New Jatrophane-Type Diterpenoids from Euphorbia kansui as Potential MDR Reversal Agents
Qiong Zheng
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorNing-Yu Chen
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorSi-Qing Lou
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorQian-Qing Liu
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorYi-Tong Luo
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorDong-E Liang
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorZha-Jun Zhan
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorCorresponding Author
Lie-Feng Ma
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorQiong Zheng
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorNing-Yu Chen
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorSi-Qing Lou
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorQian-Qing Liu
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorYi-Tong Luo
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorDong-E Liang
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorZha-Jun Zhan
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorCorresponding Author
Lie-Feng Ma
College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, 310014 P. R. China
Search for more papers by this authorAbstract
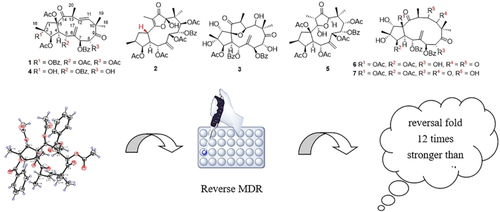
A serial jatrophane-type diterpenoids, comprised with three undescribed compounds kanesulones C−E (1–3) and four known ones (4–7), were obtained from the roots of Euphorbia kansui. The structures of compounds 1–3 were elucidated by detailed interpretation of their spectroscopic data, especially 2D-NMR and HR-ESI-MS, the absolute configuration of 1 was revealed by single crystal X-ray diffraction. These isolates were assayed for their multidrug resistance reversing activities on human breast adenocarcinoma cell line MCF-7/ADR. Compound 1 possessed potential as low toxic MDR modulator that could promote the efficacy of anticancer drug adriamycin ca. 85-fold at 5 μM, as 12 times stronger than the positive drug verapamil.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202200660-sup-0001-misc_information.pdf2.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. F. Higgins, ‘Multiple molecular mechanisms for multidrug resistance transporters’, Nature 2007, 446, 749–757.
- 2J. Zhu, R. Wang, L. Lou, W. Li, G. Tang, X. Bu, S. Yin, ‘Jatrophane Diterpenoids as Modulators of P-Glycoprotein-Dependent Multidrug Resistance (MDR): Advances of Structure-activity Relationships and Discovery of Promising MDR Reversal Agents’, J. Med. Chem. 2016, 59, 6353–6369.
- 3R. Hu, J. Gao, R. Rozimamat, H. A. Aisa, ‘Jatrophane diterpenoids from Euphorbia sororia as potent modulators against P-glycoprotein-based multidrug resistance’, Eur. J. Med. Chem. 2018, 146, 157–170.
- 4G. Figueroa-Gonzalez, N. Jacobo-Herrera, A. Zentella-Dehesa, R. Pereda-Miranda, ‘Reversal of multidrug resistance by morning glory resin glycosides in human breast cancer cells’, J. Nat. Prod. 2012, 75, 93–97.
- 5S. G. Aller, J. Yu, A. Ward, Y. Weng, S. Chittaboina, R. Zhuo, P. M. Harrell, Y. T. Trinh, Q. Zhang, I. L. Urbatsch, G. Chang, ‘Structure of P-Glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding’, Science 2009, 323, 1718–1722.
- 6I. J. Sousa, M. J. Ferreira, J. Molnar, M. X. Fernandes, ‘QSAR studies of macrocyclic diterpenes with P-glycoprotein inhibitory activity’, Eur. J. Pharm. Sci. 2013, 48, 542–553.
- 7S. Shaker, J. Sang, X. L. Yan, R. Z. Fan, G. H. Tang, Y. K. Xu, S. Yin, ‘Diterpenoids from Euphorbia royleana reverse P-glycoprotein-mediated multidrug resistance in cancer cells’, Phytochemistry 2020, 176, 112395.
- 8R. J. Kathawala, P. Gupta, C. R. Ashby Jr, Z. S. Chen, ‘The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade’, Drug Resist. Updates 2015, 18, 1–17.
