Synthesis, Biological Activity Evaluation, Docking and Molecular Dynamics Studies of New Triazole-Tetrahydropyrimidinone(thione) Hybrid Scaffolds as Urease Inhibitors
Sadaf Rezvanpoor
Department of Medicinal Chemistry, School of Pharmacy, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Search for more papers by this authorNeda Shakour
Department of Medicinal Chemistry, School of Pharmacy, Mashhad University of Medical Sciences, 9138813944 Mashhad, Iran
Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, 9138813944 Mashhad, Iran
Search for more papers by this authorNazli Ahangarzadeh
Department of Medicinal Chemistry, School of Pharmacy, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Search for more papers by this authorHamid Bakherad
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Isfahan University of Medical Sciences, 81746-73461 Isfahan, Iran
Search for more papers by this authorCorresponding Author
Saghi Sepehri
Department of Medicinal Chemistry, School of Pharmacy, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Pharmaceutical Sciences Research Center, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Search for more papers by this authorGhazaleh Farhadi
Department of Medicinal Chemistry, School of Pharmacy, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Search for more papers by this authorMohammad Hosein Pakdel
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Isfahan University of Medical Sciences, 81746-73461 Isfahan, Iran
Search for more papers by this authorMehrdad Iranshahi
Biotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, 9138813944 Mashhad, Iran
Search for more papers by this authorSadaf Rezvanpoor
Department of Medicinal Chemistry, School of Pharmacy, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Search for more papers by this authorNeda Shakour
Department of Medicinal Chemistry, School of Pharmacy, Mashhad University of Medical Sciences, 9138813944 Mashhad, Iran
Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, 9138813944 Mashhad, Iran
Search for more papers by this authorNazli Ahangarzadeh
Department of Medicinal Chemistry, School of Pharmacy, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Search for more papers by this authorHamid Bakherad
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Isfahan University of Medical Sciences, 81746-73461 Isfahan, Iran
Search for more papers by this authorCorresponding Author
Saghi Sepehri
Department of Medicinal Chemistry, School of Pharmacy, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Pharmaceutical Sciences Research Center, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Search for more papers by this authorGhazaleh Farhadi
Department of Medicinal Chemistry, School of Pharmacy, Ardabil University of Medical Sciences, 56189-53141 Ardabil, Iran
Search for more papers by this authorMohammad Hosein Pakdel
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Isfahan University of Medical Sciences, 81746-73461 Isfahan, Iran
Search for more papers by this authorMehrdad Iranshahi
Biotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, 9138813944 Mashhad, Iran
Search for more papers by this authorAbstract
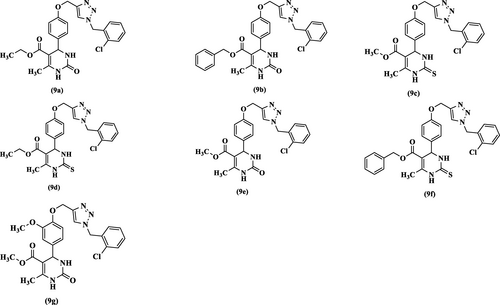
New series of triazole-tetrahydropyrimidinone(thione) hybrids (9a–g) were synthesized. FT-IR, 1H-NMR, 13C-NMR, elemental analysis and mass spectroscopic studies characterized the structures of the synthesized compounds. Then, the synthesized compounds were screened to determine the urease inhibitory activity. Methyl 4-(4-((1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (9c) exhibited the highest urease inhibitory activity (IC50=25.02 μM) among the compounds which was almost similar to thiourea as standard (IC50=22.32 μM). The docking study of the screened compounds demonstrated that these compounds fit well in the urease active site. Based on the docking study, compound 9c with the highest urease inhibitory activity showed chelates with both Ni2+ ions of the urease active site. Moreover, the molecular dynamic study of the most potent compounds showed that they created important interactions with the active site flap residues, His322, Cys321, and Met317.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202300054-sup-0001-misc_information.pdf3.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1I. P. Kalatuwawege, M. J. Gunaratna, D. N. Udukala, Mol. (Basel, Switzerland). 2021, 26.
- 2J. K. Y. Hooi, W. Y. Lai, W. K. Ng, M. M. Y. Suen, F. E. Underwood, D. Tanyingoh, P. Malfertheiner, D. Y. Graham, V. W. S. Wong, J. C. Y. Wu, F. K. L. Chan, J. J. Y. Sung, G. G. Kaplan, S. C. Ng, Gastroenterology. 2017, 153, 420–429.
