Antibacterial Triketone-Phloroglucinol-Triketone Adducts from Myrtus communis
Yan Wu
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Co-first authors.
Search for more papers by this authorJiao-Wen Liu
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Co-first authors.
Search for more papers by this authorChao Liu
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorXiao-Jun Huang
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorNi-Ping Li
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorCorresponding Author
Wen-Cai Ye
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorCorresponding Author
Lei Wang
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorYan Wu
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Co-first authors.
Search for more papers by this authorJiao-Wen Liu
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Co-first authors.
Search for more papers by this authorChao Liu
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorXiao-Jun Huang
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorNi-Ping Li
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorCorresponding Author
Wen-Cai Ye
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorCorresponding Author
Lei Wang
Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorAbstract
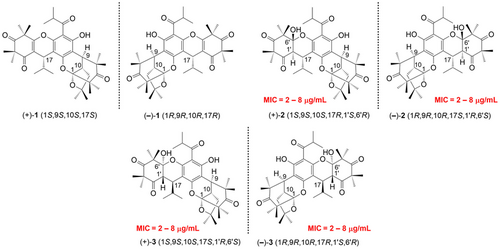
Myrtucyclitones A–C ((+)- and (−)-1–3), three pairs of new triketone-phloroglucinol-triketone hybrids were isolated from the plant Myrtus communis. Their structures with absolute configurations were established by NMR analysis and chemical calculations. Myrtucyclitones B and C exhibited remarkable antibacterial effect.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202000708-sup-0001-misc_information.pdf3.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. B. Levy, B. Marshall, ‘Antibacterial resistance worldwide: causes, challenges and responses’, Nat. Med. 2004, 10, S122–S129.
- 2T. F. Schäberle, I. M. Hack, ‘Overcoming the current deadlock in antibiotic research’, Trends Microbiol. 2014, 22, 165–167.
- 3I.-P. Singh, S.-B. Bharate, ‘Phloroglucinol compounds of natural origin’, Nat. Prod. Rep. 2006, 23, 558–591.
- 4M.-J. Cheng, J.-Q. Cao, X.-Y. Yang, L.-P. Zhong, L.-J. Hu, X. Lu, B.-L. Hou, Y.-J. Hu, Y. Wang, X.-F. You, L. Wang, W.-C. Ye, C.-C. Li, ‘Catalytic Asymmetric Total Syntheses of Myrtucommuacetalone, Myrtucommuacetalone B and Callistrilones A, C, D, E’, Chem. Sci. 2018, 9, 1488–1495.
- 5L. Wu, Y.-L. Zhang, X.-B. Wang, R.-H. Liu, M.-H. Yang, L.-Y. Kong, L. Jun, ‘Acylphloroglucinols from the fruits of Callistemon viminalis’, Phytochem. Lett. 2017, 20, 61–65.
- 6H.-X. Liu, K. Chen, Y. Yuan, Z.-F. Xu, H.-B. Tan, S.-X. Qiu, ‘Rhodomentones A and B, novel meroterpenoids with unique NMR characteristics from Rhodomyrtus tomentosa’, Org. Biomol. Chem. 2016, 47, 7354–7360.
- 7H.-X. Liu, W.-M. Zhang, Z.-F. Xua, Y.-C. Chen, H.-B. Tan, S.-X. Qiu, ‘Isolation, synthesis, and biological activity of tomentosenol A from the leaves of Rhodomyrtus tomentosa’, RSC Adv. 2016, 6, 25882–25886.
- 8X.-J. Qina, H. Liu, Q. Yu, H. Yan, J.-F. Tang, L.-K. An, A. Khan, Q.-R. Chen, X.-J. Hao, H.-Y. Liu, ‘Acylphloroglucinol derivatives from the twigs and leaves of Callistemon salignus’, Tetrahedron 2017, 73, 1803–1811.
- 9M. Leonti, L. Casu, F. Sanna, L. Bonsignore, ‘A comparison of medicinal plant use in Sardinia and Sicily-De Materia Medica revisited?’, J. Ethnopharmacol. 2009, 121, 255–267.
- 10G. Alipour, S. Dashti, H. Hosseinzadeh, ‘Review of pharmacological effects of Myrtus communis L. and its active constituents’, Phytother. Res. 2014, 28, 1125–1136.
- 11A. Rotstein, A. Lifshitz, Y. Kashman, ‘Isolation and antibacterial activity of acylphloroglucinols from Myrtus communis’, Antimicrob. Agents Chemother. 1974, 6, 539–542.
- 12M.-I. Choudhary, N. Khan, M. Ahmad, S. Yousuf, H.-K. Fun, S. Soomro, M. Asif, M.-A. Mesaik, F. Shaheen, ‘New inhibitors of ROS generation and T-cell proliferation from Myrtus communis’, Org. Lett. 2013, 15, 1862–1865.
