Novel Benzotriazole-β-lactam Derivatives as Antimalarial Agents: Design, Synthesis, Biological Evaluation and Molecular Docking Studies
Dr. Malihe Aye
Department of Chemistry, College of Sciences, Shiraz University, Shiraz, 71946-84795 Iran
Department of Civil and Environmental Engineering, Shiraz University, Shiraz, Iran
Search for more papers by this authorCorresponding Author
Prof. Aliasghar Jarrahpour
Department of Chemistry, College of Sciences, Shiraz University, Shiraz, 71946-84795 Iran
Search for more papers by this authorDr. Zahra Haghighijoo
Department of pharmacology and Toxicology, University of Texas Medical Branch, Galveston, TX, 77555 USA.
Search for more papers by this authorCorresponding Author
Dr. Roghayeh Heiran
Estahban Higher Education Center- Shiraz University, Estahban, Iran
Search for more papers by this authorDr. Roya Pournejati
Department of Biology, College of Sciences, Shiraz University, PO Box: 71467-13565, Shiraz, 71454 Iran
Search for more papers by this authorDr. Hamid Reza Karbalaei-Heidari
Department of Biology, College of Sciences, Shiraz University, PO Box: 71467-13565, Shiraz, 71454 Iran
Search for more papers by this authorDr. Veronique Sinou
Aix Marseille Univ, INSERM, SSA, MCT, Faculté de Pharmacie, 27 bd Jean Moulin, 13385 Marseille, France
Search for more papers by this authorProf. Jean Michel Brunel
Aix Marseille Univ, INSERM, SSA, MCT, Faculté de Pharmacie, 27 bd Jean Moulin, 13385 Marseille, France
Search for more papers by this authorProf. Mehmet Akkurt
Department of Physics, Faculty of Sciences, Erciyes University, 38039, Kayseri, Turkey
Search for more papers by this authorDr. Namık Özdemir
Division of Physics Education, Department of Mathematics and Science Education, Faculty of Education, Ondokuz Mayıs University, TR-55139 Samsun, Turkey
Search for more papers by this authorProf. Edward Turos
Center for Molecular Diversity in Drug Design, Discovery, and Delivery, Department of Chemistry, CHE 207, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620 USA
Search for more papers by this authorDr. Malihe Aye
Department of Chemistry, College of Sciences, Shiraz University, Shiraz, 71946-84795 Iran
Department of Civil and Environmental Engineering, Shiraz University, Shiraz, Iran
Search for more papers by this authorCorresponding Author
Prof. Aliasghar Jarrahpour
Department of Chemistry, College of Sciences, Shiraz University, Shiraz, 71946-84795 Iran
Search for more papers by this authorDr. Zahra Haghighijoo
Department of pharmacology and Toxicology, University of Texas Medical Branch, Galveston, TX, 77555 USA.
Search for more papers by this authorCorresponding Author
Dr. Roghayeh Heiran
Estahban Higher Education Center- Shiraz University, Estahban, Iran
Search for more papers by this authorDr. Roya Pournejati
Department of Biology, College of Sciences, Shiraz University, PO Box: 71467-13565, Shiraz, 71454 Iran
Search for more papers by this authorDr. Hamid Reza Karbalaei-Heidari
Department of Biology, College of Sciences, Shiraz University, PO Box: 71467-13565, Shiraz, 71454 Iran
Search for more papers by this authorDr. Veronique Sinou
Aix Marseille Univ, INSERM, SSA, MCT, Faculté de Pharmacie, 27 bd Jean Moulin, 13385 Marseille, France
Search for more papers by this authorProf. Jean Michel Brunel
Aix Marseille Univ, INSERM, SSA, MCT, Faculté de Pharmacie, 27 bd Jean Moulin, 13385 Marseille, France
Search for more papers by this authorProf. Mehmet Akkurt
Department of Physics, Faculty of Sciences, Erciyes University, 38039, Kayseri, Turkey
Search for more papers by this authorDr. Namık Özdemir
Division of Physics Education, Department of Mathematics and Science Education, Faculty of Education, Ondokuz Mayıs University, TR-55139 Samsun, Turkey
Search for more papers by this authorProf. Edward Turos
Center for Molecular Diversity in Drug Design, Discovery, and Delivery, Department of Chemistry, CHE 207, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620 USA
