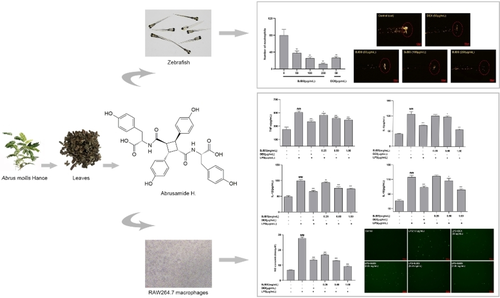
Abrusamide H Impairs the Secretion of the Cytokines in RAW264.7 Cells and the Inflammatory Infiltration in Tail Transection-Induced Zebrafish
Roujia Liu
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorFeirong Zhou
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorXinru Wei
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorXiangying Liu
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorCorresponding Author
Xujiang Yuan
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorCorresponding Author
Chuqin Yu
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorRoujia Liu
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorFeirong Zhou
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorXinru Wei
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorXiangying Liu
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorCorresponding Author
Xujiang Yuan
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorCorresponding Author
Chuqin Yu
Guangdong Provincial Key Laboratory of Advanced Drug Delivery Systems, Guangdong Pharmaceutical University, Center for Drug Research and Development, Guangdong Pharmaceutical University, Guangdong Engineering & Technology Research Center of Topical Precise Drug Delivery System, Guangdong Pharmaceutical University, East Waihuan Road 280, Guangzhou, P. R. China
Search for more papers by this authorAbstract
Abrus mollis Hance (Leguminosae) has a variety of biological activities, including anti-inflammatory, antioxidant, antibacterial, antiviral, and antitumor activities. However, the specific substances responsible for the anti-inflammatory effects are unknown. Abrusamide H (BJBS) is a truxillic acid derivative obtained from the leaves of Abrus mollis Hance and has potential anti-inflammatory effects. In this study, we aimed to estimate the potential effect and mechanism of BJBS in inflammation by establishing lipopolysaccharide (LPS)-stimulated RAW264.7 cells in vitro and an injured zebrafish tail fin in vivo. The RAW264.7 cells were treated with different concentrations of BJBS after LPS stimulation. The production of nitric oxide (NO) was detected by Griess reaction, and reactive oxygen species (ROS) were detected by an ROS assay kit. The levels of proinflammatory cytokines, including interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interleukin 18 (IL-18) were measured by ELISA. Results showed that BJBS at all concentrations inhibited the proliferation of RAW264.7 macrophages after LPS stimulation by cell counting kit-8 and the production of NO and ROS. In the BJBS treatment group, the levels of IL-6, TNF-α, IL-1β, and IL-18 decreased in a concentration-dependent manner. The results in vivo showed that no significant difference in the survival of zebrafish between the BJBS and blank groups and BJBS inhibited the migration and aggregation of zebrafish neutrophils in a dose-dependent manner in inflammation induced by tail transection-induced inflammation. In conclusion, BJBS inhibited the production of NO and ROS, decreased the levels of secreted IL-6, TNF-α, IL-1β, and IL-18, and reduced the migration and aggregation of zebrafish neutrophils.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
References
- 1X. Cai, F. Sha, C. Zhao, Z. Zheng, S. Zhao, Z. Zhu, H. Zhu, J. Chen and Y. Chen, ‘Synthesis and anti-inflammatory activity of novel steroidal chalcones with 3β-pregnenolone ester derivatives in RAW 264.7 cells in vitro’, Steroids 2021, 171, 108830.
- 2M. E. Rubio-Ruiz, A. E. Peredo-Escárcega, A. Cano-Martínez and V. Guarner-Lans, ‘An Evolutionary Perspective of Nutrition and Inflammation as Mechanisms of Cardiovascular Disease’, Int J Evol Biol 2015, 2015, 179791.
- 3A. Alfaddagh, S. S. Martin, T. M. Leucker, E. D. Michos, M. J. Blaha, C. J. Lowenstein, S. R. Jones and P. P. Toth, ‘Inflammation and cardiovascular disease: From mechanisms to therapeutics’, American Journal of Preventive Cardiology 2020, 4, 100130.
