Proanthocyanidins in Pruning Wood Extracts of Four European Plum (Prunus domestica L.) Cultivars and Their hLDHA Inhibitory Activity
Abstract
European plum tree (Prunus domestica L.) is cultivated in many countries for its delicious and nutritive fruit and, accordingly, certain amounts of wood (from pruning works) are generated every year. The main objective of this work was to value this agricultural woody residue, for which the chemical composition of pruning wood extracts from four European plum cultivars was investigated, and the human lactate dehydrogenase A (hLDHA) inhibitory activity of plum wood extracts and pure proanthocyanidins present in those extracts was measured. For the chemical characterization, total phenolic content and DPPH radical-scavenging assays and HPLC-DAD/ESI-MS analyses were performed, the procyanidin (−)-ent-epicatechin-(2α→O→7,4α→8)-catechin (4), the phenolic glucoside (−)-annphenone (3) and the flavan-3-ol catechin (1) being the major components of the wood extracts. Some quantitative and qualitative differences were found among plum cultivars, and the content of proanthocyanidins ranged from 1.51 (cv. ‘Claudia de Tolosa’) to 8.51 (cv. ‘De la Rosa’) mg g−1 of dry wood. For the hLDHA inhibitory activity, six wood extracts and six proanthocyanidins were evaluated by a UV spectrophotometric assay, compound 4 showing the highest inhibitory activity (IC50 3.2 μM) of this enzyme involved on the excessive production of oxalate in the liver of patients affected by the rare disease Primary Hyperoxaluria.
Graphical Abstract
Introduction
Prunus domestica L., known as European plum tree, is a deciduous tree that is mainly cultivated in countries, such as China, Romania, USA and Serbia, for its delicious and nutritive fruits (plums). In Europe, it is also cultivated in France, Italy and Spain. European plum tree, together with Japanese plum tree (Prunus salicina Lindl.), is the source of the plums and prunes (dehydrated plums) consumed in the world.1 According to the statistics of the Food and Agriculture Organization of the United Nations, around 12 million of metric tons of plums, including P. domestica and P. salicina, are produced each year in the world.1, 2
Numerous cultivars (cv.) of European plum tree are known, such as ‘D'Agen prune’, ‘Italian prune’ and ‘German prune’, used to produce prunes, or cultivars belonging to the group of ‘Reine Claude’ and ‘Mirabelles’, cultivated for their (fresh) plums.1, 3 In Spain, the main cultivars grown are ‘De la Rosa’, ‘Reina Claudia Verde’, ‘President’, and ‘Stanley’, among others.4 In general, the consumption of plums and prunes from any cultivar provide beneficial properties, evaluated by in vitro and in vivo assays,5, 6 that afford benign effects on cardiovascular and osteoporosis diseases,7, 8 among others.
Regarding the phenolic composition of P. domestica, it has been studied in several occasions. Thus, flavonoids have been reported in fruits,9-14 leaves,15 stems,16, 17 and heartwood,18-21 and procyanidin A2, B1, B2 and B7 have been identified in plums.10, 22 More recently, our research group has isolated from the woody biomass generated during the pruning works on P. domestica, cv. ‘De la Rosa’, six dimeric A-type proanthocyanidins (PACs) (4−9), together with the phenolic glucoside (−)-annphenone (3). In addition, two flavan-3-ols, catechin (1) and epicatechin (2), have also been identified by HPLC-MS (Figure 1).23 In that work, compounds 3–9 were reported for the first time in P. domestica, and heteroside 3 and propelargonidins 6 and 7 showed noticeable activities in disruption of preformed biofilms of a resistant strain of Enterobacter sp.23
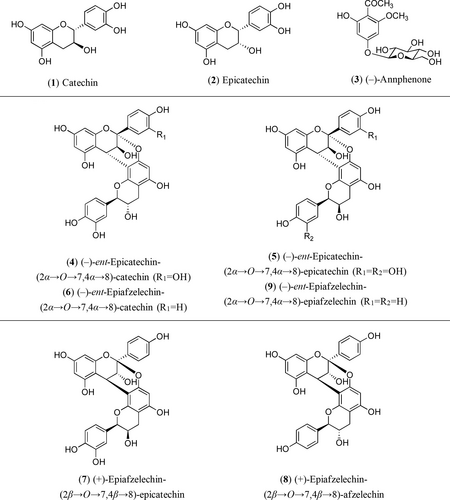
Compounds previously identified by the authors in the wood of an European plum tree (Prunus domestica L.).23
Agricultural works on fruit crops generate every year big amounts of pruning wood, which is an interesting (renewable, cheap and abundant) waste to get bioactive compounds like flavonoids, among many other compounds.24 The valorization of agro-industrial residues has been reported in numerous occasions,25-28 and our group has made some contributions on the recovery of natural products from plant materials obtained from certain crops and wild plants.23, 29-32 In these works, we have focused our attention on the antioxidant and antimicrobial activities of isolated phenolic compounds, but more recently, we have started to explore the applicability of small organic molecules in the treatment of primary hyperoxalurias (PHs).
