Biomimetic Chemical Reactions with Natural Products Using Metalloporphyrins and Salen Complexes as Catalysts: A Brief Review
Rita C. N. Sousa
Postgraduate Program in Chemistry, Chemistry Institute, Federal University of Rio Grande do Norte, 59078-900 Natal-RN, Brazil
Search for more papers by this authorVitor P. P. Confessor
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorAntonio K. B. Da Silva
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorAddison R. Almeida
Postgraduate Program in Chemistry, Chemistry Institute, Federal University of Rio Grande do Norte, 59078-900 Natal-RN, Brazil
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorFrancisco A. S. D. Pinheiro
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorCorresponding Author
Leandro S. Ferreira
Postgraduate Program in Chemistry, Chemistry Institute, Federal University of Rio Grande do Norte, 59078-900 Natal-RN, Brazil
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorRita C. N. Sousa
Postgraduate Program in Chemistry, Chemistry Institute, Federal University of Rio Grande do Norte, 59078-900 Natal-RN, Brazil
Search for more papers by this authorVitor P. P. Confessor
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorAntonio K. B. Da Silva
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorAddison R. Almeida
Postgraduate Program in Chemistry, Chemistry Institute, Federal University of Rio Grande do Norte, 59078-900 Natal-RN, Brazil
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorFrancisco A. S. D. Pinheiro
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorCorresponding Author
Leandro S. Ferreira
Postgraduate Program in Chemistry, Chemistry Institute, Federal University of Rio Grande do Norte, 59078-900 Natal-RN, Brazil
Pharmacy Department, Federal University of Rio Grande do Norte, 59012-570 Natal-RN, Brazil
Search for more papers by this authorAbstract
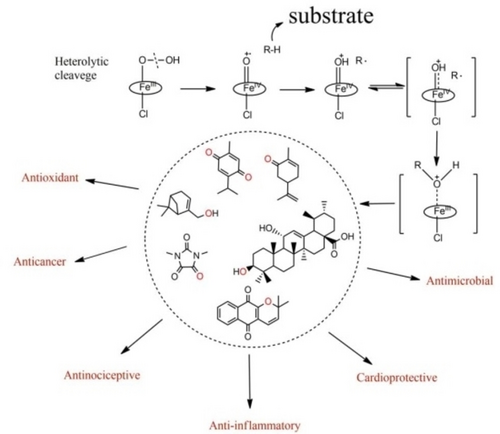
The cytochrome P450 is a superfamily of hemoproteins mainly present in the liver and are versatile biocatalysts. They participate in the primary metabolism and biosynthesis of various secondary metabolites. Chemical catalysts are utilized to replicate the activities of enzymes. Metalloporphyrins and Salen complexes can contribute to the products’ characterization and elucidate biotransformation processes, which are investigated during pre-clinical trials. These catalysts also help discover biologically active compounds and get better yields of products of industrial interest. This review aims to investigate which natural product classes are being investigated by biomimetic chemical models and the functionalities applied in the use of these catalysts. A limited number of studies were observed, with terpenes and alkaloids being the most investigated natural product classes. The research also revealed that Metalloporphyrins are still the most popular in the studies, and the identity and yield of the products obtained depend on the reaction system conditions.
Graphical Abstract
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202400668-sup-0001-misc_information.pdf1.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. Chen, in ‘Chapter 3 – Xenobiotic metabolism and disposition, An Introduction to Interdisciplinary Toxicology, 2020, Vol. 11, p. 31–42.
- 2L. B. Bolzon, J. S. dos Santos, D. B. Silva, E. J. Crevelin, L. A. Moraes, N. P. Lopes, M. D. Assis, J. Inorg. Biochem. 2017, 170, 117–124.
- 3Z. Li, Y. Jiang, F. P. Guengerich, L. Ma, S. Li, W. Zhang, J Biol Chem. 2020, 295, 833–849.
- 4B. Bhushan, Phil. Trans. R. Soc. A 2009, 367, 1446–1486.
- 5G. Di Nardo, G. Gilardi, Trends Biochem Sci. 2020, 45, 511–525.
- 6J. D. Rudolf, C. Y. Chang, M. Ma, B. Shen, Nat. Prod. Rep. 2017, 34, 1141.
- 7A. Kanwal, M. Bilal, N. Rasool, M. Zubair, S. A. A. Shah, Z. A. Zakaria, Pharmaceuticals 2022, 15, 1392.
- 8A. Masyita, R. M. Sari, A. D. Astuti, B. Yasir, N. R. Rumata, T. B. Emran, F. Nainu, J. Simal-Gandara, Food Chem. 2022, 13, 100217.
- 9B. Hamberger, S. Bak, Phil Trans R Soc B 2013, 368, 20120426.
- 10H. Kries, S. E. O'Connor, CRCHBI 2016, 31, 22–30.
- 11X. Zhang, S. Li, Nat. Prod. Rep. 2017, 34, 1061–1089.
- 12T. D. Nguyen, T. T. T. Dang, Front. Plant Sci. 2021, 12.
