated and identified for the first time. Compound 1 demonstrated dual inhibitory effects against ATP-citrate lyase (ACL, IC50: 0.92 μM) and acetyl-CoA carboxylase 1 (ACC1, IC50: 10.76 μM). Compounds 2 and 11 exclusively inhibited ACL, exhibiting IC50 values of 2.64 and 6.35 μM, respectively. Compound 1 is classified among the fortunefuroic acid-type compounds, previously isolated from K. fortunei, distinguished by the presence of a rare furoic acid moiety in their lateral chain. The chemotaxonomic significance of the 9βH-lanost-26-oic acids in Keteleeria was briefly discussed. These findings highlight the importance of conserving plant species diversity, thereby enhancing the exploration of structurally diverse compounds and potential avenues for developing new therapeutics targeting ACL/ACC1-associated diseases.
Graphical Abstract
Conflict of Interests
There are no conflicts to declare.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202401520-sup-0001-misc_information.pdf1.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1The IUCN Red List of Threatened Specieshttps://www.iucnredlist.org/species/32416/2818304(accessed 2023−3–2).
- 2J. L. Fox, Science 1984, 226, 150.
- 3M. A. Ibrahim, M. Na, J. Oh, R. F. Schinazi, T. R. McBrayer, T. Whitaker, R. J. Doerksen, D. J. Newman, L. G. Zachos, M. T. Hamann, Proc. Natl. Acad. Sci. USA 2013, 110, 16832–16837
- 4F. Zhu, C. Qin, L. Tao, X. Liu, Z. Shi, X. Ma, J. Jia, Y. Tan, C. Cui, J. Lin, C. Tan, Y. Jiang, Y. Chen, Proc. Natl. Acad. Sci. USA 2011, 108, 12943–12948
- 5L. G. Fu, J. M. Jin, China Plant Red Data Book. Rare and Endangered Plants I Science Press, Beijing, 1992, 68−78.
- 6Z.-W. Ge, M. E. Smith, Q.-Y. Zhang, Z. L. Yang, Mycorrhiza 2012, 22, 403–408.
- 7D.-L. Li, Y. Yang, S. Yang, Y.-K. Chen, Mitochondrial DNA B Resour. 2019, 4, 2934–2935.
- 8National forestry and grassland administration, 2021. List of national key protected wild plants 2021. (http://www.gov.cn/zhengce/zhengceku/2021-09/09/content 5636409.htm) (Accessed 7 September 2021).
- 9Published on the Internet http://www.worldfloraonline.org/taxon/wfo-00003569002024′WFO, 2024. Keteleeria hainanensis Chun & Tsiang. Accessed on: 15 Jun.
- 10G.-S. Yang, Y. Qiu, Z.-A. Yang, Mitochondrial DNA B Resour. 2024, 9, 557–562.
- 11Y. Q. Li, J. F. Li, D. Wang, Y. J. Zhu, D. W. Wang, Y. L. Xu, N. H. Cai, Mitochondrial DNA B Resour. 2021, 9, 2650–2651.
10.1080/23802359.2021.1920488 Google Scholar
- 12T. H. Thai, N. T. Hien, N. Cuong, J. Casanova, F. Tomi, M. Paoli, J. Essent. Oil Res. 2022, 34, 148–154.
- 13W. J. He, Z. H. Fu, H. J. Han, H. Yan, G. Z. Zeng, C. J. Jia, H. B. Chu, Y. M. Zhang, N. H. Tan, Z. Naturforsch. 2011, 66, 733–739.
- 14Z.-Y. Zhao, Y.-P. Tong, W. Jiang, Y. Zang, J. Xiong, J. Li, J.-F. Hu, J. Nat. Prod. 2023, 86, 1487–1499.
- 15P.-J. Zhou, X.-Y. Wu, Z.-Y. Zhao, Y. Zang, Z.-S. Sun, Y.-L. Li, N. Li, J. Xiong, Y. M. Choo, Z.-X. Jin, J. Li, J.-F. Hu, Phytochemistry 2024, Manuscript Number: PHYTOCHEM−D- 24–00793R3, revised).
- 16Z.-Y. Zhao, J. Wan, H.-W. Chen, Z.-S. Sun, Y.-T. Tao, Y. P. Tong, Y. Zang, Y.-M. Choo, P. Wang, Y.-L. Li, C.-X. Jiang, J. M. Li, J. Xiong, J. Li, Z.-X. Jin, J.-F. Hu, Phytochemistry 2024, 228, 114259.
- 17N. Wang, Z.-l. Li, D.-D. Song, W. Li, Y.-H. Pei, Y.-K. Jing, H.-M. Hua, Planta Med. 2012, 78, 1661–1666.
- 18S.-C. Chien, H.-K. Liu, Y.-H. Kuo, Chem. Pharm. Bull. 2004, 52, 762–763.
- 19N. H. Sa, N. T. Tam, N. T. H. Anh, T. D. Quan, D. D. Thien, D. T. Phong, T. Van Sung, T. T. Thuy, Nat. Prod. Res. 2018, 32, 341–345.
- 20X.-D. Wu, S.-Y. Wang, L. Wang, J. He, G.-T. Li, L.-F. Ding, X. Gong, L.-B. Dong, L.-D. Song, Y. Li, Q.-S. Zhao, Fitoterapia 2013, 85, 154–160.
