Cytotoxic Sesquiterpenoids from Atractylodes chinensis (DC.) Koidz.
Lei-Xin Zhuang
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorYan Liu
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorSi-Yi Wang
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorYe Sun
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorJuan Pan
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorWei Guan
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorZhi-Chao Hao
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorCorresponding Author
Hai-Xue Kuang
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorCorresponding Author
Bing-You Yang
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorLei-Xin Zhuang
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorYan Liu
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorSi-Yi Wang
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorYe Sun
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorJuan Pan
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorWei Guan
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorZhi-Chao Hao
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorCorresponding Author
Hai-Xue Kuang
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorCorresponding Author
Bing-You Yang
Key Laboratory of Basic and Application Research of Beiyao, Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040 P. R. China
Search for more papers by this authorAbstract
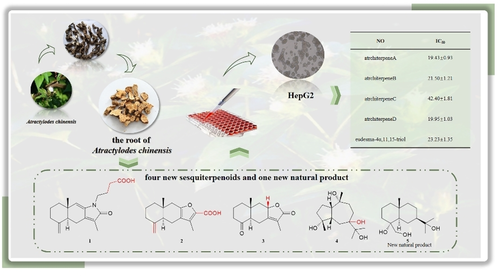
Four new sesquiterpenoids named atrchiterpenes A–D (1–4), a new natural product (5), and twelve known compounds (6–17) were isolated from Atractylodes chinensis (DC.) Koidz. Compound 1 was a rare N-containing eudesmane-type sesquiterpenoid. Structure elucidation was performed by spectroscopic techniques, including 1D, 2D NMR spectra, and HR-ESI-MS. Compounds 6–11, 14, and 17 were reported from Atractylodes for the first time. All the isolated compounds were evaluated for cytotoxicity activity. Compound 16 showed moderate cytotoxicity against HepG2 cells with an IC50 value of 5.81±0.47.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202200812-sup-0001-misc_information.pdf2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. T. Zhang, X. Peng, Z. J. Sun, C. Hu, H. H. Zhou, J. Xu, Q. Gu, ‘Diverse polyacetylenes from Atractylodes chinensis and their anti-osteoclastogenesis activity’, Fitoterapia 2022, 161, 105233.
- 2H. Meng, G. Y. Li, R. H. Dai, Y. P. Ma, K. Zhang, C. Zhang, X. Li, J. H. Wang, ‘Chemical constituents of Atractylodes chinensis (DC.) Koidz’, Biochem. Syst. Ecol. 2010, 38, 1220–1223.
- 3Y. Nakai, I. Sakakibara, K. Hirakura, S. Terabayashi, S. Takeda, ‘A New Acetylenic Compound from the Rhizomes of Atractylodes chinensis and Its Absolute Configuration’, Chem. Pharm. Bull. 2005, 53, 1580–1581.
- 4S. Tang, X. T. Zhang, Y. B. Ma, X. Y. Huang, C. A. Geng, T. Z. Li, X. M. Zhang, C. Shen, L. H. Su, Z. Gao, J. J. Chen, ‘Artemyrianolides A–S, Cytotoxic Sesquiterpenoids from Artemisia myriantha’, J. Nat. Prod. 2020, 83, 2618–2630.
- 5Y. Ren, L. Shen, D. W. Zhang, S. J. Dai, ‘Two New Sesquiterpenoids from Solanum lyratum with Cytotoxic Activities’, Chem. Pharm. Bull. 2009, 57, 408–410.
- 6K. H. Kim, H. J. Noh, S. U. Choi, K. M. Park, S. J. Seok, K. R. Lee, ‘Lactarane sesquiterpenoids from Lactarius subvellereus and their cytotoxicity’, Bioorg. Med. Chem. Lett. 2010, 20, 5385–5388.
- 7W. Y. Zhou, B. Lin, Z. L. Hou, S. C. Shi, Y. X. Wang, X. X. Huang, S. J. Song, ‘Isolation of macrocarpene-type sesquiterpenes from stigma maydis with neuroprotective activities’, Fitoterapia 2020, 141, 104448.
- 8H. Liu, M. H. Chen, Y. J. Lang, X. X. Wang, P. Y. Zhuang, ‘Sesquiterpenes from the fruits of Illicium Simonsii maxim’, Nat. Prod. Res. 2020, 34, 903–908.
- 9S. Thongnest, P. Chawergrum, S. Keeratichamroen, K. Lirdprapamongkol, C. Eurtivong, J. Boonsombat, P. Kittakoop, J. Svasti, S. Ruchirawat, ‘Vernodalidimer L, a sesquiterpene lactone dimer from Vernonia extensa and anti-tumor effects of vernodalin, vernolepin, and vernolide on HepG2 liver cancer cells’, Bioorg. Chem. 2019, 92, 103197.
- 10Y. Ye, G. X. Chou, H. Wang, J. H. Chu, W. F. Fong, Z. L. Yu, ‘Effects of Sesquiterpenes Isolated From Largehead Atractylodes Rhizome on Growth, Migration, and Differentiation of B16 Melanoma Cells’, Integr. Cancer Ther. 2011, 10, 92–100.