PHs are a group of rare genetic diseases that affect the hepatic metabolism of glyoxylate giving rise to an excessive production of oxalate in the liver. The impossibility to metabolize this compound by human organism make that it should be excreted by kidneys, provoking a high concentration of oxalate in this organ, which precipitates as calcium oxalate crystals producing tissue damage. Until very recently, existing treatments were limited to palliative measures and kidney/liver transplants in extreme cases as unique solution to avoid the death of the patient. However, nowadays, several pharmacological strategies are under study for the treatment of PHs.33 One of them is the direct inhibition of human hepatic isozyme Lactate Dehydrogenase A (hLDHA), which is in charge of the transformation of glyoxylate into oxalate, by small synthetic molecules.34-38 In addition, some natural products and plant extracts rich in phenolic compounds (phenylpropanoids, flavonoids and procyanidins) have been studied for their ability to inhibit hLDHA,39-41 although it is a field still poorly explored.
Taking into account the developing connection between phenolics and hLDHA inhibitory activity, the aim of this research work was to achieve the chemical characterization of pruning wood samples of the Spanish P. domestica cultivars ‘De la Rosa’, ‘Reina Claudia Verde’, ‘Claudia de Tolosa’, and ‘Stanley’ (by total phenolic content and antioxidant assays, and HPLC-DAD/ESI-MS analyses), and the hLDHA inhibitory activity of wood extracts and pure phenolic compounds (proanthocyanidins) present in those extracts, in order to find out natural products to develop potential treatments for PHs in the future.
Results and Discussion
In this study, the woods of four European plum (P. domestica) cultivars were compared for their total phenolic content, antioxidant activity and phenolic profile. The wood samples were collected from the biomass generated during the pruning works on cultivars ‘Reina Claudia Verde’, ‘Claudia de Tolosa’, ‘Stanley’, and ‘De la Rosa’, which were grown in two farming plots located in the village of Jaraíz de la Vera (province of Cáceres), a known European plum producing area in Spain. Furthermore, the hLDHA inhibitory activity of all wood extracts and proanthocyanidins 4–9 was evaluated by a UV spectrophotometric assay. There are no previous studies on the chemical composition of fruits or any organ of these European plum cultivars.
Extraction of European Plum Woods
The sequential extraction of the six European plum wood samples (Table 1) for 2 h at reflux, with dichloromethane (DCM) in first time and then with ethyl acetate (AcOEt), was an efficient method to obtain the phenolic fraction of woods as reported by us in previous works.29, 30, 32 The prior extraction with DCM allowed us to withdraw non-phenolic compounds from wood samples,29 and the subsequent AcOEt extraction of the residual wood material (already free of non-phenolic compounds) led to concentrate extracts in phenolic compounds, which were the subject of this work. This sequential extraction allowed us to obtain simpler extracts from a chemical viewpoint in order to be later analyzed by HPLC-DAD and HPLC-DAD/ESI-MS. In all cases, extraction yields with DCM were lower (0.6–1.2 %, with regard to dry wood) than with AcOEt (1.2–3.3 %) (Table 1). Regarding AcOEt extracts, cultivar ‘De la Rosa’ (3.3 %) showed the higher yield followed by the two samples of ‘Reina Claudia Verde’ (2.4 % and 1.8 %). These extraction yields are higher than those of other woods from agricultural wastes processed by us in the past, such as olive wood (1.6 % yield),29 sweet cherry wood (0.9 % yield)32 or laurel wood (0.3–0.8 % yield),31 and of the same order of magnitude than other useful woods like, for example, Phoebe zhennan wood residues (3.8 % yield).42
Cultivars |
Reference |
DCMa |
AcOEtb |
|---|---|---|---|
‘Reina Claudia Verde’c |
S1 |
1.1 % |
1.8 % |
‘Claudia de Tolosa’c |
S2 |
0.6 % |
1.2 % |
‘Stanley’c |
S3 |
0.9 % |
1.7 % |
‘De la Rosa’c |
S4 |
1.2 % |
3.3 % |
‘Reina Claudia Verde’d |
S5 |
1.2 % |
2.4 % |
‘Claudia de Tolosa’d |
S6 |
1.1 % |
1.2 % |
- a Extraction yield (grams of extract per 100 grams of wood) for the dichloromethane extraction of the wood sample; b Extraction yield (grams of extract per 100 grams of wood) for the ethyl acetate extraction of the residual wood material; c Wood sample collected in farming plot A; d Wood sample collected in farming plot B.
