New Antiparasitic Bis-Naphthoquinone Derivatives
Abstract
A series of bis-naphthoquinone derivatives prepared by condensation of aryl aldehydes with lawsone were tested for antiparasitic activities against Toxoplasma gondii and Trypanosoma brucei parasites. Monofluorophenyl derivative 1a, 3,4-difluorophenyl analog 1c and furyl compound 1l exhibited significant activity against T. gondii cells and appear to be new promising drug candidates against this parasite. The 3,4,5-trifluorophenyl derivative 1g and the isovanillyl derivative 1j displayed selective activity against Leishmania major amastigotes.
Graphical Abstract
Introduction
Parasitic diseases still represent an enormous world-wide medical challenge.1 Neglected tropical diseases (NTDs) pose a particularly great danger both for local people and travelers in many tropical and subtropical countries.2 Due to the climate change, further regions of the world will likely be affected by these parasitic diseases in the future.2 There are only a few drugs available against NTDs because big pharma is reluctant to develop new and more potent drugs against diseases that mainly poor people suffer from.3 Vice versa, poor people affected by NTDs are in want of affordable drugs, which could, however, arise from tapping into natural resources. Many natural product derived drug candidates, active against various NTDs, have emerged.3 In addition, natural products constitute sustainable, cheap materials.
Lawsone, for instance, is a natural 2-hydroxy-1,4-naphthoquinone isolated from the Henna plant (Lawsonia inermis) which is being applied for the management of skin diseases in South Asia as a component of the local Ayurveda and Unani folk medicine.4, 5 Lawsone (2-hydroxy-1,4-naphthoquinone) is also a useful starting material for the preparation of various related quinones with proven bioactivity such as lapachol or atovaquone.4, 5 Arylmethylene-substituted bis-naphthoquinone derivatives have been reported to exhibit only moderate activities against human cancer cells which makes them interesting candidates for the design of selective antiparasitic drugs.6 Indeed, a small series of substituted bis-2-hydroxy-1,4-naphthoquinones including 1a (Figure 1) was obtained with distinct activity against Leishmania parasites.7 Herein, we report on expanded series of such new derivatives (e. g., fluoroarenes, vanillins, furans) with optimized activity and selectivity against various parasites including Toxoplasma gondii, Trypanosoma brucei and Leishmania.
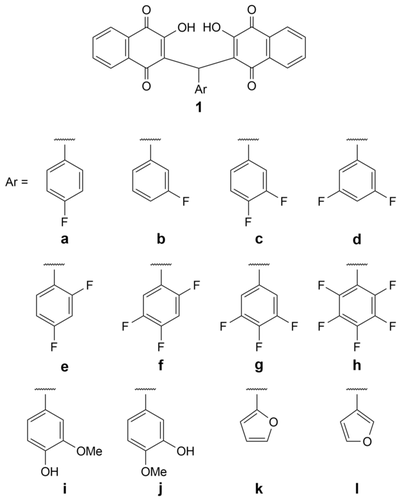
Structures of target compounds 1a–1l.
Results and Discussion
The known compounds 1a and 1b and the new analogs 1c–1l were obtained as solids from the one-pot condensation reaction of two equivalents of 2-hydroxy-1,4-naphthoquinone (lawsone) with the corresponding aryl aldehyde and a catalytic amount of β-alanine in warm acetic acid (Scheme 1).7, 8 The structures of the target compounds 1a–1l are shown in Figure 1.
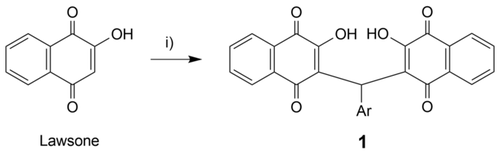
Synthesis of bis-naphthoquinone derivatives. Reagents and conditions: i) Aryl aldehyde (0.5 equiv.), cat. β-alanine, AcOH, 50 °C, 2 h, 30–62 %.
