Five New Phenylpropanoyl Phloroglucinol Derivatives from Leptospermum scoparium
Ji-Hong Gu
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJun-Shan Liu
Guangdong Provincial Key Laboratory of Chinese Medicine Pharmaceutics, School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, 510515 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJia-Hui Lin
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorFen Liu
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorZhen-Long Wu
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorYuan-Ru Zheng
Guangdong Provincial Key Laboratory of Chinese Medicine Pharmaceutics, School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, 510515 P. R. China
Search for more papers by this authorCorresponding Author
Wen-Cai Ye
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorCorresponding Author
Lei Wang
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorJi-Hong Gu
Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, 510405 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJun-Shan Liu
Guangdong Provincial Key Laboratory of Chinese Medicine Pharmaceutics, School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, 510515 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJia-Hui Lin
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorFen Liu
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorZhen-Long Wu
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorYuan-Ru Zheng
Guangdong Provincial Key Laboratory of Chinese Medicine Pharmaceutics, School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, 510515 P. R. China
Search for more papers by this authorCorresponding Author
Wen-Cai Ye
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorCorresponding Author
Lei Wang
Center for Bioactive Natural Molecules and Innovative Drugs Research, and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorAbstract
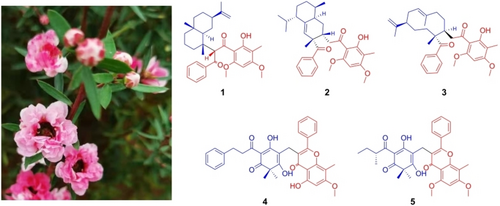
Leptosperols C−G (1–5), five new phenylpropanoyl phloroglucinol derivatives were isolated from the leaves of Leptospermum scoparium. Compounds 1–3 are phenylpropanoyl phloroglucinol-sesquiterpene adducts with new carbon skeletons. Their structures with absolute configurations were elucidated by detailed spectroscopic analyses, single-crystal X-ray diffraction, and electronic circular dichroism (ECD) calculation. Compounds 2 and 3 exhibited moderate anti-inflammatory activity in zebrafish acute inflammatory models.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202201111-sup-0001-misc_information.pdf2.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1X.-J. Qin, H. Liu, Q. Yu, H. Yan, J.-F. Tang, L.-K. An, A. Khah, Q.-R. Chen, X. J. -Hao, H.-Y. Liu, ‘Acylphloroglucinol derivatives from the twigs and leaves of Callistemon salignus’, Tetrahedron 2017, 73, 1803–1811.
- 2J.-C. Su, S. Wang, W. Cheng, X.-J. Huang, M.-M. Li, R.-W. Jiang, Y.-L. Li, L. Wang, W.-C. Ye, Y. Wang, ‘Phloroglucinol derivatives with unusual skeletons from Cleistocalyx operculatus and their in vitro antiviral activity’, J. Org. Chem. 2018, 83, 8522–8532.
- 3J.-Q. Hou, C. Guo, J.-J. Zhao, Y.-Y. Dong, X.-L. Hu, Q.-W. He, B.-B. Zhang, M. Yan, H. Wang, ‘Anti-inflammatory meroterpenoids from Baeckea frutescens’, J. Nat. Prod. 2017, 80, 2204–2214.
- 4C. Liu, S. Ang, X.-J. Huang, H.-Y. Tian, Y.-Y. Deng, D.-M. Zhang, Y. Wang, W.-C. Ye, L. Wang, ‘Meroterpenoids with new skeletons from myrtus communis and structure revision of myrtucommulone K’, Org. Lett. 2016, 18, 4004–4007.
- 5X.-J. Qin, T.-J. Rauwolf, P.-P. Li, H. Liu, J. McNeely, Y. Hua, H.-Y. Liu, J.-A.-J. Porco, ‘Isolation and synthesis of novel meroterpenoids from Rhodomyrtus tomentosa: investigation of a reactive enetrione intermediate’, Angew. Chem. Int. Ed. 2019, 58, 4291–4296.
- 6Y.-H. Guo, Y.-H. Zhang, M.-X. Xiao, Z.-G. Xie, ‘Biomimetic syntheses of callistrilones A−E via an oxidative [3 + 2] cycloaddition’, Org. Lett. 2018, 20, 2509–2512.