- 9P. D. W. Eckford, F. J. Sharom, ‘ABC Efflux Pump-Based Resistance to Chemotherapy Drugs’, Chem. Rev. 2009, 109, 2989–3011.
- 10H. Yang, A. Mamatjan, D. Tang, H. A. Aisa, ‘Jatrophane diterpenoids as multidrug resistance modulators from Euphorbia sororia’, Bioorg. Chem. 2021, 112, 104989.
- 11H. Zhu, Z. Liu, L. Tang, J. Liu, M. Zhou, F. Xie, Z. Wang, Y. Wang, S. Shen, L. Hu, L. Yu, ‘Reversal of P-gp and MRP1-mediated multidrug resistance by H6, a gypenoside aglycon from Gynostemma pentaphyllum, in vincristine-resistant human oral cancer (KB/VCR) cells’, Eur. J. Pharmacol. 2012, 696, 43–53.
- 12J. W. Lee, Q. Jin, H. Jang, D. Lee, S. B. Han, Y. Kim, J. T. Hong, M. K. Lee, B. Y. Hwang, ‘Jatrophane and ingenane-type diterpenoids from Euphorbia kansui inhibit the LPS-induced NO production in RAW 264.7 cells’, Bioorg. Med. Chem. Lett. 2016, 26, 3351–3354.
- 13K. Bukowski, M. Kciuk, R. Kontek, ‘Mechanisms of Multidrug Resistance in Cancer Chemotherapy’, Int. J. Mol. Sci. 2020, 21.
- 14L. Galluzzi, J. M. Bravo-San Pedro, I. Vitale, S. A. Aaronson, J. M. Abrams, D. Adam, E. S. Alnemri, L. Altucci, D. Andrews, M. Annicchiarico-Petruzzelli, E. H. Baehrecke, N. G. Bazan, M. J. Bertrand, K. Bianchi, M. V. Blagosklonny, K. Blomgren, C. Borner, D. E. Bredesen, C. Brenner, M. Campanella, E. Candi, F. Cecconi, F. K. Chan, N. S. Chandel, E. H. Cheng, J. E. Chipuk, J. A. Cidlowski, A. Ciechanover, T. M. Dawson, V. L. Dawson, V. De Laurenzi, R. De Maria, K. M. Debatin, N. Di Daniele, V. M. Dixit, B. D. Dynlacht, W. S. El-Deiry, G. M. Fimia, R. A. Flavell, S. Fulda, C. Garrido, M. L. Gougeon, D. R. Green, H. Gronemeyer, G. Hajnoczky, J. M. Hardwick, M. O. Hengartner, H. Ichijo, B. Joseph, P. J. Jost, T. Kaufmann, O. Kepp, D. J. Klionsky, R. A. Knight, S. Kumar, J. J. Lemasters, B. Levine, A. Linkermann, S. A. Lipton, R. A. Lockshin, C. Lopez-Otin, E. Lugli, F. Madeo, W. Malorni, J. C. Marine, S. J. Martin, J. C. Martinou, J. P. Medema, P. Meier, S. Melino, N. Mizushima, U. Moll, C. Munoz-Pinedo, G. Nunez, A. Oberst, T. Panaretakis, J. M. Penninger, M. E. Peter, M. Piacentini, P. Pinton, J. H. Prehn, H. Puthalakath, G. A. Rabinovich, K. S. Ravichandran, R. Rizzuto, C. M. Rodrigues, D. C. Rubinsztein, T. Rudel, Y. Shi, H. U. Simon, B. R. Stockwell, G. Szabadkai, S. W. Tait, H. L. Tang, N. Tavernarakis, Y. Tsujimoto, T. Vanden Berghe, P. Vandenabeele, A. Villunger, E. F. Wagner, H. Walczak, E. White, W. G. Wood, J. Yuan, Z. Zakeri, B. Zhivotovsky, G. Melino, G. Kroemer, ‘Essential versus accessory aspects of cell death: recommendations of the NCCD 2015’, Cell Death Differ. 2015, 22, 58–73.