- 3W. Y. Li, W. W. Ni, Y. X. Ye, H. L. Fang, X. M. Pan, J. L. He, T. L. Zhou, J. Yi, S. S. Liu, M. Zhou, Z. P. Xiao, H. L. Zhu, J. Enzyme Inhib. Med. Chem. 2020, 35, 404–413.
- 4E. Menteşe, M. Emirik, B. B. Sökmen, Bioorg. Chem. 2019, 86, 151–158.
- 5C. Montecucco, R. Rappuoli, Nat. Rev. Mol. Cell Biol. 2001, 2, 457–466.
- 6M. Ahmed, M. A. Qadir, A. Hameed, M. N. Arshad, A. M. Asiri, M. Muddassar, Biochem. Biophys. Res. Commun. 2017, 490, 434–440.
- 7Z. P. Xiao, Z. Y. Peng, J. J. Dong, R. C. Deng, X. D. Wang, H. Ouyang, P. Yang, J. He, Y. F. Wang, M. Zhu, X. C. Peng, W. X. Peng, H. L. Zhu, Eur. J. Med. Chem. 2013, 68, 212–221.
- 8R. Mamidala, S. R. S. Bhimathati, A. Vema, Bioorg. Chem. 2021, 114, 105010.
- 9K. Bozorov, J. Zhao, H. A. Aisa, Bioorg. Med. Chem. 2019, 27, 3511–3531.
- 10E. Bonandi, M. S. Christodoulou, G. Fumagalli, D. Perdicchia, G. Rastelli, D. Passarella, Drug Discovery Today 2017, 22, 1572–1581.
- 11M. V. Slivka, N. I. Korol, M. M. Fizer, J. Heterocycl. Chem. 2020, 57, 3236–3254.
- 12S. Moghimi, F. Goli-Garmroodi, M. Allahyari-Devin, H. Pilali, M. Hassanzadeh, S. Mahernia, M. Mahdavi, L. Firoozpour, M. Amanlou, A. Foroumadi, Arch. Pharm. 2018, 351, e1800005.
- 13N. Karaali, M. Menteşe, N. Baltas, E. Menteşe, J. Heterocycl. Chem. 2018, 55, 2571–2577.
- 14E. Menteşe, G. Akyüz, M. Emirik, N. Baltaş, Bioorg. Chem. 2019, 83, 289–296.
- 15A. Saeed, F. A. Larik, P. A. Channar, H. Mehfooz, M. Ashraf, Q. Abbas, M. Hassan, S.-Y. Seo, Chem. Biol. Drug Des. 2017, 90, 764–777.
- 16M. S. Asgari, H. Azizian, M. Nazari Montazer, M. Mohammadi-Khanaposhtani, M. Asadi, S. Sepehri, P. R. Ranjbar, R. Rahimi, M. Biglar, B. Larijani, M. Amanlou, M. Mahdavi, Arch. Pharm. 2020, 353, e2000023.
- 17A. Khan, J. Hashim, N. Arshad, I. Khan, N. Siddiqui, A. Wadood, M. Ali, F. Arshad, K. M. Khan, M. I. Choudhary, Bioorg. Chem. 2016, 64, 85–96.
- 18M. T. Muhammad, K. M. Khan, A. Tabassum, A. Khan, F. Arshad, B. Fatima, M. I. Choudhary, N. Syed, S. T. Moin, Bioorg. Chem. 2017, 75, 317–331.
- 19F. Iftikhar, Y. Ali, F. Ahmad Kiani, S. Fahad Hassan, T. Fatima, A. Khan, B. Niaz, A. Hassan, F. Latif Ansari, U. Rashid, Bioorg. Chem. 2017, 74, 53–65.
- 20S. Shamim, K. M. Khan, U. Salar, F. Ali, M. A. Lodhi, M. Taha, F. A. Khan, S. Ashraf, Z. Ul-Haq, M. Ali, S. Perveen, Bioorg. Chem. 2018, 76, 37–52.
- 21A. A. E. Mourad, A. E. Khodir, S. Saber, M. A. E. Mourad, Pharmaceuticals (Basel, Switzerland) 2021, 14, 144.
- 22K. Gong, H. Wang, S. Wang, X. Ren, Tetrahedron. 2015, 71, 4830–4834.
- 23S. Putatunda, A. Chakraborty, C. R. Chim. 2014, 17, 1057–1064.
- 24S. Safari, R. Ghavimi, N. Razzaghi-Asl, S. Sepehri, J. Heterocycl. Chem. 2020, 57, 1023–1033.