Total Phenolic Content and Antioxidant Activity of European Plum Wood Extracts
As a first approach to characterize the wood of European plum cultivars, the total phenolic content (TPC) and the antioxidant activity of the six AcOEt extracts obtained were determined. The TPC of extracts was estimated according to the Folin-Ciocalteu colorimetric methodology and the radical-scavenging activity by the DPPH-scavenging assay. TPC values ranged from 333.1 to 487.3 mg GAE g−1 dry extract (Figure 2) and cv. ‘Stanley’ (S3) showed the highest number followed by cv. ‘De la Rosa’ (S4). On the contrary, cv. ‘Claudia de Tolosa’ showed in both farming plots the lowest TPC values. According to a reported quality assessment of plum fruits from 178 different cultivars, the TPC values found ranged from 38.5 to 841.5 mg GAE per 100 g of fruit.43 Comparing these values with those found for the six woods studied here (Figure 2), expressed as mg GAE per 100 g of wood (taking into account extraction yields gathered in Table 1), it can be said that wood of cv. ‘De la Rosa’, for example, contains around twice (1508.8 mg GAE per 100 g of wood) as many phenolics as any other plum fruit.

Total phenolic content in Prunus domestica L. wood AcOEt extracts (see Table 1 for cultivar names), expressed as mean of three replicates. Error bars show the standard deviation (SD) of measurements; mean values not sharing the same letter are significantly different by ANOVA Tukey test (p<0.05).
Regarding the antioxidant activity, the radical-scavenging percentages (RSP) values ranged from 47.2 % to 72.9 % (Figure 3), cv. ‘De la Rosa’ showing the highest value, followed by cv. ‘Reina Claudia Verde’, while cv. ‘Claudia de Tolosa’ presented in both farming plots the lowest RSP values. For comparative purposes, the activity of a commercial sample of rosemary (Rosmarinus officinalis L.) extract has been measured, observing that all plum woods have somewhat less activity than rosemary, except cv. ‘De la Rosa′, which is above. Taking into account that rosemary is a plant known for its outstanding antioxidant activity, we can say that these plum woods, and especially one of them, could a priori compete with rosemary in activity and applications.
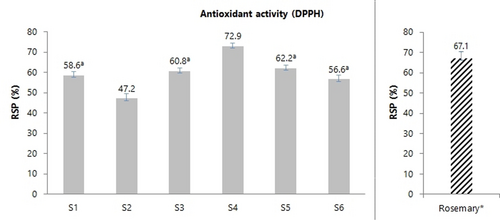
Radical-scavenging percentages of Prunus domestica L. wood AcOEt extracts (see Table 1 for cultivar names) and commercial extract of rosemary* used as reference (right), expressed as mean of three replicates. Error bars show the standard deviation (SD) of measurements; mean values not sharing the same letter are significantly different by ANOVA Tukey test (p<0.05).
Comparing both TPC and RSP values of the studied wood extracts, it can be said that cultivars ‘De la Rosa’ (S4), ‘Stanley’ (S3), and ‘Reina Claudia Verde’ (S1 and S5) hold the first positions in both assays, while cv. ‘Claudia de Tolosa’ (S2 and S6) holds down the last position. This reasonably good agreement between both parameters is in accordance with the known fact that phenolic compounds contribute to the radical-scavenging activity of most plant extracts.
Identification of Phenolic Components in European Plum Wood Extracts
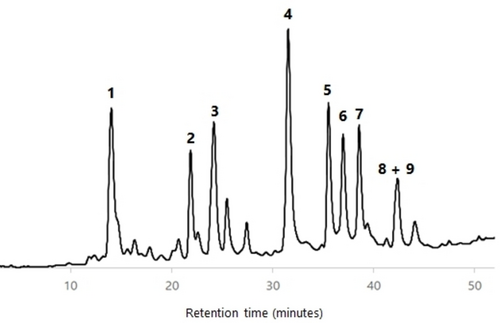
All wood AcOEt extracts were analyzed by HPLC-DAD and HPLC-DAD/ESI-MS in order to characterize their phenolic profile. The identification of components was carried out by comparison of the HPLC retention time, UV spectrum and MS/MS fragmentation data of each HPLC peak with compounds previously isolated and fully characterized by us from cv. ‘De la Rosa’ wood (see the Supporting Information).23 Figures 456789 show the HPLC chromatograms of all the wood samples analyzed, in which most of the peaks have been identified. Compounds 1, 3–6 and a mixture of 8 and 9 are present in all samples, with the exception of cv. ‘Claudia de Tolosa’ (both farming plots), where compound 2 is absent. Although each sample has a characteristic HPLC profile, the comparison between all of them revealed that (a) there are no remarkable differences between samples of the same cultivar collected in two different farming plots (Figures 4 and 8 for cv. ‘Reina Claudia Verde’; Figures 5 and 9 for cv. ‘Claudia de Tolosa’); (b) cultivars ‘Reina Claudia Verde’ (Figures 4 and 8) and cv. ‘Stanley′ (Figure 6) are very similar to each other; and (c) both cultivars ‘Claudia de Tolosa’ (Figures 5 and 9) are more different from the rest.