The antiparasitic activity of the compounds 1a–1l was initially tested against Toxoplasma gondii and Trypanosoma brucei parasites and compared with the toxicity against normal kidney epithelial Vero cells (Table 1). The best performing compounds 1a and 1c exhibited good and similar activities against T. gondii cells with 1c showing a better selectivity for T. gondii cells over Vero cells. Like 1a, the pentafluorophenyl derivative 1h was more toxic to normal Vero cells than to T. gondii cells. Atovaquone, which is commonly applied for the treatment of toxoplasmosis, was used as positive control and displayed the highest activity and selectivity.9 However, the toxicity of the most bis-naphthoquinones (except for 1a and 1h) to Vero cells was distinctly lower when compared with atovaquone. In contrast to this varied spectrum of activities against T. gondii, all compounds, except 1a and 1i which displayed low double-digit IC50 values, were virtually inactive against T. b. brucei cells. Pentamidine, an approved drug for the treatment of sleeping sickness, was applied as positive control with activity in the nano-molar concentration range.10
Compound |
IC50 (Vero, [μm]) |
EC50 (T. gondii, [μm]) |
SI (Vero/T. gondii)[b] |
IC50 (T. b. brucei, [μm]) |
SI (Vero/T. b. brucei)[c] |
|---|---|---|---|---|---|
1a |
6.78 |
11.64 |
0.58 |
15.6 |
0.43 |
1b |
53.48 |
26.63 |
2.0 |
>10 |
– |
1c |
17.63 |
13.95 |
1.26 |
>10 |
– |
1d |
27.54 |
34.53 |
0.80 |
>100 |
– |
1e |
39.37 |
27.94 |
1.41 |
>10 |
– |
1f |
24.06 |
23.86 |
1.01 |
>10 |
– |
1g |
142.3 |
48.94 |
2.91 |
– |
– |
1h |
6.88 |
21.47 |
0.32 |
72.3 |
0.10 |
1i |
>100 |
109.0 |
– |
27.8 |
>3.60 |
1j |
>100 |
59.28 |
– |
– |
– |
1k |
57.41 |
25.33 |
2.27 |
>100 |
– |
1l |
68.25 |
23.22 |
2.94 |
>10 |
– |
AmB |
7.7 |
– |
– |
– |
– |
Pentamidine |
– |
– |
– |
0.042[d] |
. |
ATO |
9.5 |
0.07 |
136 |
– |
– |
- [a] Values are the means of at least three independent experiments (SD ±15 %). They were derived from concentration–response curves obtained by measuring the percentage of vital cells relative to untreated controls after 72 h. [b] Selectivity index (EC50/IC50) calculated from the corresponding EC50 (T. gondii) and IC50 (Vero) values. [c] Selectivity index calculated from the corresponding IC50 (T. b. brucei) and IC50 (Vero) values. [d] Value is taken from ref. [8].
The compounds 1a–1l were also tested against Leishmania major promastigotes and amastigotes in comparison to normal Vero cells (Table 2). Compounds 1a and 1h showed similar moderate activities against the promastigotes (EC50=35.0 μm for 1a and 35.5 μm for 1h). Compounds 1c and 1f were most active against the amastigotes (EC50=23.7 μm for 1c and 22.6 μm for 1f) and more active than the known compound 1a. However, these compounds showed only poor selectivity. In contrast to that, compounds 1g and 1j displayed only slightly weaker activities than 1c and 1f against the amastigotes (EC50=34.1 μm for 1g and 37.9 μm for 1j) but reasonable selectivity when compared with their effects on Vero cells (SI=4.18 for 1g and more than 2.64 for 1j). The activity of the known compound 1a against L. major promastigotes (EC50=35.0 μm) was much lower when compared with its published activities against L. amazonensis (IC50=0.6 μm) and L. braziliensis promastigotes (IC50=0.8 μm).7 Amphotericin B was applied as a proper positive control for the tests with L. major parasites despite of its severe side-effects.11 In this study, the toxicity of amphotericin B to Vero cells was distinctly higher when compared with 1g and 1j, for example, which did not harm Vero cells at doses of 100 μm and still showed moderate activity against amastigotes.