Search for more papers by this authorAbstract
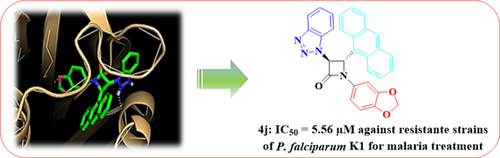
Many people around the world suffer from malaria, especially in tropical or subtropical regions. While malaria medications have shown success in treating malaria, there is still a problem with resistance to these drugs. Herein, we designed and synthesized some structurally novel benzotriazole-β-lactams using 2-(1H-benzo[d][1,2,3]triazol-1-yl)acetic acid as a key intermediate. To synthesize the target molecules, the ketene-imine cycloaddition reaction was employed. First, The reaction of 1H-benzo[d][1,2,3]triazole with 2-bromoacetic acid in aqueous sodium hydroxide yielded 2-(1H-benzo[d][1,2,3]triazol-1-yl)acetic acid. Then, the treatment of 2-(1H-benzo[d][1,2,3]triazol-1-yl)acetic acid with tosyl chloride, triethyl amine, and Schiff base provided new β-lactams in good to moderate yields.The formation of all cycloadducts was confirmed by elemental analysis, FT-IR, NMR and mass spectral data. Moreover, X-ray crystallography was used to determine the relative stereochemistry of 4a compound. The in vitro antimalarial activity test was conducted for each compound against P. falciparum K1. The IC50 values ranged from 5.56 to 25.65 μM. A cytotoxicity profile of the compounds at 200 μM final concentration revealed suitable selectivity of the compounds for malaria treatment. Furthermore, the docking study was carried out for each compound into the P. falciparum dihydrofolate reductase enzyme (PfDHFR) binding site to analyze their possible binding orientation in the active site.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202301745-sup-0001-misc_information.pdf894.5 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. E. Hyde, FEBS J. 2007, 274, 4688–4698.
- 2F. V. Rahmasari, P. B. S. Asih, F. K. Dewayanti, C. Rotejanaprasert, P. Charunwatthana, M. Imwong, D. Syafruddin, Malar. J. 2022, 21, 354.
- 3World malaria report 2022, https://www.who.int/publications/i/item/9789240064898.
- 4Talapko, Škrlec, Alebić, Jukić, Včev, Microorganisms 2019, 7, 179.
- 5A. Jarrahpour, Z. Jowkar, Z. Haghighijoo, R. Heiran, J. A. Rad, V. Sinou, F. Rouvier, C. Latour, J. M. Brunel, N. Özdemir, Med. Chem. Res. 2022, 31, 1026–1034.
- 6A. Sanei-Dehkordi, R. Heiran, M. D. Moemenbellah-Fard, S. Sayah, M. Osanloo, Bionanoscience 2022, 12, 359–369.
- 7A. Sanei-Dehkordi, R. Heiran, G. Roozitalab, N. Elahi, M. Osanloo, Psyche A J. Entomol. 2022, 2022, 1–8.
10.1155/2022/9991238 Google Scholar
- 8I. Seck, I. Ciss, A. Diédhiou, M. Baldé, S. Ka, L. A. Ba, S. F. Ndoye, B. Figadère, B. Seon-Meniel, G. Gomez, S. Cojean, S. Pomel, P. M. Loiseau, Y. Fall, M. Seck, Med. Chem. Res. 2023, 32, 158–164.
- 9P. D. Mehta, N. P. S. Sengar, A. K. Pathak, Eur. J. Med. Chem. 2010, 45, 5541–5560.
- 10N. S. Vatmurge, B. G. Hazra, V. S. Pore, F. Shirazi, P. S. Chavan, M. V. Deshpande, Bioorg. Med. Chem. Lett. 2008, 18, 2043–2047.
- 11S. B. Rosenblum, T. Huynh, A. Afonso, H. R. Davis, N. Yumibe, J. W. Clader, D. A. Burnett, J. Med. Chem. 1998, 41, 973–980.
- 12D. A. Burnett, M. A. Caplen, H. R. Davis, R. E. Burrier, J. W. Clader, J. Med. Chem. 1994, 37, 1733–1736.
- 13W. A. Slusarchyk, S. A. Bolton, K. S. Hartl, M.-H. Huang, G. Jacobs, W. Meng, M. L. Ogletree, Z. Pi, W. A. Schumacher, S. M. Seiler, J. C. Sutton, U. Treuner, R. Zahler, G. Zhao, G. S. Bisacchi, Bioorg. Med. Chem. Lett. 2002, 12, 3235–3238.
- 14W. T. Han, A. K. Trehan, J. J. Kim Wright, M. E. Federici, S. M. Seiler, N. A. Meanwell, Bioorg. Med. Chem. 1995, 3, 1123–1143.
- 15C. D. Guillon, G. A. Koppel, M. J. Brownstein, M. O. Chaney, C. F. Ferris, S. Lu, K. M. Fabio, M. J. Miller, N. D. Heindel, D. C. Hunden, R. D. G. Cooper, S. W. Kaldor, J. J. Skelton, B. A. Dressman, M. P. Clay, M. I. Steinberg, R. F. Bruns, N. G. Simon, Bioorg. Med. Chem. 2007, 15, 2054–2080.