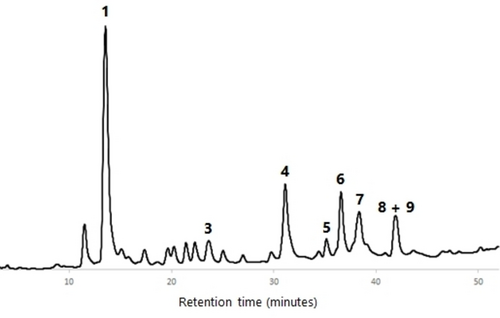

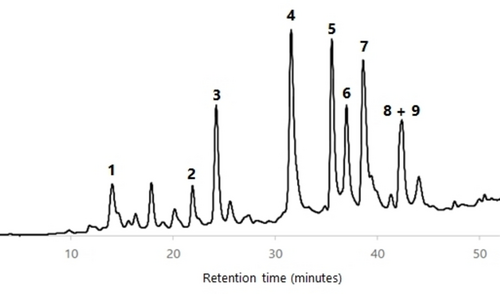
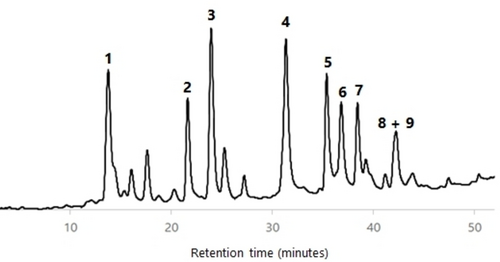
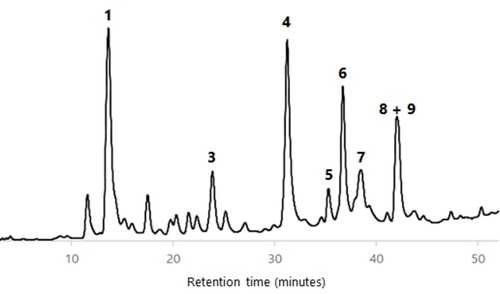
Quantification of Phenolic Components in European Plum Wood Extracts
The quantification (Table 2) of the nine compounds (1–9) identified in the European plum wood AcOEt extracts was achieved using the external-standard methodology, correlating HPLC peak areas with the concentration for each compound. The highest total amount of phenolic compounds was found in cv. ‘De la Rosa’ (12.28 mg g−1 DW), followed by the two samples of ‘Reina Claudia Verde’ (8.26 mg g−1 DW for plot B; 6.14 mg g−1 DW for plot A) matching with the AcOEt extraction yields of those same samples (Table 1). Proanthocyanidin 4 was the major phenolic compound in cultivars ‘De la Rosa’ and ‘Stanley’ and the second one in the rest. Heteroside 3 was the major component in cv. ‘Reina Claudia Verde’ (both plots) and the second one in cultivars ‘De la Rosa’ and ‘Stanley’, but one of the minor compounds in cv. ‘Claudia de Tolosa’ (both plots). Flavan-3-ol 1 was also quite abundant, being the first one in cv. ‘Claudia de Tolosa’ (both plots), and the third one in cultivars ‘Reina Claudia Verde’ (both plots) and ‘Stanley’.