- 4V. Deretic, ‘Autophagy in inflammation, infection, and immunometabolism’, Immunity 2021, 54, 437–453.
- 5D. Cianciosi, T. Y. Forbes-Hernandez, J. M. Alvarez-Suarez, J. Ansary, D. Quinzi, A. Amici, M. D. Navarro-Hortal, A. Esteban-Muñoz, J. L. Quiles, M. Battino and F. Giampieri, ‘Anti-inflammatory activities of Italian Chestnut and Eucalyptus honeys on murine RAW 264.7 macrophages’, Journal of Functional Foods 2021, 87, 104752.
- 6Y. Yang, K. Zheng, W. Mei, Y. Wang, C. Yu, B. Yu, S. Deng and J. Hu, ‘Anti-inflammatory and proresolution activities of bergapten isolated from the roots of Ficus hirta in an in vivo zebrafish model’, Biochem. Biophys. Res. Commun. 2018, 496, 763–769.
- 7J.-P. Lévesque, K. M. Summers, K. Bisht, S. M. Millard, I. G. Winkler and A. R. Pettit, ‘Macrophages form erythropoietic niches and regulate iron homeostasis to adapt erythropoiesis in response to infections and inflammation’, Experimental Hematology 2021, 103, 1–14.
- 8C. D. Ellson, I. G. Riça, J. S. Kim, Y.-m. M. Huang, D. Lim, T. Mitra, A. Hsu, E. X. Wei, C. D. Barrett, L. E. Otterbein, C. J. Hauser, M. Wahl, H. Delbrück, U. Heinemann, H. Oschkinat, C.-e. A. Chang and M. B. Yaffe, ‘An Integrated Pharmacological, Structural, and Genetic Analysis of Extracellular Versus Intracellular ROS Production in Neutrophils’, J. Mol. Biol. 2022, 434, 167533.
- 9L. Dong, L. Yin, R. Chen, Y. Zhang, S. Hua, H. Quan and X. Fu, ‘Anti-inflammatory effect of Calycosin glycoside on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells’, Gene 2018, 675, 94–101.
- 10Y. Zheng, C. Tian, C. Fan, N. Xu, J. Xiao, X. Zhao, Z. Lu, H. Cao, J. Liu and L. Yu, ‘Sheng-Mai Yin exerts anti-inflammatory effects on RAW 264.7 cells and zebrafish’, J. Ethnopharmacol. 2021, 267, 113497.
- 11H. Wyns, E. Plessers, P. De Backer, E. Meyer and S. Croubels, ‘In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs’, Vet. Immunol. Immunopathol. 2015, 166, 58–69.
- 12S. Afrin, M. Gasparrini, T. Y. Forbes-Hernández, D. Cianciosi, P. Reboredo-Rodriguez, P. P. Manna, M. Battino and F. Giampieri, ‘Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 1: Enhancement of cellular viability, regulation of cellular apoptosis and improvement of mitochondrial functionality’, Food Chem. Toxicol. 2018, 121, 203–213.
- 13J. Karinchai, P. Budluang, P. Temviriyanukul, P. Ting, O. Nuchuchua, A. Wongnoppavich, A. Imsumran and P. Pitchakarn, ‘Bioassay-guided study of the anti-inflammatory effect of Anoectochilus burmannicus ethanolic extract in RAW 264.7 cells’, J. Ethnopharmacol. 2021, 280, 114452.
- 14M. Allegra, F. D'Acquisto, L. Tesoriere, A. Attanzio and M. A. Livrea, ‘Pro-oxidant activity of indicaxanthin from Opuntia ficus indica modulates arachidonate metabolism and prostaglandin synthesis through lipid peroxide production in LPS-stimulated RAW 264.7 macrophages’, Redox Biology 2014, 2, 892–900.
- 15M. A. Cinelli, H. T. Do, G. P. Miley and R. B. Silverman, ‘Inducible nitric oxide synthase: Regulation, structure, and inhibition’, Med. Res. Rev. 2020, 40, 158–189.