- 11G. K. Wang, N. Zhang, J. N. Yao, Y. Yu, G. Wang, C. C. Huang, Y. Y. Cheng, S. L. Morros-Natschke, Z, Y, Zhou, J. S. Liu, K. H. Lee, ‘Kalshinoids A−F, Anti-inflammatory Sesquiterpenes from Kalimeris shimadae’, J. Nat. Prod. 2019, 82, 3372–3378.
- 12L. G. Chen, Y. S. Jan, P. W. Tsai, H. Norimoto, S. Michihara, C. Murayama, C. C. Wang, ‘Anti-inflammatory and Antinociceptive Constituents of Atractylodes japonica Koidzumi’, J. Agric. Food Chem. 2016, 64, 2254–2262.
- 13L. S. Hoang, M. H. Tran, J. S. Lee, Q. M. T. Ngo, M. H. Woo, B. S. Min, ‘Inflammatory Inhibitory Activity of Sesquiterpenoids from Atractylodes macrocephala Rhizomes’, Chem. Pharm. Bull. 2016, 64, 507–511.
- 14P. Wang, Z. S. Xie, J. Y. Song, H. H. Zeng, L. P. Dai, H. C. E, Z. P. Ye, S. Gao, J. Y. Xu, Z. Q. Zhang, ‘Four new sesquiterpene lactones from Atractylodes macrocephala and their CREB agonistic activities’, Fitoterapia 2020, 147, 104730.
- 15X. S. Li, X. J. Zhou, X. J. Zhang, J. Su, X. J. Li, Y. M. Yan, Y. T. Zheng, Y. Li, L. M. Yang, Y. X. Cheng, ‘Sesquiterpene and Norsesquiterpene Derivatives from Sanicula lamelligera and Their Biological Evaluation’, J. Nat. Prod. 2011, 74, 1521–1525.
- 16M. Morita, H. Nakanishi, H. Morita, S. Mihashi, H. Itokawa, ‘Structures and Spasmolytic Activities of Derivatives from Sesquiterpenes of Alpinia speciosa and Alpinia japonica’, Chem. Pharm. Bull. 2008, 44, 1603–1606.
10.1248/cpb.44.1603 Google Scholar
- 17X. J. Dong, S. D. Luo, ‘Six Novel Eudesmane-Like Sesquiterpenes from Illicium spathulatum’, Helv. Chim. Acta 2013, 96, 445–451.
- 18M. Okasaka, Y. Takaishi, Y. Kashiwada, O. K. Kodzhimatov, O. Ashurmetov, A. J. Lin, L. M. Consentino, K. H. Lee, ‘Terpenoids from Juniperus polycarpus var. Seravschanica’, Phytochemistry 2006, 67, 2635–2640.
- 19S. S. Afiyatullov, E. V. Leshchenko, M. P. Sobolevskaya, A. S. Antonov, V. A. Denisenko, R. S. Popov, Y. V. Khudyakova, N. N. Kirichuk, A. S. Kuzmich, E. A. Pislyagin, N. Y. Kim, D. V. Berdyshev, ‘New Thomimarine E from Marine Isolate of the Fungus Penicillium thomii’, Chem. Nat. Compd. 2017, 53, 290–294.
- 20W. M. Huang, F. Y. Chen, Y. T. Bian, Z. C. Chen, P. C. Shuang, J. Zhou, Y. M. Luo, ‘A pair of sesquiterpene enantiomers from Chloranthus multispike’, Chin. Herb. Med. 2021, 52, 925–930.
- 21Y. B. Cheng, C. Y. Chen, Y. H. Kuo, Y. C. Shen, ‘New Nitrogen-Containing Sesquiterpenoids from the Taiwanese Soft Coral Cespitularia taeniata May’, Chem. Biodiversity 2009, 6, 1266–1272.
- 22H. S. Park, T. Ohama, ‘Biotransformation of a Herb Plant Metabolite by a Cell Disruptant of Chlamydomonas reinhardtii’, Biosci. Biotechnol. Biochem. 2009, 73, 2803–2805.
- 23Y. Li, X. W. Yang, ‘Five new eudesmane-type sesquiterpenoid lactones biotransformed from atractylenolide I by rat hepatic microsomes’, Fitoterapia 2013, 85, 95–100.
- 24H. Y. Ding, Y. C. Wu, H. C. Lin, ‘Phytochemical and Pharmacological Studies on Chinese Changzhu’, J. Chin. Chem. Soc. 2000, 47, 561–566.
- 25T. A. Zamzami, H. M. Abdallah, I. A. Shehata, G. A. Mohamed, M. Y. Alfaifi, S. E. I. Elbehairi, A. E. Koshak, S. R. M. Ibrahim, ‘Macrochaetosides A and B, new rare sesquiterpene glycosides from Echinops macrochaetus and their cytotoxic activity’, Phytochem. Lett. 2019, 30, 88–92.
- 26B. Wang, T. Y. Zhou, C. H. Nie, D. L. Wan, S. S. Zheng, ‘Bigelovin, a sesquiterpene lactone, suppresses tumor growth through inducing apoptosis and autophagy via the inhibition of mTOR pathway regulated by ROS generation in liver cancer’, Biochem. Biophys. Res. Commun. 2018, 499, 156–163.