Compound |
tR (min) |
λmax (nm) |
Extracts (cultivar names in Table 1) and concentration of components (milligrams of compound per gram of DW) |
|||||
|---|---|---|---|---|---|---|---|---|
S1 |
S2 |
S3 |
S4 |
S5 |
S6 |
|||
Flavan-3-ols |
||||||||
Catechin (1) |
13.5 |
279.3 |
1.00±0.02a |
1.37±0.01 |
1.05±0.01a |
0.82±0.01 |
1.32±0.03 |
1.07±0.03a |
Epicatechin (2) |
21.8 |
279.3 |
0.44±0.01 |
– |
0.40±0.04 |
0.61±0.02 |
0.77±0.02 |
– |
Total* |
|
|
1.44a |
1.37 |
1.45a |
1.43a |
2.09 |
1.07 |
(−)-Annphenone (3) |
23.5 |
222.7, 285.2 |
1.37±0.05 |
0.25±0.03 |
1.52±0.02 |
2.34±0.08 |
2.21±0.04 |
0.49±0.02 |
Proanthocyanidins |
||||||||
(−)-ent-Epicatechin-(2α→O→7,4α→8)-catechin (4) |
30.6 |
278.1 |
1.23±0.09 |
0.50±0.07 |
1.56±0.05 |
2.49±0.07 |
1.41±0.03 |
0.94±0.05 |
(−)-ent-Epicatechin-(2α→O→7,4α→8)-epicatechin (5) |
35.0 |
276.9 |
0.64±0.04 |
0.12±0.02 |
0.55±0.03 |
1.72±0.04 |
0.75±0.04 |
0.16±0.04 |
(−)-ent-Epiafzelechin-(2α→O→7,4α→8)-catechin (6) |
36.6 |
278.1 |
0.51±0.03 |
0.33±0.03 |
0.63±0.02a |
1.04±0.01 |
0.65±0.02a |
0.56±0.01 |
(+)-Epiafzelechin-(2β→O→7,4β→8)-epicatechin (7) |
38.2 |
276.9 |
0.50±0.06 |
0.27±0.02 |
N.D. |
1.95±0.09 |
0.55±0.02 |
0.10±0.02 |
(+)-Epiafzelechin-(2β→O→7,4β→8)-afzelechin (8) and (−)-ent-Epiafzelechin-(2α→O→7,4α→8)-epiafzelechin (9) |
42.1 |
222.7, 272.2 |
0.45±0.01 |
0.29±0.04 |
0.33±0.05 |
1.31±0.03 |
0.60±0.01 |
0.56±0.02 |
Total** |
|
|
3.33 |
1.51 |
3.07 |
8.51 |
3.96 |
2.32 |
- DW: Dry wood; values (mg g−1 DW) are mean±SD of three replicates. N.D. (not determined): quantification was not possible due to the overlapping of HPLC peaks. *Sum of 1 and 2. **Sum of 4–9. Mean values in the same line (row) not sharing the same letter are significantly different by ANOVA Tukey test (p<0.05).
Focusing on flavan-3-ols, the amount of catechin (1) in all cultivars was higher than that of epicatechin (2), and the sum of both compounds was lower than the total amount of A-type proanthocyanidins (PACs) (4–9) in all samples (Table 2). With respect to the latter group (4–9), cv. ‘De la Rosa’ showed the largest amount followed by both ‘Reina Claudia Verde’ cultivars. Regarding procyanidins (4 and 5) and propelargonidins (6–9), the first sub-group predominates over the second one in cultivars ‘Reina Claudia Verde’ (both plots) and ‘Stanley’, while both sub-groups are present in a similar amount in the rest of woods. Comparing with the literature, the total amount of A-type PACs (1.51–8.51 mg g−1 DW) in the studied woods is considerably higher than in other woods, such as those from Quercus robur, Quercus petrea or Castanea sativa,44 and it is in the same range of special A-type PACs-rich plant materials, such as cranberry extracts45 and derived products46 or peanut skins.47 In addition, the A-type PACs present in these plum wood extracts have a low polymerization degree, which would afford better bioavailability than other natural extracts with a higher degree of polymerization.48 Taking into account the quantitative analysis shown here, the pruning wood of cv. ‘De la Rosa’ seems to be the best natural source of A-type PACs among the cultivars studied. Since a standard plum crop can generate around 6–8 kilograms of pruning material per tree each year (according to Eng. Ángel Valderas-Sabido, supervisor of the cultivation plots from which the European plum wood samples were obtained), cv. ‘De la Rosa’, for instance, could yield every agricultural year up to 265 g of extract from the pruning remains of just a tree.
Inhibitory Activity of European Plum Wood Extracts and Proanthocyanidins 4–9 against hLDHA
The inhibitory effects on the activity of hLDHA, according to the reduction of pyruvate to lactate, of both AcOEt extracts of European plum cultivars studied here and A-type PACs isolated previously by us (compounds 4–9)23 were evaluated by a UV spectrophotometric assay.40 For it, the decrease in the co-substrate β-NADH absorbance at 340 nm (due to its oxidation into NAD+) of the solution containing the inhibitor (extract or isolated compound) was monitored for 10 min after adding β-NADH. The slope was compared with that obtained from the solution without inhibitor (100 % enzymatic activity). The method was validated by measuring the inhibitory activity of the commercial compound 3-[[3[(cyclopropylamino)sulfonyl]-7-(2,4-dimethoxy-5-pyrimidinyl)-4-quinolinyl]amino]-5-(3,5-difluorophenoxy)benzoic acid (GSK-2837808A).49
IC50 values of all plum wood extracts, compounds 4–9, and standard procyanidin A-2 (Table 3) were determined using a dose response curve, plotting the logarithm of inhibitor concentration vs. normalized enzymatic activity. For that, eight solutions at different concentrations of each sample were prepared: wood extracts in the range of 500–0.23 μg mL−1, and pure compounds in the range of 200–0.09 μM. According to these IC50 values (Table 3), all the extracts are less active than the pure compounds studied, except extract of cultivar ‘De la Rosa’ (sample S4), whose IC50 value (19.2 μg mL−1) is between those of compounds 5 (14.2 μg mL−1) and 8 (23.4 μg mL−1). Cultivar ‘De la Rosa’ contains higher amounts of compounds 4–9 than the rest of plum cultivars (Table 2), which could justify that its activity is of the same order of magnitude as that of some pure compounds.