- 16M. P. van den Berg, H. Meurs and R. Gosens, ‘Targeting arginase and nitric oxide metabolism in chronic airway diseases and their co-morbidities’, Curr. Opin. Pharmacol. 2018, 40, 126–133.
- 17M. Lee, K. Rey, K. Besler, C. Wang and J. Choy, ‘Immunobiology of Nitric Oxide and Regulation of Inducible Nitric Oxide Synthase’, Results Probl. Cell Differ. 2017, 62, 181–207.
- 18J. Ren, D. Su, L. Li, H. Cai, M. Zhang, J. Zhai, M. Li, X. Wu and K. Hu, ‘Anti-inflammatory effects of Aureusidin in LPS-stimulated RAW264.7 macrophages via suppressing NF-κB and activating ROS- and MAPKs-dependent Nrf2/HO-1 signaling pathways’, Toxicol. Appl. Pharmacol. 2020, 387, 114846.
- 19C. Wang, L. Yang, Y. Hu, J. Zhu, R. Xia, Y. Yu, J. Shen, Z. Zhang and S. L. Wang, ‘Isoliquiritigenin as an antioxidant phytochemical ameliorates the developmental anomalies of zebrafish induced by 2,2′,4,4′-tetrabromodiphenyl ether’, Sci. Total Environ. 2019, 666, 390–398.
- 20X.-Y. Zhu, B. Xia, Y.-Y. Wu, H. Yang, C.-Q. Li and P. Li, ‘Fenobucarb induces heart failure and cerebral hemorrhage in zebrafish’, Aquat. Toxicol. 2019, 209, 34–41.
- 21J. E. Kim, S. K. Min, J. M. Hong, K. H. Kim, S. J. Han, J. H. Yim, H. Park and I. C. Kim, ‘Anti-inflammatory effects of methanol extracts from the Antarctic lichen, Amandinea sp. in LPS-stimulated raw 264.7 macrophages and zebrafish’, Fish Shellfish Immunol. 2020, 107, 301–308.
- 22M. Cafora, A. Brix, F. Forti, N. Loberto, M. Aureli, F. Briani and A. Pistocchi, ‘Phages as immunomodulators and their promising use as anti-inflammatory agents in a cftr loss-of-function zebrafish model’, J. Cystic Fibrosis 2021, 20, 1046–1052.
- 23H. Zhou, H. Cao, Y. Zheng, Z. Lu, Y. Chen, D. Liu, H. Yang, J. Quan, C. Huo, J. Liu and L. Yu, ‘Liang-Ge-San, a classic traditional Chinese medicine formula, attenuates acute inflammation in zebrafish and RAW 264.7cells’, J. Ethnopharmacol. 2020, 249, 112427.
- 24K. Howe, M. D. Clark, C. F. Torroja, J. Torrance, C. Berthelot, M. Muffato, J. E. Collins, S. Humphray, K. McLaren, L. Matthews, S. McLaren, I. Sealy, M. Caccamo, C. Churcher, C. Scott, J. C. Barrett, R. Koch, G. J. Rauch, S. White, W. Chow, B. Kilian, L. T. Quintais, J. A. Guerra-Assuncao, Y. Zhou, Y. Gu, J. Yen, J. H. Vogel, T. Eyre, S. Redmond, R. Banerjee, J. X. Chi, B. Y. Fu, E. Langley, S. F. Maguire, G. K. Laird, D. Lloyd, E. Kenyon, S. Donaldson, H. Sehra, J. Almeida-King, J. Loveland, S. Trevanion, M. Jones, M. Quail, D. Willey, A. Hunt, J. Burton, S. Sims, K. McLay, B. Plumb, J. Davis, C. Clee, K. Oliver, R. Clark, C. Riddle, D. Eliott, G. Threadgold, G. Harden, D. Ware, B. Mortimer, G. Kerry, P. Heath, B. Phillimore, A. Tracey, N. Corby, M. Dunn, C. Johnson, J. Wood, S. Clark, S. Pelan, G. Griffiths, M. Smith, R. Glithero, P. Howden, N. Barker, C. Stevens, J. Harley, K. Holt, G. Panagiotidis, J. Lovell, H. Beasley, C. Henderson, D