Compound |
EC50 (promastigotes, [μm]) |
EC50 (amastigotes, [μm]) |
SI (Vero/promastigotes)[b] |
SI (Vero/amastigotes)[c] |
|---|---|---|---|---|
1a |
35.0 |
32.4 |
0.19 |
0.21 |
1b |
116.0 |
48.0 |
0.46 |
1.12 |
1c |
92.5 |
23.7 |
0.19 |
0.74 |
1d |
89.1 |
77.2 |
0.31 |
0.36 |
1e |
83.0 |
36.4 |
0.48 |
1.08 |
1f |
76.5 |
22.6 |
0.32 |
1.06 |
1g |
108 |
34.1 |
1.32 |
4.18 |
1h |
35.5 |
30.1 |
0.19 |
0.23 |
1i |
>200 |
106.0 |
– |
>0.94 |
1j |
97.4 |
37.9 |
>1.03 |
>2.64 |
1k |
101 |
33.5 |
0.57 |
1.71 |
1l |
94.1 |
53.7 |
1.27 |
0.73 |
AmB |
0.83 |
0.47 |
9.6 |
16.4 |
- [a] Values are the means of at least three independent experiments (SD ±15 %). They were derived from concentration−response curves obtained by measuring the percentage of vital cells relative to untreated controls after 72 h. [b] Selectivity index (IC50/EC50) calculated from the corresponding IC50 values for Vero cells and the EC50 values against L. major promastigotes. [c] Selectivity index calculated from the corresponding IC50 values for Vero cells and the EC50 values for L. major amastigotes.
Conclusions
A series of bis-naphthoquinone derivatives with variance in the aryl residue at the bridging methylene carbon were prepared and tested on various protozoal parasites against which they showed distinctly different activities. We identified compounds that were remarkably active against T. gondii cells (e. g., the fluorophenyl derivatives 1a and 1c, and the furan 1l), and sufficiently selective for L. major amastigotes (e. g., the fluorophenyl derivative 1g and isovanillyl derivative 1j) to warrant further drug optimization studies. It should be noted that in our tests with different parasites we could not confirm the excellent activities published for 1a.7 Although this compound was among the most active ones of this series, its toxic effect on normal epithelial Vero cells is unfavorable for an antiparasitic drug. It might hold, however, like the new compound 1h, some potential as an antitumoral drug candidate.
Experimental Section
General
All starting compounds were purchased from Aldrich. The known compounds 1a and 1b were prepared according to literature procedures.7, 12 The following instruments were used: melting points (uncorrected), Gallenkamp; IR spectra, PerkinElmer Spectrum One FT-IR spectrophotometer with ATR sampling unit; nuclear magnetic resonance spectra, Bruker Avance 300 spectrometer; chemical shifts are given in parts per million (δ) downfield from tetramethylsilane as internal standard; mass spectra, Varian MAT 311A (EI), Q Exactive (ESI, HRMS, solutions of MeCN); microanalyses, PerkinElmer 2400 CHN elemental analyzer. All tested compounds are >95 % pure by elemental analysis.
2,2′-[(4-Fluorophenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1a). Yield: 69 mg (0.15 mmol, 30 %). MS: 454 (21) [M+⋅], 436 (21), 408 (31), 252 (100), 224 (22), 196 (27), 174 (63), 105 (68), 76 (46).
2,2′-[(3-Fluorophenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1b). Yield: 68 mg (0.15 mmol, 30 %). MS: 454 (83) [M+⋅], 436 (57), 408 (84), 280 (33), 251 (100), 174 (70), 105 (87), 76 (56).
