Prenylated Acetophenone Derivatives from the Leaves of Acronychia pedunculata and Their Anti-Proliferative Activities
Zhen-Zhen Yang
College of Pharmacy, Shenzhen Technology University, Shenzhen, 518118 People's Republic of China
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
These authors contributed equally to this work.
Search for more papers by this authorNi-Ping Li
State Key Laboratory of Bioactive Molecules and Druggability Assessment, Jinan University, Guangzhou, 510632 People's Republic of China
These authors contributed equally to this work.
Search for more papers by this authorLi-Xia Lv
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
Search for more papers by this authorYi-Yi Li
State Key Laboratory of Bioactive Molecules and Druggability Assessment, Jinan University, Guangzhou, 510632 People's Republic of China
Search for more papers by this authorGe Ge
College of Pharmacy, Shenzhen Technology University, Shenzhen, 518118 People's Republic of China
Search for more papers by this authorYue Zhuo
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
Search for more papers by this authorCorresponding Author
Qi Wang
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
Search for more papers by this authorCorresponding Author
Ji-Hong Gu
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
Search for more papers by this authorCorresponding Author
Yan Wu
College of Pharmacy, Shenzhen Technology University, Shenzhen, 518118 People's Republic of China
Search for more papers by this authorZhen-Zhen Yang
College of Pharmacy, Shenzhen Technology University, Shenzhen, 518118 People's Republic of China
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
These authors contributed equally to this work.
Search for more papers by this authorNi-Ping Li
State Key Laboratory of Bioactive Molecules and Druggability Assessment, Jinan University, Guangzhou, 510632 People's Republic of China
These authors contributed equally to this work.
Search for more papers by this authorLi-Xia Lv
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
Search for more papers by this authorYi-Yi Li
State Key Laboratory of Bioactive Molecules and Druggability Assessment, Jinan University, Guangzhou, 510632 People's Republic of China
Search for more papers by this authorGe Ge
College of Pharmacy, Shenzhen Technology University, Shenzhen, 518118 People's Republic of China
Search for more papers by this authorYue Zhuo
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
Search for more papers by this authorCorresponding Author
Qi Wang
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
Search for more papers by this authorCorresponding Author
Ji-Hong Gu
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 People's Republic of China
Search for more papers by this authorCorresponding Author
Yan Wu
College of Pharmacy, Shenzhen Technology University, Shenzhen, 518118 People's Republic of China
Search for more papers by this authorAbstract
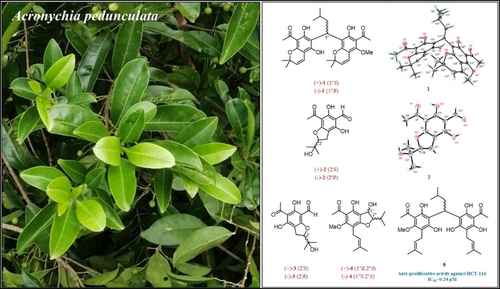
Four new prenylated acetophenone derivatives, including one acetophenone dimer [acronyrone D (1)] and three acetophenone monomers [acronyrones E−G (2–4)], along with seven known analogues (5–11) were obtained from the leaves of Acronychia pedunculata. Their structures and absolute configurations were established by analysis of HRMS and NMR data, single crystal X-ray diffraction studies and quantum chemical calculations. In addition, the isolates were tested for their anti-proliferative acivity against HCT-116, RKO and SW480 cancer cell lines. Compound 5 exhibited significant anti-proliferative effects on the three cell lines, with IC50 values ranging from 0.24–5.3 μM.
Graphical Abstract
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202401027-sup-0001-misc_information.pdf5.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Miyake, C. Morita, A. Suzuki, N. Matsushita, Y. Saito, M. Goto, D. J. Newman, B. R. O'Keefe, K. H. Lee, K. Nakagawa-Goto, J. Nat. Prod. 2019, 82, 2852–2858.
- 2R. Raju, S. Mathew, A. Singh, P. Reddell, G. Münch, Nat. Prod. Res. 2022, 36, 4364–4370.
- 3T. D. Tran, M. A. Olsson, D. J. McMillan, J. K. Cullen, P. G. Parsons, P. W. Reddell, S. M. Ogbourne, Antibiotics (Basel). 2020, 9, 487.
- 4L. P. Robertson, L. Lucantoni, S. Duffy, V. M. Avery, A. R. Carroll, J. Nat. Prod. 2019, 82, 1019–1023.
- 5K. Miyake, A. Suzuki, C. Morita, M. Goto, D. J. Newman, B. R. O'Keefe, S. L. Morris-Natschke, K. H. Lee, K. Nakagawa-Goto, J. Nat. Prod. 2016, 79, 2883–2889.
- 6M. Oyama, K. F. Bastow, Y. Tachibana, Y. Shirataki, S. Yamaguchi, G. M. Cragg, T. S. Wu, K. H. Lee, Chin. Pharm. J. (Taipei, Taiwan) 2023, 55, 239–245.
