The Effect of Dried Ginger (Gan Jiang) on Stomach Energy Metabolism and the Related Mechanism in Rats Based on Metabonomics
Limei Chen
The Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang, 330004 Jiangxi, China
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Institute of Chinese Materia Medica China Academy of Chinese Medical, Dongcheng, 100700 Beijing, China
Authors contributed equally.
Search for more papers by this authorHui Wang
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Qidong People's Hospital, Qidong, 226200 Jiangsu, China
Authors contributed equally.
Search for more papers by this authorZhao Chen
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Authors contributed equally.
Search for more papers by this authorWenhao Zhuo
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorRuixiang Xu
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorXin Zeng
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorQirui He
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorCorresponding Author
Yongmei Guan
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorCorresponding Author
Hui Li
Institute of Chinese Materia Medica China Academy of Chinese Medical, Dongcheng, 100700 Beijing, China
Search for more papers by this authorCorresponding Author
Hongning Liu
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorLimei Chen
The Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang, 330004 Jiangxi, China
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Institute of Chinese Materia Medica China Academy of Chinese Medical, Dongcheng, 100700 Beijing, China
Authors contributed equally.
Search for more papers by this authorHui Wang
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Qidong People's Hospital, Qidong, 226200 Jiangsu, China
Authors contributed equally.
Search for more papers by this authorZhao Chen
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Authors contributed equally.
Search for more papers by this authorWenhao Zhuo
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorRuixiang Xu
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorXin Zeng
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorQirui He
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorCorresponding Author
Yongmei Guan
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorCorresponding Author
Hui Li
Institute of Chinese Materia Medica China Academy of Chinese Medical, Dongcheng, 100700 Beijing, China
Search for more papers by this authorCorresponding Author
Hongning Liu
Jiangxi University of Chinese Medicine, Nanchang, 330006 Jiangxi, China
Search for more papers by this authorAbstract
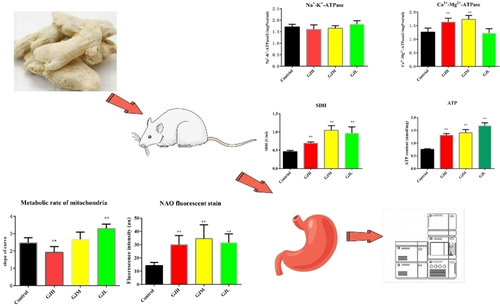
Dried ginger is a commonly used stomachic. Dried ginger is often used as a gastric protector to treat stomach-related diseases. However, the effect of dried ginger on energy metabolism in stomach tissue of rats under physiological condition has not been studied. In this study, different doses of water extract of dried ginger were given to rats for 4 weeks. The activity of Na+-K+-ATPase, Ca2+-Mg2+-ATPase, SDH (succinate dehydrogenase) enzyme, ATP content, mitochondrial metabolic rate and mitochondrial number in stomach tissue of rats were measured. Analysis of potential biomarkers related to the effect of dried ginger on energy metabolism in stomach tissue of rats by metabonomics, and their metabolic pathways were also analyzed. The results revealed that there was no significant difference in Na+-K+-ATPase in high-dose group (GJH), medium-dose group (GJM) and low-dose group (GJL) compared to the Control group. The Ca2+-Mg2+-ATPase activity was significantly increased in stomach tissue of GJH group and GJM group, but there were no significant changes in stomach tissue of GJL group. The SDH activity and the ATP levels were significantly increased in stomach tissue of GJH group, GJM group and GJL group. The mitochondrial metabolic rate was significantly increased in GJL group, but there was no significant change in GJM group and was inhibited in GJH group. These effects might be mediated by arginine biosynthesis, glutathione metabolism, arachidonic acid metabolism, glycerophospholipid metabolism, arginine and proline metabolism, purine metabolism pathway.
Graphical Abstract
Conflict of interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202200757-sup-0001-misc_information.pdf548.5 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. Pertz, J. Lehmann, R. Roth-Ehrang, S. Elz, ‘Effects of ginger constituents on the gastrointestinal tract: role of cholinergic M3 and serotonergic 5-HT3 and 5-HT4 receptors’, Planta Med. 2011, 77, 973–978.
- 2A. Someya, S. Horie, H. Yamamoto, T. Murayama, ‘Modifications of capsaicin-sensitive neurons in isolated guinea pig ileum by gingerol and lafutidine’, Journal of pharmacological sciences 2003, 92, 359–366.
- 3R. Gadekar, P. K. Singour, P. K. Chaurasiya, R. S. Pawar, U. K. Patil, ‘A potential of some medicinal plants as an antiulcer agents’, Pharmacogn. Rev. 2010, 4, 136–146.