- 13G. Appendino, F. Bianchi, A. Minassi, O. Sterner, M. Ballero, S. Gibbons, ‘Oligomeric acylphloroglucinols from myrtle (Myrtus communis)’, J. Nat. Prod. 2002, 65, 334–338.
- 14J.-Q. Cao, X.-J. Huang, Y.-T. Li, Y. Wang, L. Wang, R.-W. Jiang, W.-C. Ye, ‘Callistrilones A and B, Triketone-Phloroglucinol-Monoterpene Hybrids with a New Skeleton from Callistemon rigidus’, Org. Lett. 2016, 18, 120–123.
- 15J.-G. Song, J.-C. Su, Q.-Y. Song, R.-L. Huang, W. Tang, L.-J. Hu, X.-J. Huang, R.-W. Jiang, Y.-L. Li, W.-C. Ye, Y. Wang, ‘Cleistocaltones A and B, Antiviral Phloroglucinol−Terpenoid Adducts from Cleistocalyx operculatus’, Org. Lett. 2019, 21, 9579–9583.
- 16F. Liu, H.-Y. Tian, X.-L. Huang, W.-J. Wang, N.-P. Li, J. He, W.-C. Ye, L. Wang, ‘Xanthchrysones A–C: Rearranged Phenylpropanoyl-Phloroglucinol Dimers with Unusual Skeletons from Xanthostemon chrysanthus’, J. Org. Chem. 2019, 84, 15355–15361.
- 17F. Liu, Y. Wu, N.-P. Li, J.-W. Liu, L. Wang, W.-C. Ye, ‘Chiral Isolation and Absolute Configuration of (+)- and (−)-Xanchryones F and G from Xanthostemon chrysanthus’, Chem. Biodiversity 2019, 17, e1900683.
- 18J.-Q. Cao, Y. Wu, Y.-L. Zhong, N.-P. Li, M. Chen, M.-M. Li, W.-C. Ye, L. Wang, ‘Antiviral Triketone-Phloroglucinol-Monoterpene Adducts from Callistemon rigidus’, Chem. Biodiversity 2018, 15, e1800172.
- 19J.-H. Gu, W.-J. Wang, J.-Z. Chen, J.-S. Liu, N.-P. Li, M.-J. Cheng, L.-J. Hu, C.-C. Li, W.-C. Ye, L. Wang, ‘Leptosperols A and B, Two Cinnamoylphloroglucinol−Sesquiterpenoid Hybrids from Leptospermum scoparium: Structural Elucidation and Biomimetic Synthesis’, Org. Lett. 2020, 22, 1796–1800.
- 20S. Rattanaburi, W. Mahabusarakam, S. Phongpaichit, A.-R. Carrolld, ‘Acylphloroglucinols from Callistemon lanceolatus DC’, Tetrahedron 2013, 69, 6070–6075.
- 21A.-R. Carroll, J. Lamb, R. Moni, G.-P. Guymer, P.-I. Forster, R.-J. Quinn, ‘Myrtucommulones F–I, phloroglucinols with thyrotropin-releasing hormone receptor-2 binding affinity from the seeds of Corymbia scabrida’, J. Nat. Prod. 2008, 71, 1564–1568.
- 22F. Cottiglia, L. Casu, M. Leonti, P. Caboni, C. Floris, B. Busonera, P. Farci, A. Ouhtit, G. Sanna, ‘Cytotoxic phloroglucinols from the leaves of Myrtus communis’, J. Nat. Prod. 2012, 75, 225–229.
- 23L.-B. Dong, J. Yang, J. He, H.-R. Luo, X.-D. Wu, X. Deng, L.-Y. Peng, X. Cheng, Q.-S. Zhao, ‘Lycopalhine A, a Novel Sterically Congested Lycopodium Alkaloid with an Unprecedented Skeleton from Palhinhaea cernua’, Chem. Commun. 2012, 48, 9038–9040.
- 24C.-F. Ding, H.-X. Ma, J. Yang, X.-J. Qin, G. S. S. Njateng, H.-F. Yu, X. Wei, Y.-P. Liu, W.-Y. Huang, Z.-F. Yang, X.-H. Wang, X.-D. Luo, ‘Antibacterial Indole Alkaloids with Complex Heterocycles from Voacanga Africana’, Org. Lett. 2018, 20, 2702–2706.
- 25Gaussian 09, Revision A.02, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
- 26T. Bruhn, A. Schaumlöffel, Y. Hemberger, G. Bringmann, SpECDis version 1.60, University of Wuerzburg, Germany, 2012.
- 27CLSI, ‘Performance Standards for Antimicrobial Susceptibility Testing’, 27th edn., CLSI supplement M100, Wayne, PA, Clinical and Laboratory Standards Institute, 2017.