- 7E. Kouloura, M. Halabalaki, M. C. Lallemand, F. Tillequin, A. L. Skaltsounis, Planta Med. 2008, 74, 1051–1052.
10.1055/s-0028-1084423 Google Scholar
- 8B. Cui, H. Chai, Y. Dong, F. D. Horgen, B. Hansen, D. A. Madulid, D. D. Soejarto, N. R. Farnsworth, G. A. Cordell, J. M. Pezzuto, A. D. Kinghorn, Phytochemistry 1999, 52, 95–98.
- 9S. Nathabumroong, A. Wisetsai, R. Lekphrom, T. Suebrasri, F. T. Schevenels, Nat. Prod. Res. 2023, 37, 1098–1105.
- 10A. Wisetsai, R. Lekphrom, T. Suebrasri, F. T. Schevenels, Nat. Prod. Res. 2022, 36, 5330–5336.
- 11N. T. Son, Mini-Rev. Org. Chem. 2023, 20, 818–841.
- 12A. Wisetsai, R. Lekphrom, T. Suebrasri, S. Tontapha, T. Senawong, K. Pudhom, S. Choodej, F. T. Schevenels, Planta Med. 2023, 89, 416–422.
- 13C. Ito, T. Matsui, Y. Ban, T. S. Wu, M. Itoigawa, Nat. Prod. Commun. 2016, 11, 83–86.
- 14S. Kozaki, Y. Takenaka, Y. Mizushina, T. Yamaura, T. Tanahashi, J. Nat. Med. 2014, 68, 421–426.
- 15E. Kouloura, M. Halabalaki, M. C. Lallemand, S. Nam, R. Jove, M. Litaudon, K. Awang, H. A. Hadi, A. L. Skaltsounis, J. Nat. Prod. 2012, 75, 1270–1276.
- 16P. Panyasawat, A. Wisetsai, R. Lekphrom, T. Senawong, F. T. Schevenels, Nat. Prod. Res. 2022, 36, 2743–2752.
- 17C. Ito, M. Hosono, H. Tokuda, T. S. Wu, M. Itoigawa, Nat. Prod. Commun. 2016, 11, 1299–1302.
- 18V. Kumar, V. Karunaratne, M. R. Sanath, K. Meeegalle, Phytochemistry 1989, 28, 1278–1279.
- 19M. Tanjung, I. Nurmalasari, A. K. Wilujeng, R. Dewi Saputri, F. Rachmadiarti, S. T. Tjahjandarie, Nat. Prod. Sci. 2018, 24, 284–287.
- 20Editorial Committee of Flora of China, Flora of China, Science Press, Beijing, 1997, 43, 106.
- 21S. K. Panda, Y. K. Mohanta, L. Padhi, Y. H. Park, T. K. Mohanta, H. Bae, Molecules 2016, 21, 293.
- 22F. D. Horgen, R. A. Edrada, G. de los Reyes, F. Agcaoili, D. A. Madulid, V. Wongpanich, C. K. Angerhofer, J. M. Pezzuto, D. D. Soejarto, N. R. Farnsworth, Phytomedicine. 2001, 8, 71–81.
- 23C. R. Su, P. C. Kuo, M. L. Wang, M. J. Liou, A. G. Damu, T. S. Wu, J. Nat. Prod. 2003, 66, 990–993.
- 24W. M. K. M. Ratnayake, T. S. Suresh, A. M. Abeysekera, N. Salim, U. G. Chandrika, J. Ethnopharmacol. 2019, 238, 111827.
- 25L. X. Lv, Y. Wu, H. X. He, N. P. Li, W. Zhao, Y. Q. Fan, X. Wei, J. C. Su, Q. Wang, J. H. Gu, Fitoterapia. 2022, 163, 105303.
- 26T. S. Wu, M. L. Wang, T. T. Jong, A. T. McPhail, D. R. McPhail, K. H. Lee, J. Nat. Prod. 1989, 52, 1284–1289.
- 27L. J. Yang, K. Jiang, J. J. Tan, S. J. Qu, H. F. Luo, C. H. Tan, D. Y. Zhu, Helv. Chim. Acta. 2013, 96, 119–123.
- 28X. Yang, Y. B. Zhang, Z. N. Wu, X. Q. Zhang, J. W. Jiang, Y. L. Li, G. C. Wang, Fitoterapia. 2015, 105, 156–159.
- 29J. Xu, X. C. Sun, X. Y. Liu, M. Q. Peng, S. Li, D. Q. Jin, D. Lee, M. Bartlam, Y. Q. Guo, J. Funct. Foods 2016, 23 : 565–572.
- 30Z. Y. Cheng, X. Sun, P. Liu, B. Lin, L. Z. Li, G. D. Yao, X. X. Huang, S. J. Song, Phytochemistry 2020, 179, 112503.
- 31Z. Y. Zhao, Y. P. Tong, W. Jiang, Y. Zang, J. Xiong, J. Li, J. F. Hu, J. Nat. Prod. 2023, 86, 1487–1499.
- 32Y. Wu, S. X. Bi, Z. Huang, J. Qi, B. Y. Yu, RSC Adv. 2018, 8, 2498–2505.