Synthesis of Taxol and Docetaxel by Using 10-Deacetyl-7-xylosyltaxanes
Corresponding Author
Baoyu Xue
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorJunhong Zhao
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorYange Fan
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorShipeng Chen
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorWenfeng Li
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorJin Chen
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorZheng Li
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorHongxing Wang
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorHongjun Kong
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorCorresponding Author
Baoyu Xue
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorJunhong Zhao
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorYange Fan
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorShipeng Chen
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorWenfeng Li
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorJin Chen
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorZheng Li
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorHongxing Wang
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorHongjun Kong
Henan Institute of Chemistry, Henan Academy of Sciences, No. 56 Hongzhuan Road, Zhengzhou, 450003 P. R. China
Search for more papers by this authorAbstract
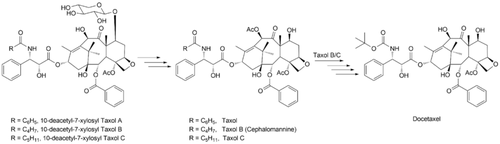
A mixture of taxols was prepared from 10-deacetyl-7-xylosyltaxanes by three-step reactions: redox, acetylation, and deacetylation. The mixture was separated by column chromatography on silica gel to afford Taxol, Taxol B (Cephalomannine) and Taxol C. The mixture of Taxol B and Taxol C was converted to Docetaxel by Schwartz's reagent. The structures of Taxol and Docetaxel were characterized by HPLC, 1H-NMR, 13C-NMR and MS. This synthetic process has expanded the source of biomass for the chemical semi-synthesis of Taxol and Docetaxel, reduced the production costs, and increased the biomass resource of taxanes.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv201900631-sup-0001-misc_information.pdf561.9 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. C. Wani, H. L. Taylor, M. E. Wall, P. Coggon, A. T. McPhail, ‘Plant antitumor agents. VI. The isolation and structure of Taxol, a novel antileukemic and antitumor agent from Taxus brevifolia’, J. Am. Chem. Soc. 1971, 93, 2325–2327.
- 2M. E. Wall, M. C. Wani, ‘Camptothecin and Taxol: discovery to clinic thirteenth Bruce F. Cain Memorial Award Lecture’, Cancer Res. 1995, 55, 753–760.
- 3E. K. Rowinsky, R. C. Donehower, N. B. Rosenshein, D. S. Ettinger, W. P. McGuire, ‘Phase II study of Taxol in advanced ovarian epithelial malignancies’, Proc. Am. Soc. Clin. Oncol. 1988, 136.
- 4D. Z. Chen, L Wu, D. M. Chen, ‘Cultivation and utilization of imported tree species’, Yunnan Sci. Technol. Press, 2010, pp. 111–117.
- 5Y. Li, ‘Design, Synthesis and Evaluation of Novel Taxane-Based Anticancer Agents’, State University of New York at Stony Brook, 2011.
- 6J. N. Denis, A. E. Greene, D. Guenard, F. Gueritte-Voegelein, L. Mangatal, P. Potier, ‘Highly efficient, practical approach to natural Taxol’, J. Am. Chem. Soc. 1988, 110, 5917–5919.
- 7D. Guenard, F. Gueritte-Voegelein, P. Potier, ‘Taxol and taxotere: discovery, chemistry, and structure−activity relationships’, Acc. Chem. Res. 1993, 26, 160–167.
- 8A. H. Wang, ‘The Synthesis Research of Docetaxel’, Nanjing University of Science and Technology, 2012.
- 9M. A. Strelkova, N. V. Kirillova, ‘Anti-tumour activity of extracts from biomass of Taxus baccata L. cell culture’, Rastit. Resur. 2002, 38, 70–77.
- 10P. G. Xiao, ‘New Chinese Medicine (Volume III)’, Chemical Industry Press, 2002, pp. 731–732.
- 11G. Strobel, X. Yang, J. Sears, R. Kramer, R. S. Sidhu, W. M. Hess, ‘Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana’, Microbiology 1996, 142, 435–440.