- 13H. Hasnat, S. A. Shompa, M. M. Islam, S. Alam, F. T. Richi, N. U. Emon, S. Ashrafi, N. U. Ahmed, M. N. R. Chowdhury, N. Fatema, M. S. Hossain, A. Ghosh, F. Ahmed, Heliyon 2024, 10, e27533.
- 14S. A. Mir, A. Dar, L. Hamid, N. Nisar, J. A. Malik, T. Ali, G. N. Bader, CRPHAR 2024, 6, 100167.
- 15W. Ren, Z, Qiao, H. Wang, L. Zhu, L. Zhang, Med. Res. Rev. 2003, 23, 519–534.
- 16R. Ushimaru, CRCHBI 2024 , 80, 102462.
- 17S. Faizan, M. M. A. Mohsen, C. Amarakanth, A. Justin, R. R. Rahangdale, H. R. Chandrashekar, B. R. P. Kumar, Results Chem. 2024, 7, 101432.
- 18H. Zhao, J. Dietrich, Expert Opin Drug Discov 2015, 10, 781–790.
- 19M. Trytek, J. Fiedurek, K. Polska, S. Radzki, Catal letters. 2005, 105, 119–126.
- 20D. C. da Silva Martins, F. C. Silva, A. M. Meireles, E. A. R. Soares, G. D. F. Silva, S. A. Vieira-Filho, Y. M. Idemori, Catal Commun 2016, 86, 104–107.
10.1016/j.catcom.2016.08.014 Google Scholar
- 21M. Milos, Appl. Catal. A Gen. 2001, 216(1-2), 157–161.
10.1016/S0926-860X(01)00560-9 Google Scholar
- 22E. F. A. Fernandes, A. R. de Oliveira, V. P. Barros, T. Guaratini, N. P. Lopes, Rev. Bras Farmacogn 2020, 30, 551–558.
- 23I. V. Rodrigues, Doctoral dissertation, Universidade de São Paulo, Brasil, 2014.
- 24S. M. S. Chauhan, P. P. Mohapatra, S. Parkash, M. Azam, Indian J Chem 2000, 39B, 183–189.
- 25J. Chen, J. Yao, X. X. Li, Y. Wang, W. Song, K. B. Cho, B. Wang, ACS Catal. 2022, 12, 6756–6769.
- 26L. R. Sartori, ‘Avaliação do metabolismo in vitro da budleína A e correlatos’, Doctoral dissertation, Universidade de São Paulo, Brasil, 2014.
- 27T. Mutsvairo, ‘Isolation, Characterization and Biomimetic Oxidation of Selected Marine Natural Products and their Analogues’, Master's thesis, Rhodes University, South Africa, 2015.
- 28W. J. Yang, N. Y. Tao, C. C. Guo, J Cent South Univ of Technol 2007, 14(5), p. 660–665.
- 29M. Trytek, M. Majdan, A. Lipke, J. Fiedurek, J catal 2012, 286, 193–205.
- 30R. Hajian, E. Bahrami, Catal. Letters 2022, 152, 2445–2456.
- 31K. Tanaka, K. Mazumder, E. R. Siwu, S. Nozaki, Y. Watanabe, K. Fukase, Tetrahedron Lett. 2012, 53, 1756–1759.
- 32K. S. Cho, Y. R. Lim, K. Lee, J. Lee, J. H. Lee, I. S. Lee, Toxicol. Res. 2017, 33, 97–106.
- 33A. Aberkouks, A. A. Mekkaoui, B. Boualy, S. E. Houssame, M. A. Ali, L. El Firdoussi, Mater. Today 2019, 13, 453–457.
- 34S. Ogawa, Y. Wakatsuki, M. Makino, Y. Fujimoto, K. Yasukawa, T. Kikuchi, T. Iida, Chem phys lipids 2010, 163, 165–171.
- 35N. Bribi, Asian J Botany 2018, 1(1), 1–6.
- 36E. H. Schaab, A. E. M. Crotti, Y. Iamamoto, M. J. Kato, L. V. C. Lotufo, N. P. Lopes, Biol Pharm Bull 2010, 33(5), 912–916.
- 37A. Bauermeister, F. A. Aguiar, L. M. M. Marques, J. D. S. Malta Jr, F. Barros, D. R. Callejon, N. P. Lopes, Planta med 2016, 82(15), 1368–1373.
- 38C. M. Neves, M. M. Simões, I. C. Santos, F. M. Domingues, M. G. P. Neves, F. A. A. Paz, J. A. Cavaleiro, Tetrahedron Lett. 2011, 52, 2898–2902.
- 39M. B. Chagas, D. O. Pontes, A. V. Albino, E. J. Ferreira, J. S. Alves, A. S. Paiva, L. S. Ferreira, Rapid Commun Mass Spectrom 2020, e8757.
- 40J. N. Sousa-Junior, B. A. Rocha, M. D. Assis, A. P. Peti, L. A. Moraes, Y. Iamamoto, N. P. Lopes, Rev. Bras. Farmacog. 2013, 23, 621–629.