. Gordon, K. Auger, D. Wright, J. Collins, C. Raisen, L. Dyer, K. Leung, L. Robertson, K. Ambridge, D. Leongamornlert, S. McGuire, R. Gilderthorp, C. Griffiths, D. Manthravadi, S. Nichol, G. Barker, S. Whitehead, M. Kay, J. Brown, C. Murnane, E. Gray, M. Humphries, N. Sycamore, D. Barker, D. Saunders, J. Wallis, A. Babbage, S. Hammond, M. Mashreghi-Mohammadi, L. Barr, S. Martin, P. Wray, A. Ellington, N. Matthews, M. Ellwood, R. Woodmansey, G. Clark, J. Cooper, A. Tromans, D. Grafham, C. Skuce, R. Pandian, R. Andrews, E. Harrison, A. Kimberley, J. Garnett, N. Fosker, R. Hall, P. Garner, D. Kelly, C. Bird, S. Palmer, I. Gehring, A. Berger, C. M. Dooley, Z. Ersan-Urun, C. Eser, H. Geiger, M. Geisler, L. Karotki, A. Kirn, J. Konantz, M. Konantz, M. Oberlander, S. Rudolph-Geiger, M. Teucke, K. Osoegawa, B. L. Zhu, A. Rapp, S. Widaa, C. Langford, F. T. Yang, N. P. Carter, J. Harrow, Z. M. Ning, J. Herrero, S. M. J. Searle, A. Enright, R. Geisler, R. H. A. Plasterk, C. Lee, M. Westerfield, P. J. de Jong, L. I. Zon, J. H. Postlethwait, C. Nusslein-Volhard, T. J. P. Hubbard, H. Roest Crollius, J. Rogers, D. L. Stemple, ‘The zebrafish reference genome sequence and its relationship to the human genome’, Nature 2013, 496, 498–503.
- 25G. R. Kim, J. Y. Yang, K. S. Hwang, S. S. Kim, J. S. Chae, H. Kan, J. H. Ahn, W. M. Lee, S. H. Ahn, Y. M. Lee, M. A. Bae and D. S. Shin, ‘Anti-inflammatory effect of a novel synthetic compound 1-((4-fluorophenyl)thio)isoquinoline in RAW264.7 macrophages and a zebrafish model’, Fish Shellfish Immunol. 2019, 87, 395–400.
- 26W. Liu, L. Zhang, S. Sun, L.-s. Tang, S.-m. He, A.-q. Chen, L.-n. Yao and D.-L. Ren, ‘Cordycepin inhibits inflammatory responses through suppression of ERK activation in zebrafish’, Dev. Comp. Immunol. 2021, 124, 104178.
- 27Y. Guo, Y. Liu, C. Zhang, Z. Y. Su, W. Li, M. T. Huang and A. N. Kong, ‘The epigenetic effects of aspirin: the modification of histone H3 lysine 27 acetylation in the prevention of colon carcinogenesis in azoxymethane- and dextran sulfate sodium-treated CF-1 mice’, Carcinogenesis 2016, 37, 616–624.
- 28L. Zhang, J. Chen, H. Liao, C. Li and M. Chen, ‘Anti-inflammatory effect of lipophilic grape seed proanthocyanidin in RAW 264.7 cells and a zebrafish model’, Journal of Functional Foods 2020, 75, 104217.
- 29P. G. Conaghan, ‘A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity’, Rheumatol. Int. 2012, 32, 1491–1502.
- 30L. Zong, J. Zhang, L. Dai, J. Liu, Y. Yang, J. Xie and X. Luo, ‘The Anti-Inflammatory Properties of Rhododendron molle Leaf Extract in LPS-Induced RAW264.7’, Chem. Biodiversity 2020, 17, e2000477.
- 31X. Yao, Z. Li, X. Gong, X. Fu, X. Xiao, M. He, B. Huang and Z. Xu, ‘Total saponins extracted from Abrus cantoniensis Hance suppress hepatitis B virus replication in vitro and in rAAV8-1.3HBV transfected mice’, J. Ethnopharmacol. 2020, 249, 112366.