- 12X. L. Gou, J. Jing, H. C. Bi, G. P. Zhong, Z. L. Li, M. Huang, ‘Study on antitumor activity of extracts of branches and leaves of Taxus chinensis and taxoids on four cell lines’, Chin. Pharmacol. Bull. 2016, 32, 591–592.
- 13J. Crown, M. O′Leary, W. S. Ooi, ‘Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience’, Oncologist 2004, 9, 24–32.
- 14H. W. Lu, ‘Medication of Exemestane and Docetaxel on the Growth of Human Endometrial Carcinoma Xenograft in Nude Mice’, China Medical University, 2010.
- 15N. Cong, ‘Studies on quality control of docetaxel for injection and pharmacokinetics’, Shen Yang Pharmaceutical University, 2007.
- 16H. S. Jin, H. Q. Lou, S. C. Jiang, ‘Study of Technology of Isolated Paclitaxel from Taxus Chinensis’, Guangzhou Chem. Ind. 2014, 10, 88–91.
- 17J. H. Zhao, W. F. Li, Y. G. Fan, B. Y. Xue, J. Yang, ‘Determination of Taxol in Chinese Yew Planted in Xinxiang by RP-HPLC’, Henan Sci. 2011, 29, 288–290.
- 18Y. Zhao, D. Y. Zhang, X. M. Wu, W. Y. Hua, ‘Technological Improvement of the Semisynthesis of Taxol’, Fine Chemicals 2013, 30, 555–560.
- 19Y. Y. Wang, F. Feng, L. Z. Tian, Q. Wang, Q. L. Yu, ‘Study on the Process Related Impurities of Semisynthetic Paclitaxel’, Chin. J. Modern Appl. Pharm. 2014, 12, 154–158.
- 20M. D. Jiang, ‘Semisynthesis and quality standards research of Taxol’, Jilin University, China, 2015.
- 21D. G. I. Kingston, P. G. Jagtap, H. Yuan, L. Samala, ‘The chemistry of Taxol and related taxoids’, Fortschr. Chem. Org. Naturst. 2002, 84, 53–225.
- 22H. Zhang, H. Gan, Z. N. Wu, Z. Y. Meng, G. F. Dou, ‘Biotransformation of 7-xylose taxanes’, Chin. J. New Drugs 2013, 22, 1029–1033.
- 23S. G. Jiang, ‘Study on 7-xylosyl-10-deacetylpaclitaxel antitumor activity and mechanism’, Northeast Forestry University, 2008.
- 24H. Zhang, ‘Study on biotransformation of 7-xylosyl-10-deacetyltaxol’, Guangxi Medical University, 2013.
- 25X. L. Tong, W. S. Fang, J. Y. Zhou, C. H. He, W. M. Chen, Q. C. Fang, ‘Studies on the chemical constituents of leaves and twigs of Taxus guspidata’, Acta Pharm. Sin. 1994, 29, 55–60.
- 26J. H. Li, ‘Microbial transformation of cephalomannine and the enzymatic synthesis of 10-deacetyltaxol’, Chinese Academy of Medical Sciences-Peking Union, 2007.
- 27Z. Y. Chen, Y. P. Chen, ‘Study on Chemical Composition of Yunnan Taxus chinensis’, China J. Chin. Mater. Med. 1996, 21, 230–232.
- 28B. Y. Xue, W. F. Li, Y. G. Fan, J. H. Zhao, ‘Method for preparing Taxol by using 7-xylosyl-10-deacetylated taxane’, CN 201110425004.0, 2012-06-27.
- 29B. Y. Xue, W. F. Li, Y. G. Fan, J. H. Zhao, J. H. Cao, K. L. Ding, ‘A method for industrial separation and purification of paclitaxel’, CN 201110121650.8, 2011-10-19.
- 30D. H. Chen, ‘Study of docetaxel synthesis process’, Chongqing Medical University, 2009.
- 31X. M. Deng, ‘Study on the Synthesis Process and Activity of Paclitaxel and Its Derivatives’, Zhejiang University of Technology, 2018.
- 32H. C. Zhang, ‘Synthesis of Docetaxel’, Zhejiang University, 2013.
- 33D. H. Chen, ‘Study of docetaxel synthesis process’, Chongqing Medical University, 2009.