- 41B. A. Rocha, A. R. M. de Oliveira, M. Pazin, D. J. Dorta, A. P. N. Rodrigues, A. A. Berretta, P. J. Gates, Biomed Res. Int. 2014, 152102.
- 42M. Niehues, V. P. Barros, F. da Silva Emery, M. Dias-Baruffi, M. das Dores Assis, N. P. Lopes, Eur J Med Chem 2012, 54, 804–812.
- 43M. D. dos Santos, P. R. Martins, P. A. dos Santos, R. Bortocan, Y. Iamamoto, N. P. Lopes, Eur J Pharm Sci 2005, 26, 62–70.
- 44S. N. Kim, H. Y. Choi, W. Lee, G. M. Park, W. S. Shin, Y. K. Kim, Phil. Trans. R. Soc. A 2008, 582, 3465–3472.
- 45L. S. Ferreira, D. R. Callejon, A. Engemann, B. Cramer, H. U. Humpf, V. P. de Barros, N. P. Lopes, Planta med 2012, 78, 1939–1941.
- 46J. N. D. P. Souza, R. M. Silva, S. S. Fortes, A. R. M. de Oliveira, L. S. Ferreira, R. Vessecchi, N. P. Lopes, D. B. Silva, Planta Med 2023, 89, 700–708.
- 47M. Linhares, S. L. Rebelo, M. M. Simoes, A. M. Silva, M. G. P. Neves, J. A. Cavaleiro, C. Freire, Appl. Catal. A Gen. 2014, 470, 427–433.
- 48P. Mondal, S. Rajapakse, G. B. Wijeratne, J Am Chem Soc 2022, 144, 3843–3854.
- 49H. M. Shen, M. Y. Hu, L. Liu, B. Qi, H. L. Ye, Y. B. She, App Catal A Gen 2020, 599, 117599.
- 50H.M Shen, L. Liu, B. Qi, M. Y. Hu, H. L. Ye, Y. B. She, Mol Catal 2020, 493, 111102.
- 51X. T. Zhou, H. B. Ji, Green Chem Eng 2020, 2, 217–223.
10.1016/j.gce.2020.10.009 Google Scholar
- 52Y. Li, C. Liu, W. Yang, New J Chem 2017, 41, 8214–8221.
- 53H. Y. Chen, M. Lv, X. T. Zhou, J. X. Wang, Q. Han, H. B. Ji, Catal Commun 2018, 109, 76–79.
- 54Y. Xie, Y. Huang, C. Wu, W. Yuan, Y. Xia, X. Liu, H. Wang, Mol Catal 2018, 452, 20–27.
- 55Y. Yang, G. Li, X. Mao, Y. She, Org Process Res Dev 2019, 23, 1078–1086.
- 56H. M. Shen, X. Wang, L. Ning, A. B. Guo, J. H. Deng, Y. B. She, Appl Catal A-Gen 2021, 609, 117904.
- 57L. D. Zanatta, I. A. Barbosa, P. C. de Sousa Filho, F. B. Zanardi, L. B. Bolzon, O. A. Serra, Y. Iamamoto, Mini-Rev Org Chem 2016, 13, 281–288.
- 58A. L. A. Lage, A. M. Meireles, A. C. Marciano, J. M. Ribeiro, E. M. de Souza-Fagundes, D. C. da Silva Martins, J Hazard Mater 2018, 360, 445–451.
- 59A. L. A. Lage, J. M. Ribeiro, E. M. de Souza-Fagundes, M. F. Brugnera, D. C. da Silva Martins, J Hazard Mater 2019, 378, 120748.
- 60M. N. Paludetto, C. Bijani, F. Puisset, V. Bernardes-Génisson, C. Arellano, A. Robert, J Med Chem 2018, 61(17), 7849–7860.
- 61S. L. Rebelo, S. M. Pires, M. M. Simões, B. de Castro, M. Neves, C. J. Medforth, Catalysts 2020, 10, 62.
10.3390/catal10010062 Google Scholar
- 62H. Y. Yin, J. Tang, J. L. Zhang, Eur J Inorg Chem 2017, 5085–5093.
- 63A. Erxleben, Inorg Chim Acta 2018, 472, 40–57.
- 64J. C. Pessoa, I. Correia, Coord Chem Rev 2019, 388, 227–247.
- 65B. Ali, M. A. Iqbal, ChemistrySelect 2017, 2(4), 1586–1604.
- 66B. K. Kundu, S. M. Mobin, S. Mukhopadhyay, Dalton Trans 2020, 49, 15481–15503.
- 67D. Majumdar, J. E. Philip, S. Das, B. K. Kundu, R. V. Saini, G. Chandan, D. Mishra, J Mol Struct 2021, 1225, 129189.
- 68A. Gualandi, M. Marchini, L. Mengozzi, H. T. Kidanu, A. Franc, P. Ceroni, P. G. Cozzi, Eur J Org Chem 2020, 10, 1486–1490.
10.1002/ejoc.201901086 Google Scholar