- 7S.-P. Yang, X.-W. Zhang, J. Ai, L.-S. Gan, J.-B. Xu, Y. Wang, Z.-S. Su, L. Wang, J. Ding, M.-Y. Geng, J. M. Yue, ‘Potent HGF/c-Met axis inhibitors from Eucalyptus globulus: the coupling of phloroglucinol and sesquiterpenoid is essential for the activity’, J. Med. Chem. 2012, 55, 8183–8187.
- 8J. H. Gu, W.-J. Wang, J.-Z. Chen, J.-S. Liu, N.-P. Li, M.-J. Cheng, L.-J. Hu, C.-C. Li, W.-C. Ye, L. Wang, ‘Leptosperols A and B, two cinnamoylphloroglucinol-sesquiterpenoid hybrids from Leptospermum scoparium: structural elucidation and biomimetic synthesis’, Org. Lett. 2020, 22, 1796–1800.
- 9F. Liu, H.-Y. Tian, X.-L. Huang, W.-J. Wang, N.-P. Li, J. He, W.-C. Ye, L. Wang, ‘Xanthchrysones A−C: rearranged phenylpropanoyl-phloroglucinol dimers with unusual skeletons from Xanthostemon chrysanthus’, J. Org. Chem. 2019, 84, 15355–15361.
- 10M.-J. Cheng, X.-Y. Yang, J.-Q. Cao, C. Liu, L.-P. Zhong, Y. Wang, X.-F. You, C.-C. Li, L. Wang, W.-C. Ye, ‘Isolation, structure elucidation and total synthesis of myrtuspirone A from Myrtus communis’, Org. Lett. 2019, 21, 1583–1587.
- 11M.-J. Cheng, J.-Q. Cao, X.-Y. Yang, L.-P. Zhong, L.-J. Hu, B.-L. Hou, Y.-J. Hu, Y. Wang, X.-F. You, L. Wang, W.-C. Ye, C.-C. Li, ‘Catalytic asymmetric total syntheses of myrtucommuacetalone, myrtucommuacetalone B and callistrilones A, C, D, E’, Chem. Sci. 2018, 9, 1488–1495.
- 12J.-Q. Cao, X.-J. Huang, Y.-T. Li, Y. Wang, L. Wang, R.-W. Jiang, W.-C. Ye, ‘Callistrilones A and B, triketone-phloroglucinol-monoterpene hybrids with a new skeleton from Callistemon rigidus’, Org. Lett. 2016, 18, 120–123.
- 13J.-Q. Cao, H.-Y. Tian, M.-M. Li, W. Zhang, Y. Wang, L. Wang, W.-C. Ye, ‘Rearranged phloroglucinol-monoterpenoid adducts from Callistemon rigidus’, J. Nat. Prod. 2018, 81, 57–62.
- 14S.-G. Brooker, R.-C. Cooper, ‘New Zealand medicinal plants’, Econ. Bot. 1961, 15, 1–10.
- 15K. Xia, J.-H. Gu, X.-X. Fu, N.-P. Li, M. Chen, Q. Huang, W.-J. Wang, W.-C. Ye, L. Wang, ‘Dimeric acylphloroglucinol derivatives with new skeletons from Leptospermum scoparium’, Chem. Biodiversity 2021, 18, e2100252.
- 16R. Mayer, ‘A β-hydroxychalcone from Leptospermum scoparium’, Planta Med. 1993, 59, 269–271.
- 17J.-H. Gu, W. Zhang, W.-Y. Cai, X.-X. Fu, H.-L. Zhou, N.-P. Li, H.-Y. Tian, J.-S. Liu, W.-C. Ye, L. Wang, ‘Gelserancines A−E, monoterpenoid indole alkaloids with unusual skeletons from Gelsemium elegans’, Org. Chem. Front. 2021, 8, 1918–1925.
- 18N.-P. Li, J.-S. Liu, J.-W. Liu, H.-Y. Tian, H.-L. Zhou, Y.-R. Zheng, X.-J Huang, J.-Q. Cao, W.-C. Ye, L. Wang, ‘Monoterpenoid indole alkaloids from the fruits of Gelsemium elegans and their anti-inflammatory activities’, Bioorg. Chem. 2021, 107, 104624.