- 21X.-W. Yang, S.-M. Li, L. Wu, Y.-L. Li, L. Feng, Y.-H. Shen, J.-M Tian, J. Tang, N. Wang, Y. H. Liu, W.-D. Zhang, Org. Biomol. Chem. 2010, 8, 2609–2616.
- 22G. M. Cabrera, M. Gallo, A. M. Seldes, J. Nat. Prod. 1996, 59, 343–347.
- 23C. Escobedo-Martínez, M. C. Lozada, S. Hernández-Ortega, M. L. Villarreal, D. Gnecco, R. G. Enríqueza, W Reynoldse, Magn. Reson. Chem. 2012, 50, 52–57.
- 24L.-K. Sy, R. M. K. Saunders, G. D. Brown, Phytochemistry 1997, 44, 1099–1108.
- 25S. Furukawa, N. Takagi, T. Ikeda, M. Ono, A. M. Nafady, T. Nohara, H. Sugimoto, S. Doi, H. Yamada, Chem. Pharm. Bull. 2002, 50, 439–440.
- 26A. I. Lisina, S. M. Yasnetskaya, V. A. Pentegova, Chem. Nat. Compd. 1972, 8, 296–299.
10.1007/BF00563733 Google Scholar
- 27J. S. Calderón, L. Quijano, F. Gómez-Garibay, M. Morán, T. Ríos, Phytochemistry 1987, 26, 2639–2641.
- 28Y.-L. Li, Y.-X. Gao, H.-Z. Jin, L. Shan, W.-L. Chang, X.-W. Yang, H.-W. Zeng, N. Wang, A. Steinmetz, W.-D. Zhang, Phytochemistry 2015, 117, 135–143.
- 29T. Norin, S. Sundin, O. Theander, Acta Chem. Scand. 1971, 25, 607–610.
- 30S. M. Puente-Villegas, L. A. Ticona, A. R. Sanchez, J.-L. Acebes, J. Ethnopharmacol. 2024, 318, 117021.
- 31C.-L. Lee, L.-C. Chiang, L.-H. Cheng, C.-C. Liaw, M. H. Abd El-Razek, F.-R. Chang, Y.-C. Wu, J. Nat. Prod. 2009, 72, 1568–1572.
- 32H. T. A. Cheung, T. Miyase, M. P. Lenguyen, M. A. Smal, Tetrahedron 1993, 49, 7903–7915.
- 33P. Bhan, B. S. Pande, R. Soman, N. P. Damodaran, S. Dev, Tetrahedron 1984, 40, 2961–2965.
- 34L. G. Esteban, P. de Palacios, A. García-Iruela, F. García-Fernández, L. García-Esteban, D. G. de Vega, Forests 2021, 12, 1706.
10.3390/f12121706 Google Scholar
- 35X.-D. Wu, J. He, Y. Shen, L.-B. Dong, Z.-H. Pan, G. Xu, X. Gong, L.-D. Song, Y. Leng, Y. Li, L.-Y. Peng, Q.-S. Zhao, Tetrahedron Lett. 2012, 53, 800–803.
- 36S.-P. Yang, J.-M. Yue, Bioorg. Med. Chem. Lett. 2001, 11, 3119–3122.
- 37J.-H. Ran, T.-T. Shen, H. Wu, X. Gong, X.-Q Wang, Mol. Phylogenet. Evol. 2018, 129, 106–116.
- 38K. E. Wellen, G. Hatzivassiliou, U. M. Sachdeva, T. V. Bui, J. R. Cross, C. B. Thompson, Science 2009, 324, 1076–1080.
- 39C. Granchi, Expert Opin. Ther. Pat. 2022, 32, 731–742.
- 40M. R. Morrow, B. Batchuluun, J. H. Wu, E. Ahmadi, J. M. Leroux, P. Mohammadi-Shemirani, E. M. Desjardins, Z. Wang, E. E. Tsakiridis, D. C. T. Lavoie, A. Reihani, B. K. Smith, J. M. Kwiecien, J. S. V. Lally, T. L. Nero, M. W. Parker, K. Ask, J. W. Scott, L. Jiang, Cell Metab. 2022, 34, 919–936.
- 41A. Markham, Drugs 2020, 80, 747–753.
- 42K. Paponja, I. Pećin, Ž. Reiner, M. Banach, Curr. Opin. Lipidol. 2024, 35, 41–50.
- 43A. Veprik, G. Denwood, D. Liu, R. B. Bakar, V. Morfin, K. McHugh, N. N. Tebeka, L. Vetterli, E. Yonova-Doing, F. Gribble, F. Reimann, K. L. Hoehn, P. A. Hemsley, J. Ahnfelt-Rønne, P. Rorsman, Q. Zhang, H. de Wet, J. Cantley, Biol. 2022, 5, 238.
- 44X. Wu, T. H. Huang, Future Med. Chem. 2020, 12, 533–561.
- 45M. Hunkeler, A. Hagmann, E. Stuttfeld, M. Chami, Y. Guri, H. Stahlberg, T. Maier Nature 2018, 558, 470–474.
- 46T. Lu, F. W. Chen, J. Comput. Chem. 2012, 33, 580–592.
- 47S. K. Koerner, J.-I. Hanai, S. Bai, F. E. Jernigan, M. Oki, C. Komaba, E. Shuto, V. P. Sukhatme, L. Sun, Eur. J. Med. Chem. 2017, 126, 920–928.