AcOEt extract (see Table 1) |
IC50 (μg mL−1)* |
IC50 (μM)* |
R2 |
ClogP |
|---|---|---|---|---|
‘Reina Claudia Verde’ (S1) |
>150 |
– |
– |
– |
‘Claudia de Tolosa’ (S2) |
124.5±4.4 |
– |
0.93 |
– |
‘Stanley’ (S3) |
69.0±2.7 |
– |
0.90 |
– |
‘De la Rosa’ (S4) |
19.2±0.7 |
– |
0.97 |
– |
‘Reina Claudia Verde’ (S5) |
112.6±4.2 |
– |
0.80 |
– |
‘Claudia de Tolosa’ (S6) |
54.6±4.0 |
– |
0.93 |
– |
Compound (see Table 2) |
|
|
|
|
4 |
1.9±0.2 |
3.2±0.3 |
0.95 |
3.3 |
5 |
14.2±1.6a |
24.6±2.8a |
0.96 |
3.3 |
6 |
8.3±1.3b |
16.0±1.8b |
0.95 |
3.7 |
7 |
7.2±0.1b |
12.8±0.1b |
0.98 |
3.7 |
8 |
23.4±1.4 |
43.0±2.5 |
0.97 |
4.1 |
9 |
12.1±0.9a |
22.1±1.7a |
0.94 |
4.1 |
Procyanidin A-2** |
31.3±3.7 |
54.2±6.4 |
0.96 |
3.3 |
- *Results expressed as mean±SD of three replicates; mean values in the same column not sharing the same letter are significantly different by ANOVA Tukey test (p<0.05). **Compound used as reference.
Among the pure compounds, procyanidin 4 showed the highest inhibitory activity against hLDHA (1.9 μg mL−1), followed by compounds 7 (7.2 μg mL−1), 6 (8.3 μg mL−1), 9 (12.1 μg mL−1) and 5 (14.2 μg mL−1). It is worth noting that all these A-type PACs (4–9) are significantly more active than the A-type procyanidin A-2 used as reference (31.3 μg mL−1) and, according to the literature, also more active than other natural products isolated, for example, from the aerial parts of Polygala flavescens ssp. flavescens50 or Phlomis kurdica.41 To the best of our knowledge, this is the first time that pure A-type PACs have been evaluated on their hLDHA inhibitory activity, since only one work has been reported before with PACs-enriched fractions.40 Furthermore, in order to estimate the lipophilicity and cell membrane permeability51 of the hLDHA inhibitors 4–9, theoretical partition coefficients, expressed as ClogP, were calculated, finding favorable values (less than 5) for all of them (Table 3).
Conclusions
Pruning wood of European plum tree (Prunus domestica L.) is an excellent natural source of A-type proanthocyanidins, the cultivar ‘De la Rosa’ showing the higher amount among all studied samples. The characterization of the wood extracts of four plum cultivars was performed by the total phenolic content and DPPH radical-scavenging assays and by HPLC-DAD and HPLC-DAD/ESI-MS analyses. It allowed for the identification of flavan-3-ols catechin (1) and epicatechin (2), the phenolic glucoside annphenone (3), and six A-type proanthocyanidins (4–9). After quantification of components by an external standard method, (−)-ent-epicatechin-(2α→O→7,4α→8)-catechin (4), (−)-annphenone (3) and catechin (1) were the major components in most of the plum cultivars, although every wood extract seemed to have a particular phenolic profile.
Regarding the inhibitory activity of both wood extracts and pure compounds 4–9 against hLDHA, it is noteworthy that all those individual compounds, together with the extract of cv. ‘De la Rosa’, are significantly more active than the A-type procyanidin A-2 used as reference. Among them, the procyanidin 4 showed the IC50 value more favorable to inhibit this key enzyme in the rare disease of Primary Hyperoxaluria. These results highlight, in one hand, the potential use of A-type PACs to inhibit hLDHA and, in the other hand, the use of pruning wood from P. domestica as a renewable, cheap and abundant source to obtain these compounds.