- 4M. Al-Amin, G. N. N. Sultana, C. F. Hossain, ‘Antiulcer principle from Zingiber montanum’, J. Ethnopharmacol. 2012, 141, 57–60.
- 5M. A. Al-Yahya, S. Rafatullah, J. S. Mossa, A. M. Ageel, N. S. Parmar, M. Tariq, ‘Gastroprotective activity of ginger zingiber officinale rosc., in albino rats’, Am. J. Clin. Med. 1989, 17, 51–56.
- 6M. Khushtar, V. Kumar, K. Javed, U. Bhandari, ‘Protective Effect of Ginger oil on Aspirin and Pylorus Ligation-Induced Gastric Ulcer model in Rats’, Indian J. Pharm. Sci. 2009, 71, 554–558.
- 7M. N. Siddaraju, S. M. Dharmesh, ‘Inhibition of gastric H+-K+-ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale’, Mol. Nutr. Food Res. 2007, 51, 324–332.
- 8X. Li, M. Ao, C. Zhang, S. Fan, Z. Chen, L. Yu, ‘Zingiberis Rhizoma Recens: A review of its traditional uses, phytochemistry, pharmacology, and toxicology’, Evid.-Based Complement. Altern. Med. 2021, 2021, 01–20.
- 9Q. C. Ma, ‘Effects of Mankshood, Dry ginger, Chinese goldthread and Rhubarb on signs of cold and heat and on Energy metabolism in rats’, Journal of Shandong University of Traditional Chin. Medicine 2010.
- 10J. H. Beattie, F. Nicol, M. J. Gordon, M. D. Reid, L. Cantlay, G. W. Horgan, I. S. Kwun, J. Y. Ahn, T. Y. Ha, ‘Ginger phytochemicals mitigate the obesogenic effects of a high-fat diet in mice: a proteomic and biomarker network analysis’, Mol. Nutr. Food Res. 2011, 55: S203–S213.
- 11Q. C. Ma, H. Y. Yu, J. Zhao, N. Gao, ‘Effects of mankshood,dry ginger,Chinese goldthread and rhubarb on energy metabolism of rats’, Journal of Shandong University of Traditional Chin. Medicine 2010, 34, 379–380.
- 12N. Gao, Y. Yang, S. J. Wang, R. Rong, X. P. Wang, ‘Determination of Contents of Adenine Nucleotides and Energy Charge in Rat Liver Tissue Treated with Aconite Roots by HPLC’, Chin. Journal of Experimental Traditional Medical Formulae 2010, 16, 172–175.
- 13L. M. Chen, B. H. Qu, H. Wang, H. N. Liu, Y. M. Guan, J. Q. Zhou, J. Q. Zhang, ‘The effect of curculigo orchioides (Xianmao) on kidney energy metabolism and the related mechanism in rats based on metabolomics’, Food Sci. Nutr. 2021, 9, 6194–6212.
- 14J. K. Nicholson, J. C. Lindon, E. Holmes, ‘′Metabonomics′: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data’, Xenobiotica 1999, 29, 1181–1189.
- 15X. Rong, G. Peng, T. Suzuki, Q. Yang, J. Yamahara, Y. Li, ‘A 35-day gavage safety assessment of ginger in rats’, Regul. Toxicol. Pharmacol. 2009, 54, 118–123.
- 16M. A. Shalaby, A. R. Hamowieh, ‘Safety and efficacy of zingiber officinale roots on fertility of male diabetic rats’, Food Chem. Toxicol. 2010, 48, 2920–2924.
- 17J. M. Miao, Y. X. Yang, Y. Ma, Y. J. Xu, Y. R. Deng, J. T. Qi, H. X. Yuan, ‘Effects of Xuanfu Daizhe decoction on Na+-K+-ATP and Ca2+-Mg2+-ATP enzymes activity of plasma in reflux esophagitis rats’, Tianjin Journal of Traditional Chin. Medicine 2016, 33, 231–234.
- 18P. Jin, H. Zhu, J. Wang, J. Chen, X. Wang, Y. Zheng, ‘Effect of methyl jasmonate on energy metabolism in peach fruit during chilling stress’, J. Sci. Food Agric. 2013, 93, 1827–1832.
- 19Q. F. Zhen, ‘The objectivity study on the ′hot′ property of Aconiti Lateralis Radix based on the energy metabolism of mitochondrion’, Chengdu University of Traditional Chin. Medicine 2015.