- 16A. K. Halve, D. Bhadauria, R. Dubey, Bioorg. Med. Chem. Lett. 2007, 17, 341–345.
- 17R. Heiran, A. Jarrahpour, E. Riazimontazer, A. Gholami, A. Troudi, C. Digiorgio, J. M. Brunel, E. Turos, ChemistrySelect 2021, 6, 5313–5319.
- 18E. Riazimontazer, R. Heiran, A. Jarrahpour, A. Gholami, Z. Hashemi, A. Kazemi, ChemistrySelect 2022, 7, e202203373.
- 19S. Ranjbari, A. Jarrahpour, S. Kianpour, S. Sepehri, R. Heiran, Y. Ghasemi, E. Turos, J. Mol. Struct. 2023, 1294, 136298.
- 20M. Zarei, M. Mohamadzadeh, Tetrahedron 2011, 67, 5832–5840.
- 21A. Jarrahpour, M. Aye, V. Sinou, C. Latour, J. M. Brunel, J. Iran. Chem. Soc. 2015, 12, 2083–2092.
- 22R. Heiran, S. Sepehri, A. Jarrahpour, C. Digiorgio, H. Douafer, J. M. Brunel, A. Gholami, E. Riazimontazer, E. Turos, Bioorg. Chem. 2020, 102, 104091.
- 23P. Singh, S. Sachdeva, R. Raj, V. Kumar, M. P. Mahajan, S. Nasser, L. Vivas, J. Gut, P. J. Rosenthal, T.-S. Feng, K. Chibale, Bioorg. Med. Chem. Lett. 2011, 21, 4561–4563.
- 24P. Singh, R. Raj, V. Kumar, M. P. Mahajan, P. M. S. Bedi, T. Kaur, A. K. Saxena, Eur. J. Med. Chem. 2012, 47, 594–600.
- 25N. Borazjani, S. Sepehri, M. Behzadi, A. Jarrahpour, J. A. Rad, M. Sasanipour, M. Mohkam, Y. Ghasemi, A. R. Akbarizadeh, C. Digiorgio, J. M. Brunel, M. M. Ghanbari, G. Batta, E. Turos, Eur. J. Med. Chem. 2019, 179, 389–403.
- 26G. Dive, C. Bouillon, A. Sliwa, B. Valet, O. Verlaine, E. Sauvage, J. Marchand-Brynaert, Eur. J. Med. Chem. 2013, 64, 365–376.
- 27I. Briguglio, S. Piras, P. Corona, E. Gavini, M. Nieddu, G. Boatto, A. Carta, Eur. J. Med. Chem. 2015, 97, 612–648.
- 28A. Mermer, S. Demirci, Eur. J. Med. Chem. 2023, 259, 115655.
- 29H. K. Gulati, N. Kumar, A. Sharma, Jyoti, A. Khanna, S. Sharma, R. Salwan, P. M. S. Bedi, J. Mol. Struct. 2023, 1284, 135354.
- 30A. Singh, K. Singh, J. Kaur, R. Kaur, A. Sharma, J. Kaur, U. Kaur, R. Chadha, P. M. S. Bedi, ACS Chem. Neurosci. 2023, 14, 3291–3317.
- 31S. Zhao, J. Liu, Z. Lv, G. Zhang, Z. Xu, Eur. J. Med. Chem. 2023, 251, 115254.
- 32B. Rathod, K. Kumar, Chem. Biodiversity 2022, 19, DOI 10.1002/cbdv.202200679.
- 33N. Agouram, Med. Chem. Res. 2023, 32, 2458–2472.
- 34A. Singh, K. Singh, A. Sharma, K. Joshi, B. Singh, S. Sharma, K. Batra, K. Kaur, D. Singh, R. Chadha, P. Mohinder Singh Bedi, Chem. Biodiversity 2023, 20, DOI 10.1002/cbdv.202300024.
- 35G. Tian, Q. Song, Z. Liu, J. Guo, S. Cao, S. Long, Eur. J. Med. Chem. 2023, 259, 115603.
- 36A. Sharma, R. Dubey, R. Bhupal, P. Patel, S. K. Verma, S. Kaya, V. Asati, Mol. Diversity 2023, DOI 10.1007/s11030-023-10728-1.
- 37H. B. Bollikolla, S. N. M. Boddapati, S. Thangamani, B. R. Mutchu, M. M. Alam, M. Hussien, S. B. Jonnalagadda, J. Heterocycl. Chem. 2023, 60, 705–742.