Experimental Section
Chemicals
Solvents used for extractions and high-performance liquid chromatography (HPLC) analyses were purchased from VWR (Barcelona, Spain) as described by us.30 Ultrapure water used for HPLC and hLDHA inhibitory assay was obtained from a Milli-Q-Water (1.8 MΩ) equipment (Merck, KGaA, Darmstadt, Germany). (−)-Catechin and (−)-annphenone used as standards for quantification purposes were isolated from sweet cherry wood and European plum wood, respectively, as described by us.23, 32 The purity of these standards was calculated by HPLC as 99 % and 98 %, respectively. Procyanidin A-2 used as standard was purchased from Extrasynthese (Genay, France) with a purity of 98 % (HPLC). 2,2-Diphenyl-1-picrylhydrazyl radical (DPPH•) used for radical scavenging activity was purchased from Sigma-Aldrich Chemie (Steinheim, Germany). Rosemary extract used as reference was purchased from Evesa (Cádiz, Spain). Folin-Ciocalteu reagent used to the Total Phenolic Content colorimetric method was purchased from Merck.
Plant Material and Extraction
Pruning residues of P. domestica cultivars ‘De la Rosa’, ‘Reina Claudia Verde’, ‘Claudia de Tolosa’ and ‘Stanley’ were collected in November 2015 during the pruning works of plum trees in two farming plots (Farming plot A: N: 40° 03’ 12.97’’ W: 5° 44’ 11.24’’; Farming plot B: N: 40° 02’ 06.05’′ W: 5° 44’ 49.36’’) located in western Spain (Jaraíz de la Vera, province of Cáceres). Eng. Ángel Valderas-Sabido (agricultural engineer of a grouping of cooperatives called ‘Valle del Jerte’, Valdastillas, Spanish Autonomous Region of Extremadura, Spain), was the person in charge of the agronomic supervision of both plots and confirmed the identity and origin of each wood sample. Pieces of wood (with a diameter of 0.8–1.4 cm), without leaves, were dried and processed as reported before,32 and extracted sequentially with dichloromethane (DCM) and AcOEt for 2 h at reflux (Figure 10).31 Starting from 20 g of wood shavings, 240–660 mg of AcOEt extracts were obtained, of which around 10 mg are used for the total phenolic content and DPPH radical-scavenging activity assays and around 5 mg for the HPLC analyses and component quantification and the hLDHA inhibitory activity assay.
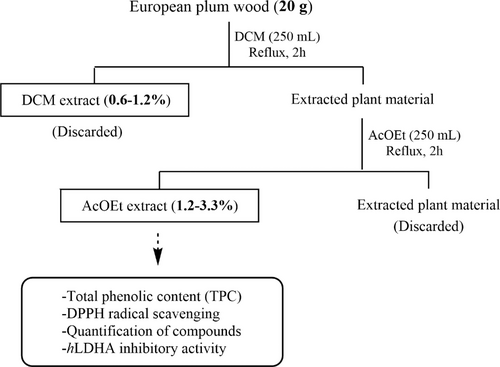
Extraction procedure of Prunus domestica L. wood samples.
Total Phenolic Content
The total phenolic content (TPC) of AcOEt extracts was determined by the Folin-Ciocalteu colorimetric method, as reported by Singleton and Rossi,52 and using gallic acid as standard. For that, a calibration curve was constructed using methanolic solutions (25–100 μg mL−1) of gallic acid, and their absorbances at 760 nm were recorded on a Varian Cary 4000 spectrophotometer.30 Briefly, a mixture of an aqueous solution (0.6 mL) of Folin-Ciocalteu reagent 0.2 N, and methanolic solutions (0.12 mL) of each AcOEt extracts (at a concentration of 10 μg mL−1) were added to a 1 cm path length semi-micro disposable plastic cuvette. The blend was incubated in the dark at room temperature for 5 min. Next, an aqueous solution (0.48 mL; 75 g L−1) of Na2CO3 was added, shaken and incubated again for 1 h in the dark at room temperature. Finally, the absorbance at 760 nm was determined. Results were expressed as mg Gallic acid equivalent (GAE) per gram of dry extract. Measurements were carried out in triplicate.
DPPH Radical-Scavenging Activity
The radical-scavenging activity value of AcOEt extracts was determined using a spectrophotometric method.29 Briefly, methanolic solutions (0.4 mL) of each extract were mixed with 0.8 mL of a methanolic solution of the stable DPPH radical (7.09×10−5 M) in semi-micro disposable plastic cuvettes, shaken and kept in dark at room temperature for 15 min. Next, the decreased of absorbance was measured at 515 nm. The concentration of DPPH radical in each solution was determined by interpolation in a calibration curve. Blank was prepared with 0.8 mL of DPPH radical solution and 0.4 mL of MeOH. Measurements were carried out in triplicate.