- 15D. J. Newman, G. M. Cragg, ‘Natural products as sources of new drugs over the 30 years from 1981 to 2010’, J. Nat. Prod. 2012, 75, 311–335.
- 16J. S. Zhang, H. Z. Weng, J. L. Huang, G. H. Tang, S. Yin, ‘Anti-inflammatory Ingenane Diterpenoids from the Roots of Euphorbia kansui’, Planta Med. 2018, 84, 1334–1339.
- 17D. Q. Fei, L. L. Dong, F. M. Qi, G. X. Fan, H. H. Li, Z. Y. Li, Z. X. Zhang, ‘Euphorikanin A, a Diterpenoid Lactone with a Fused 5/6/7/3 Ring System from Euphorbia kansui’, Org. Lett. 2016, 18, 2844–2847.
- 18J. Shen, J. Kai, Y. Tang, L. Zhang, S. Su, J. A. Duan, ‘The Chemical and Biological Properties of Euphorbia kansui’, Am. J. Chin. Med. 2016, 44, 253–273.
- 19J. Hohmann, J. Molnar, D. Redei, F. Evanics, P. Forgo, A. Kalman, G. Argay, P. Szabo, ‘Discovery and biological evaluation of a new family of potent modulators of multidrug resistance: Reversal of multidrug resistance of mouse lymphoma cells by new natural jatrophane diterpenoids isolated from Euphorbia species’, J. Med. Chem. 2002, 45, 2425–2431.
- 20T. S. Wu, Y. M. Lin, M. Haruna, D. J. Pan, T. Shingu, Y. P. Chen, H. Y. Hsu, T. Nakano, K. H. Lee, ‘Antitumor agents, 119. Kansuiphorins A and B, two novel antileukemic diterpene esters from Euphorbia kansui’, J. Nat. Prod. 1991, 54, 823–829.
- 21C. M. Wu, W. Chu, Y. L. Chen, D. E. Liang, F. J. Qiu, Z. J. Zhan, L. F. Ma, ‘Lignans and sesquiterpenes from Schisandra tomentella A. C. Smith’, Fitoterapia 2022, 158, 105142.
- 22Y. Chen, D. Luo, N.-Y. Chen, Y. Zhang, D.-E. Liang, Z.-J. Zhan, L.-F. Ma, ‘New ingenane diterpenoids from Euphorbia kansui reverse multi-drug resistance’, Phytochem. Lett. 2021, 43, 169–172.
- 23A. Vasas, E. Sulyok, D. Redei, P. Forgo, P. Szabo, I. Zupko, A. Berenyi, J. Molnar, J. Hohmann, ‘Jatrophane diterpenes from Euphorbia esula as antiproliferative agents and potent chemosensitizers to overcome multidrug resistance’, J. Nat. Prod. 2011, 74, 1453–1461.
- 24L. Y. Wang, N. L. Wang, X. S. Yao, S. Miyata, S. Kitanaka, ‘Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus’, J. Nat. Prod. 2002, 65, 1246–1251.
- 25G. D. Manners, R. Y. Wong, ‘The absolute stereochemical characterization of two new jatrophane diterpenes from Euphorbia esula’, J. Chem. Soc. Perkin Trans. 1 1985.
10.1039/p19850002075 Google Scholar
- 26Z. Q. Lu, S. H. Guan, X. N. Li, G. T. Chen, J. Q. Zhang, H. L. Huang, X. Liu, D. A. Guo, ‘Cytotoxic diterpenoids from Euphorbia heloscopia’, J. Nat. Prod. 2008, 71, 873–876.
- 27D. Uemura, Y. Hirata, Y. P. Chen, H. Y. Hsu, ‘The structure of kansuinine A, a new multi-oxygenated diterpene’, Tetrahedron Lett. 1975, 16, 1697–1700.
10.1016/S0040-4039(00)72236-2 Google Scholar
- 28J. Shi, M. Izumi, N. Baba, S. Nakajima, ‘Termiticidal activity of diterpenes from the roots of Euphorbia kansui’, Z. Naturforsch. C 2008, 63, 51–58.