- 25Y. F. Rego, M. P. Queiroz, T. O. Brito, P. G. Carvalho, V. T. de Queiroz, Â. De Fátima, F. Macedo, Jr., J. Adv. Res. 2018, 13, 69–100.
- 26A. Mourad Al-Azhar, J. Pharm. Sci. 2021, 64, 203–221.
- 27N. Ahangarzadeh, N. Shakour, S. Rezvanpoor, H. Bakherad, M. H. Pakdel, G. Farhadi, S. Sepehri, Arch. Pharm. 2022, e2200158.
- 28E. B. Rezaei, F. Abedinifar, H. Azizian, M. N. Montazer, M. Asadi, S. Hosseini, S. Sepehri, M. Mohammadi-Khanaposhtani, M. Biglar, B. Larijani, M. Amanlou, M. Mahdavi, Chem. Pap. 2021, 75, 4217–4226.
- 29S. Mirzayi, M. Kakanj, S. Sepehri, B. Alavinejad, Z. Bakherad, M. Ghazi-Khansari, Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 1109–1116.
- 30L. Mazzei, M. Cianci, F. Musiani, S. Ciurli, Dalton Trans. 2016, 45, 5455–5459.
- 31K. Macegoniuk, E. Grela, J. Palus, E. Rudzińska-Szostak, A. Grabowiecka, M. Biernat, Ł. Berlicki, J. Med. Chem. 2016, 59, 8125–8133.
- 32I. O. Isaac, M. al-Rashida, S. U. Rahman, R. D. Alharthy, A. Asari, A. Hameed, K. M. Khan, J. Iqbal, Bioorg. Chem. 2019, 82, 6–16.
- 33O.-U.-R. Abid, S. Daud, A. Sardar, W. Rehman, A. Wadood, A. U. Rehman, T. Akhter, I. Bibi, Z. Ahmad, M. Yasir, Med. Chem. Res. 2021, 30, 226–235.
- 34K. Zaman, F. Rahim, M. Taha, H. Ullah, A. Wadood, M. Nawaz, F. Khan, Z. Wahab, S. A. A. Shah, A. U. Rehman, A. N. Kawde, M. Gollapalli, Bioorg. Chem. 2019, 89, 103024.
- 35M. Biglar, R. Mirzazadeh, M. Asadi, S. Sepehri, Y. Valizadeh, Y. Sarrafi, M. Amanlou, B. Larijani, M. Mohammadi-Khanaposhtani, M. Mahdavi, Bioorg. Chem. 2020, 95, 103529.
- 36M. Taha, F. Rahim, H. Ullah, A. Wadood, R. K. Farooq, S. A. A. Shah, M. Nawaz, Z. A. Zakaria, Sci. Rep. 2020, 10, 10673.
- 37M. Nazari Montazer, M. Asadi, S. Bahadorikhalili, F. S. Hosseini, A. Amanlou, M. Biglar, M. Amanlou, Med. Chem. Res. 2021, 30, 729–742.
- 38M. Özil, Ö. Tuzcuoğlu, N. Baltaş, M. Emirik, ChemistrySelect. 2021, 6, 5307–5312.
- 39H. Liu, Y. Wang, M. Lv, Y. Luo, B.-M. Liu, Y. Huang, M. Wang, J. Wang, Bioorg. Chem. 2020, 105, 104370.
- 40F. Peytam, M. Adib, S. Mahernia, M. Rahmanian-Jazi, M. Jahani, B. Masoudi, M. Mahdavi, M. Amanlou, Bioorg. Chem. 2019, 87, 1–11.
- 41X.-D. Yu, R.-B. Zheng, J.-H. Xie, J.-Y. Su, X.-Q. Huang, Y.-H. Wang, Y.-F. Zheng, Z.-Z. Mo, X.-L. Wu, D.-W. Wu, Y.-E. Liang, H.-F. Zeng, Z.-R. Su, P. Huang, J. Ethnopharmacol. 2015, 162, 69–78.
- 42F. Peytam, M. Adib, S. Mahernia, M. Rahmanian-Jazi, M. Jahani, B. Masoudi, M. Mahdavi, M. Amanlou, Bioorg. Chem. 2019, 87, 1–11.
- 43M. J. Abraham, T. Murtola, R. Schulz, S. Páll, J. C. Smith, B. Hess, E. J. S. Lindahl, 2015, 1, 19–25.
- 44T. J. Piggot, A. Pineiro, S. J. J. O. C t Khalid, Comput. 2012, 8, 4593–4609.