HPLC Analyses of AcOEt Extracts and Quantification of Identified Compounds
Ethyl acetate extracts were analyzed by HPLC in order to know their phenolic profile. This procedure was carried out using the instrument and protocol reported previously by us.23 The mobile phase consisted of mixtures of acetonitrile (solvent A) and water (solvent B), both with 0.2 % acetic acid. Flow rate: 0.7 mL min−1. Gradient program: linear gradient from 5 % to 20 % A in 40 min; linear gradient from 20 % to 25 % A in 5 min; linear gradient from 25 % to 40 % in 15 min, and other 2 min to return to the initial conditions. Phenolic compounds were identified comparing their retention time, UV/VIS and mass spectra with those of the compounds isolated previously by us (see the Supporting Information).23
Individual quantification of identified compounds (1–9) in AcOEt extracts was carried out by an external standard method using HPLC peak areas at 230 nm. Solutions of (−)-catechin, (−)-annphenone, and procyanidin A-2 in methanol (0.1–1.0 mg mL−1) were used to make calibration curves (see the Supporting Information) by correlating the area value of each standard peak with its concentration. The concentration of target compounds was determined comparing HPLC peak area at 230 nm of each component with the calibration curve of the compound from the same phenolic group. Thus, (−)-catechin was used to quantify compounds 1 and 2 [y=40000000x–907133 (r2=0.9904)]; (−)-annphenone for compound 3 [y=30000000x–58648 (r2=0.9989)]; and procyanidin A-2 for compounds 4–9 [y=40000000x–842819 (r2=0.9986)]. Results were expressed as milligrams of compound per gram of dry wood (DW). Measurements were carried out in triplicate.
hLDHA Enzymatic Activity Assay and ClogP Estimation
Enzymatic activity of hLDHA was determined with recombinant human LDHA (95 %, specific activity>300 units mg−1 and concentration of 0.5 mg mL−1, Abcam, Cambridge, United Kingdom) in the presence of sodium pyruvate (96 %, Merck) as substrate and β-NADH (≥97 %, Merck) as cofactor in 100 mM potassium phosphate buffer, pH 7.4. The enzymatic assay was performed on 96-well microplates and the decrease in the β-NADH absorbance (340 nm) was measured in a Synergy HT Multi-Detector Microplate Reader (BioTeK Instrument, Inc.) for 10 min at 28 °C. The activity was determined using the method employed by Li et al.40 and modified as described here: in each well, the final volume was set to 200 μL and the final concentrations were 100 mM potassium phosphate buffer, 0.041 units mL−1 hLDHA, 155 μM β-NADH, 1 mM pyruvate (saturated conditions), and DMSO solutions (1 %, v/v) of wood extracts and pure compounds at concentrations in the range of 500–0.23 μg mL−1 and 200–0.09 μM, respectively. The reaction was initiated by the addition of pyruvate and a suitable linear timeframe was selected to calculate the slope of each concentration. Controls for the establishment of the 0 % and 100 % enzymatic activity were introduced in the assay and the compound 3-[[3[(cyclopropylamino)sulfonyl]-7-(2,4-dimethoxy-5-pyrimidinyl)-4-quinolinyl]amino]-5-(3,5-difluorophenoxy)benzoic acid (GSK 2837808 A, Tocris, Minneapolis, USA) was used as a positive control at 1 μM in well.54 All measurements were made in triplicate and data were expressed as the mean±SD. Nonlinear regression analysis was used for dose response curve, fitting of logarithm of inhibitor concentration vs. normalized enzymatic activity, to calculate IC50 values.
The computational tool MOE (molecular operation environment, 2020 software, Chemical Computing Group, ULC, Canada) was used to estimate log P (Clog P).
Data Analysis
The results are expressed as mean values±SD. The data were statistically analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, La Jolla, California, USA). A one-way analysis of variance (ANOVA) with Tukey's test (p<0.05) was applied to determine statistical significative differences among measurements.
Author Contribution Statement
J. O.-V. performed the experiments, analyzed the data and wrote the original draft. L. R.-M. performed part of the experiments and helped with analyses. S. S. and J. A. conceived the experiments, made review & editing of the manuscript and got funding.
Acknowledgments
This research has been supported by the Spanish Ministerio de Ciencia, Innovación y Universidades under Grant RTI2018-098560-B-C22. Authors wish to thank the Centro de Instrumentación Científico-Técnica (CICT) of the University of Jaén for partial financial support, and the Eng. Ángel Valderas-Sabido, who supplied European plum wood samples.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.