2,2′-[(3,4-Difluorophenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1c) – General Procedure. 2-Hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), 3,4-difluorobenzaldehyde (71 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) were dissolved in AcOH (8 mL). The reaction mixture was stirred at 50 °C for 2 h. The reaction mixture was poured on water (30 mL), the precipitate was collected and dried in vacuum. Yield: 70 mg (0.15 mmol, 30 %). Yellow solid. M.p. 109–110 °C. ATR-IR: 3340, 1642, 1593, 1515, 1460, 1431, 1362, 1336, 1269, 1211, 1162, 1115, 1044, 1013, 977, 918, 887, 868, 816, 756, 723, 693. 1H-NMR (300 MHz, CDCl3): 6.10 (1 H. s), 7.0–7.2 (3 H, m), 7.6–7.8 (4 H, m), 8.0–8.1 (4 H, m). 13C-NMR (75.5 MHz, CDCl3): 37.4 (CH), 110.7 (quinone-C3), 116.7–117.6 (m, fluoroaryl-C2, C5), 122.0 (fluoroaryl-C6), 124.3, 126.4, 126.5, 126.7, 127.3, 129.4, 132.7, 133.3 (aryl-carbons), 135.3–135.6 (m, fluoroaryl-C1), 147.6 (fluoroaryl-C3), 154.3 (C−OH), 181.3 (CO), 184.1 (CO). MS: 472 (63) [M+⋅], 454 (55), 426 (72), 270 (100), 242 (22), 214 (36), 174 (86), 105 (86) 76 (52). HR-ESI-MS: calc. for C27H15O6F2, [M+H]+, 473.08312; found 473.08213.
2,2′-[(3,5-Difluorophenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1d). Compound 1d was prepared analogously to 1c from 2-hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), 3,5-difluorobenzaldehyde (71 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) in AcOH (8 mL). Yield: 90 mg (0.21 mmol, 42 %). Yellow solid. M.p. 116–118 °C. ATR-IR: 3327, 1649, 1624, 1594, 1459, 1365, 1337, 1300, 1266, 1213, 1158, 1115, 1045, 990, 919, 853, 795, 725, 686. 1H-NMR (300 MHz, CDCl3): 6.14 (1 H, s), 6.6–6.7 (1 H, m), 6.7–6.8 (2 H, m), 7.6–7.8 (4 H, m), 8.0–8.1 (4 H, m). 13C-NMR (75.5 MHz, CDCl3): 37.6 (CH), 101.8–102.5 (m, fluoroaryl-C4), 110.7 (quinone-C3), 111.1–111.5 (m, fluoroaryl-C2, C6), 121.4, 126.4, 126.7, 127.3, 129.4, 132.7, 133.1, 133.3, 135.3 (aryl carbons), 142.7–142.9 (m, fluoroaryl-C1), 154.5 (C−OH), 161.2–164.6 (m, fluoroaryl-C3, C5), 181.3 (CO), 184.0 (CO). MS: 472 (92) [M+⋅], 454 (69), 426 (100), 382 (23), 359 (29), 326 (18), 298 (31), 269 (31), 214 (23), 105 (62), 104 (61), 76 (38). HR-ESI-MS: calc. for C27H15O6F2, [M+H]+, 473.08312; found 473.08267.