- 20N. Mota-Martorell, I. Pradas, M. Jové, A. Naudí, R. Pamplona, R. Pamplona, ‘De novo biosynthesis of glycerophospholipids and longevity’, Revista Espanola de Geriatria y Gerontologia 2019, 54, 88–93.
- 21A. A. Farooqui, L. A. Horrock, T. Farooqui, ‘Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders’, Chem. Phys. Lipids 2000, 106, 01–29.
- 22B. Halliwell, M. C. Gutteridge John, ‘Free radicals in biology and medicine’, Clarendon Pr. 1985, 1, 331–332.
- 23A. Triebl, ‘Glycerophospholipids’, Encyclopedia of Lipidomics 2016, pp: 01–04.
- 24H. F. Ma, F. Wei, X. Y. Dong, H. Chen, F. H. Huang, ‘Progress in Analysis Methods of Glycerophospholipid Based on Chemical Derivatization’, Journal of Instrumental Analysis 2018, 37, 1396–1404.
- 25S. Sigala, A. Imperato, P. Rizzonelli, ‘L-α-glycerylphosphorylcholine antagonizes scopolamine-induced amnesia and enhances hippocampal cholinergic transmission in the rat’, Eur. J. Pharmacol. 1992, 211, 351–358.
- 26G. P. Ceda, G. Ceresini, L. Denti, ‘Alpha-glycerylphosphorylcholine administration increases the GH responses to GHRH of young and elderly subjects’, Horm. Metab. Res. 1992, 24, 119–121.
- 27I. J. Neeland, C. Hughes, C. R. Ayers, C. R. Malloy, E. S. Jin, ‘Effects of visceral adiposity on glycerol pathways in gluconeogenesis’, Metabolism. 2017, Feb, 67, 80–89.
- 28S. H. Zeisel, ‘Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones’, Mutat. Res. 2012, 733, 34–38.
- 29H. Kenneth, K. Yashige, S. Hong, N. P. Quentin, L. W. Gemma, M. K. Lisa, T. Tahereh, A. S. Charles, K. Yoichi, N. Dai, A. F. Robert, ‘Dietary choline restriction causes complex I dysfunction and increased H2O2 generation in liver mitochondria’, Carcinogenesis 2000, 21, 983–989.
- 30Y. Wang, T. B. Jiang, Z. W. Sun, ‘Advance in animal nutritional regulation of L-arginine metabolites’, Chin. Journal of Animal Science 2017, 53, 13–17.
- 31Y. C. Jia, C. Y. Zhong, Y. M. Wang, Z. J. He, X. R. Wang, ‘Effects of aluminum on amino acid neurotransmittersin hippocampus of rats’, Chin. Journal of Preventive Medicine 2001, 35, 397–400.
- 32Y. K. Zhang, B. Wang, X. L. Jiang, B. F. Bai, J. Li, X. Z. Yue, ‘Effects of glutamine supplement on the concentration of free tryptophan in serum and brain, and 5-hydroxytryptamine in brain after exhaustion treadmill running in rat’, Chin. Journal of Sports Medicine 2004, 23, 634–638.
- 33Q. J. Wang, L. Xu, M. Z. Fan, ‘Recent advanced in transport systenma of glutamate and glutamine’, Chin. Journal of Animal Nutrition 2011, 23, 901–907.
- 34J. Fan, J. J. Kamphorst, R. Mathew, M. K. Chung, E. White, T. Shlomi, J. D. Rabinowitz, ‘Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia’, Molecular systems biology 2013, 9, 712–712.
- 35P. Newsholme, ‘Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?’, Journal nutrition 2001, 131, 2515S–2522S.
- 36D. N. Ying, H. Y. Chen, Y. Fu, H. B. Cai, W. S. Tan, ‘Promotion of NK-92 cells expansion by glutamine and sodium pyruvate’, Journal of Chemical Engineering of Chin. Universities 2020, 34, 09.
- 37T. S. Ayala, F. H. G. Tessaro, G. P. Jannuzzi, L. M. Bella, K. S. Ferreira, J. O. Martins, ‘High Glucose Environments Interfere with Bone Marrow-Derived Macrophage Inflammatory Mediator Release, the TLR4 Pathway and Glucose Metabolism’, Sci. Rep. 2019, 9 : 11447.
- 38Z. Zhang, D. Zeng, W. Zhang, A. Chen, J. Lei, F. Liu, B. Deng, J. Zhuo, B. He, M. Yan, X. Lei, S. Wang, E. W. Lam, Q. Liu, Z. Wang, ‘Modulation of oxidative phosphorylation augments antineoplastic activity of mitotic aurora kinase inhibition’, Cell Death Dis. 2021, 12 : 893.