- 32M. Yang, Q. Shen, L. Q. Li, Y. Q. Huang and H. Y. Cheung, ‘Phytochemical profiles, antioxidant activities of functional herb Abrus cantoniensis and Abrus mollis’, Food Chem. 2015, 177, 304–312.
- 33S. Wu, X. Fu, M. A. Brennan, C. S. Brennan and C. Chun, ‘The Effects of Different Purifying Methods on the Chemical Properties, in Vitro Anti-Tumor and Immunomodulatory Activities of Abrus cantoniensis Polysaccharide Fractions’, Int. J. Mol. Sci. 2016, 17, 511.
- 34X. Yuan, Y. Liu, H. Zhao, L. Men, C. He, Y. Qiu, Q. Yu, K. Li, L. Qi and D. Chen, ‘The isolation, structure and fragmentation characteristics of natural truxillic and truxinic acid derivatives in Abrus mollis leaves’, Phytochemistry 2021, 181, 112572.
- 35A. Matta, P. Chammingkwan, B. K. Singh, M. Terano, T. Kaneko and T. Taniike, ‘Truxillic and truxinic acid-based, bio-derived diesters as potent internal donor in Ziegler-Natta catalyst for propylene polymerization’, Appl. Catal. A 2018, 554, 80–87.
- 36S. Wu, X. Fu, L. You, A. M. Abbasi, H. Meng, D. Liu and R. M. Aadil, ‘Antioxidant, antitumor and immunomodulatory activities of water-soluble polysaccharides in Abrus cantoniensis’, Int. J. Biol. Macromol. 2016, 89, 707–716.
- 37W. Yan, Q. Han, P. Guo, C. Wang and Z. Zhang, ‘Simultaneous Detection of Flavonoids, Phenolic Acids and Alkaloids in Abri Herba and Abri Mollis Herba using Liquid Chromatography Tandem Mass Spectrometry’, Phytochem. Anal. 2016, 27, 50–56.
- 38M. Gasparrini, S. Afrin, T. Y. Forbes-Hernandez, D. Cianciosi, P. Reboredo-Rodriguez, A. Amici, M. Battino and F. Giampieri, ‘Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 2: Control of oxidative stress induced damage, increase of antioxidant enzyme activities and attenuation of inflammation’, Food Chem. Toxicol. 2018, 120, 578–587.
- 39M. Mokhtar, J. Soukup, P. Donato, F. Cacciola, P. Dugo, A. Riazi, P. Jandera and L. Mondello, ‘Determination of the polyphenolic content of a Capsicum annuum L. extract by liquid chromatography coupled to photodiode array and mass spectrometry detection and evaluation of its biological activity’, J. Sep. Sci. 2015, 38, 171–178.
- 40J. Y. Wang, H. L. Wang, H. L. Zhang, Z. H. Liu, C. Y. Ma and W. Y. Kang, ‘Immunomodulation of ADPs-1a and ADPs-3a on RAW264.7 cells through NF-kappa B/MAPK signaling pathway’, Int. J. Biol. Macromol. 2019, 132, 1024–1030.
- 41V. Khajuria, S. Gupta, N. Sharma, A. Kumar, N. A. Lone, M. Khullar, P. Dutt, P. R. Sharma, A. Bhagat and Z. Ahmed, ‘Anti-inflammatory potential of hentriacontane in LPS stimulated RAW 264.7 cells and mice model’, Biomed. Pharmacother. 2017, 92, 175–186.
- 42M. Gasparrini, T. Y. Forbes-Hernandez, F. Giampieri, S. Afrin, J. M. Alvarez-Suarez, L. Mazzoni, B. Mezzetti, J. L. Quiles and M. Battino, ‘Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages’, Food Chem. Toxicol. 2017, 102, 1–10.
- 43Y. Zhang, C. Liu, B. Dong, X. Ma, L. Hou, X. Cao and C. Wang, ‘Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages’, Inflammation 2015, 38, 756–764.
- 44T. Hagemann, S. K. Biswas, T. Lawrence, A. Sica and C. E. Lewis, ‘Regulation of macrophage function in tumors: the multifaceted role of NF-κB’, Blood 2009, 113, 3139–3146.