- 38S. M. Abdul Rahman, J. S. Bhatti, S. Thareja, V. Monga, Eur. J. Med. Chem. 2023, 259, 115699.
- 39L. Ravindar, S. A. Hasbullah, K. P. Rakesh, N. I. Hassan, Eur. J. Med. Chem. 2023, 259, 115694.
- 40D.-J. Fu, S.-Y. Zhang, Y.-C. Liu, L. Zhang, J.-J. Liu, J. Song, R.-H. Zhao, F. Li, H.-H. Sun, H.-M. Liu, Y.-B. Zhang, Bioorg. Med. Chem. Lett. 2016, 26, 3918–3922.
- 41P. Shiri, Mini-Rev. Med. Chem. 2021, 21, 536–553.
- 42A.-C. Vilain, B. Pirotte, I. Vergely, N. Boggetto, B. Masereel, M. Schynts, J. Delarge, M. Reboud-Ravaux, J. Pharm. Pharmacol. 2011, 45, 466–472.
10.1111/j.2042-7158.1993.tb05577.x Google Scholar
- 43S. Dhawan, P. Awolade, P. Kisten, N. Cele, A. S. Pillay, S. T. Saha, M. Kaur, S. B. Jonnalagadda, P. Singh, Chem. Biodiversity 2020, 17, e1900462.
- 44J. M. Divse, S. B. Mhaske, C. R. Charolkar, D. G. Sant, S. G. Tupe, M. V. Deshpande, V. M. Khedkar, L. U. Nawale, D. Sarkar, V. S. Pore, New J. Chem. 2017, 41, 470–479.
- 45K. Kumar, S. Carrère-Kremer, L. Kremer, Y. Guérardel, C. Biot, V. Kumar, Dalton Trans. 2013, 42, 1492–1500.
- 46K. Kumar, B. Pradines, M. Madamet, R. Amalvict, V. Kumar, Eur. J. Med. Chem. 2014, 86, 113–121.
- 47P. Singh, P. Singh, M. Kumar, J. Gut, P. J. Rosenthal, K. Kumar, V. Kumar, M. P. Mahajan, K. Bisetty, Bioorg. Med. Chem. Lett. 2012, 22, 57–61.
- 48A. Jarrahpour, M. Aye, J. A. Rad, S. Yousefinejad, V. Sinou, C. Latour, J. M. Brunel, E. Turos, J. Iran. Chem. Soc. 2018, 15, 1311–1326.
- 49A. Jarrahpour, P. Shirvani, V. Sinou, C. Latour, J. M. Brunel, Med. Chem. Res. 2016, 25, 149–162.
- 50X. Chen, C. Liu, J. Wang, Y. Li, J. Heterocycl. Chem. 2010, 47, 1225–1229.
- 51M. Akkurt, S. Karaca, A. A. Jarrahpour, M. Zarei, O. Büyükgüngör, Acta Crystallogr. Sect. E 2008, 64, o924–o924.
- 52M. Akkurt, S. Karaca, A. A. Jarrahpour, D. Khalili, O. Büyükgüngör, Acta Crystallogr. Sect. E 2006, 62, o866–o868.
- 53M. Akkurt, S. Türktekin, A. Jarrahpour, S. A. T. Badrabady, O. Büyükgüngör, Acta Crystallogr. Sect. E 2011, 67, o183–o183.
- 54B. Bananezhad, M. Islami, Synlett 2017, 28, 1453–1456.
- 55M. Alborz, A. Jarrahpour, R. Pournejati, H. R. Karbalaei-Heidari, V. Sinou, C. Latour, J. M. Brunel, H. Sharghi, M. Aberi, E. Turos, L. Wojtas, Eur. J. Med. Chem. 2018, 143, 283–291.
- 56F. Zigheimat, M. Islami, F. Nourmohammadian, Synlett 2013, 25, 229–232.
- 57W. Trager, J. B. Jensen, Science 1976, 193, 673–675.
- 58C. Lambros, J. P. Vanderberg, J. Parasitol. 1979, 65, 418–20.
- 59R. Nakweti, V. Sinou, S. Ndiku, F. Sabot, C. Franche, European J. Med. Plants 2017, 18, 1–10.
10.9734/EJMP/2017/30181 Google Scholar
- 60H. Le Nagard, C. Vincent, F. Mentré, J. Le Bras, Comput. Methods Programs Biomed. 2011, 104, 10–18.
- 61http://www.antimalarialicestimator.net.
- 62Z. Haghighijoo, O. Firuzi, B. Hemmateenejad, S. Emami, N. Edraki, R. Miri, Bioorg. Chem. 2017, 74, 126–133.