2,2′-[(2,4-Difluorophenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1e). Compound 1e was prepared analogously to 1c from 2-hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), 2,4-difluorobenzaldehyde (71 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) in AcOH (8 mL). Yield: 138 mg (0.29 mmol, 58 %). Yellow solid. M.p. 169–170 °C. ATR-IR: 3336, 1647, 1593, 1502, 1461, 1428, 1364, 1338, 1297, 1274, 1258, 1211, 1161, 1139, 1090, 1044, 1012, 967, 938, 903, 848, 816, 798, 782, 722, 693, 679, 646, 608. 1H-NMR (300 MHz, CDCl3): 6.23 (1 H, s), 6.7–6.8 (2 H, m), 7.1–7.2 (1 H, m), 7.6–7.8 (4 H, m), 8.0–8.1 (4 H, m). 13C-NMR (75.5 MHz, CDCl3): 32.1 (CH), 103.1–103.6 (m, fluoroaryl-C3), 110.5–110.8 (m, fluoroaryl-C5, quinone-C3), 121.3, 126.3, 127.2, 129.3, 130.2–130.5 (m), 132.8, 133.1, 135.2 (aryl carbons), 154.0 (C−OH), 161.0–164.0 (m, fluoroaryl-C2, C4), 181.3 (CO), 183.8 (CO). MS: 472 (4) [M+⋅], 452 (100), 426 (53), 279 (27), 251 (32), 174 (26), 105 (60), 76 (48). HR-ESI-MS: calc. for C27H15O6F2, [M+H]+, 473.08312; found 473.08247.
2,2′-[(2,4,5-Trifluorophenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1f). Compound 1f was prepared analogously to 1c from 2-hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), 2,4,5-trifluorobenzaldehyde (80 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) in AcOH (8 mL). Yield: 130 mg (0.27 mmol, 54 %). Yellow solid. M.p. 167–169 °C. ATR-IR: 3347, 1647, 1593, 1513, 1461, 1424, 1364, 1334, 1276, 1261, 1212, 1188, 1151, 1098, 1043, 1011, 970, 938, 911, 870,3 840, 816, 796, 762, 723, 688, 658, 638. 1H-NMR (300 MHz, CDCl3): 6.20 (1 H, s), 6.8–6.9 (1 H, m), 7.0–7.1 (1 H, m), 7.6–7.8 (4 H, m), 8.0–8.1 (4 H, m). 13C-NMR (75.5 MHz, CDCl3): 32.0 (CH), 104.9–105.3 (m, fluoroaryl-C3), 117.8–118.1 (m, fluoroaryl-C6), 120.7 (fluoroaryl-C6), 126.4, 127.3, 129.2, 132.8, 133.2, 135.3 (aryl carbons), 148.0 (fluoroaryl-C5), 151.0 (fluoroaryl-C4), 153.9 (C−OH), 157.5 (fluoroaryl-C2), 181.2 (CO), 183.6 (CO). MS: 490 (9) [M+⋅], 470 (100), 444 (76), 298 (37), 288 (31), 269 (47), 104 (72), 76 (54). HR-ESI-MS: calc. for C27H14O6F3, [M+H]+, 491.07370; found: 491.07311.
2,2′-[(3,4,5-Trifluorophenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1g). Compound 1f was prepared analogously to 1c from 2-hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), 3,4,5-trifluorobenzaldehyde (80 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) in AcOH (8 mL). Yield: 120 mg (0.25 mmol, 50 %). Yellow solid. M.p. 116–117 °C. ATR-IR: 3318, 1647, 1620, 1593, 1527, 1460, 1444, 1363, 1338, 1260, 1213, 1159, 1040, 998, 971, 919, 879, 868, 852, 817, 797, 725, 700. 1H-NMR (300 MHz, CDCl3): 6.08 (1 H, s), 6.9–7.0 (2 H, m), 7.7–7.8 (4 H, m), 8.1–8.2 (4 H, m). 13C-NMR (75.5 MHz, CDCl3): 37.5 (CH), 110.7 (quinone-C3), 112.4–112.7 (m, fluoroaryl-C2, C6), 121.3, 126.4, 126.5, 126.7, 127.3, 129.3, 132.7, 133.1, 133.3, 135.3 (aryl carbons), 148.9 (fluoroaryl-C4), 152.5 (fluoroaryl-C3, C5), 154.3 (C−OH), 181.3 (CO), 183.9 (CO). MS: 490 (62) [M+⋅], 472 (51), 444 (100), 416 (11), 400 (14), 359 (27), 344 (12), 288 (26), 232 (14), 174 (20), 147 (12), 104 (38), 76 (21). HR-ESI-MS: calc. for C27H14O6F3, [M+H]+, 491.07370; found: 491.07262.
2,2′-[(Pentafluorophenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1h). Compound 1h was prepared analogously to 1c from 2-hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), pentafluorobenzaldehyde (98 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) in AcOH (6 mL). Yield: 160 mg (0.30 mmol, 61 %). Yellow solid. M.p. 110–111 °C. ATR-IR: 3327, 1648, 1594, 1522, 1499, 1364, 1339, 1275, 1213, 1117, 1045, 999, 977, 911, 785, 724, 699. 1H-NMR (300 MHz, CDCl3): 6.17 (1 H, s), 7.59 (2 H, s), 7.6–7.8 (4 H, m), 8.0–8.1 (4 H, m). 13C-NMR (75.5 MHz, CDCl3): 29.1 (CH), 113.8–114.2 (m, fluoroaryl-C1), 119.7, 126.4, 126.9, 127.2, 129.2, 132.7, 133.2, 135.4 (aryl carbons), 137.8–139.2 (m, fluoroaryl-C3, C5), 141.5–142.2 (m, fluoroaryl-C2, C6), 143.7–144.6 (m, fluoroaryl-C2, C6), 147.1–148.6 (m, fluoroaryl-C4), 153.4 (C−OH), 181.1 (CO), 183.4 (CO). MS: 526 (79) [M+⋅], 506 (66), 480 (100) 434 (20), 359 (36), 334 (64), 305 (51), 174 (38), 105 (78), 104 (79), 76 (47). HR-ESI-MS: calc. for C27H12O6F5, [M+H]+, 527.05486; found 527.05347.
2,2′-[(4-Hydroxy-3-methoxyphenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1i). Compound 1i was prepared analogously to 1c from 2-hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), vanillin (76 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) in AcOH (6 mL). Yield: 80 mg (0.17 mmol, 34 %). Yellow solid. M.p. 107–108 °C. ATR-IR: 3294, 1645, 1592, 1580, 1513, 1460, 1431, 1362, 1336, 1252, 1206, 1155, 1122, 1030, 972, 915, 867, 813, 796, 722. 1H-NMR (300 MHz, CDCl3): 3.79 (3 H, s), 5.52 (1 H, s), 6.7–6.8 (3 H, m), 7.6–7.8 (4 H, m), 7.9–8.1 (6 H, m). 13C-NMR (75.5 MHz, CDCl3): 37.6 (CH), 56.0 (MeO), 110.7 (quinone-C3), 111.3 (phenyl-C2), 114.1 (phenyl-C5), 121.1 (phenyl-C6), 122.9, 126.3, 126.5, 127.2, 129.6, 132.8, 133.2, 135.1 (aryl carbons), 144.6 (phenyl-C4), 146.5 (phenyl-C3), 154.5 (quinone-C−OH), 181.4 (CO), 184.6 (CO). MS: 482 (8) [M+⋅], 464 (5), 308 (45), 280 (100), 263 (14), 249 (27), 237 (19), 209 (12), 174 (73), 146 (16), 105 (64), 76 (27). HR-ESI-MS: calc. for C28H19O8, [M+H]+, 483.10744; found 483.10624.
2,2′-[(3-Hydroxy-4-methoxyphenyl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1j). Compound 1j was prepared analogously to 1c from 2-hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), isovanillin (76 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) in AcOH (6 mL). Yield: 80 mg (0.17 mmol, 34 %). Yellow solid. M.p. 114–116 °C. ATR-IR: 3334, 1646, 1592, 1579, 1510, 1460, 1441, 1362, 1336, 1259, 1211, 1128, 1045, 1023, 981, 916, 885, 868, 798, 740, 723, 692, 656. 1H-NMR (300 MHz, CDCl3): 3.83 (3 H, s), 5.53 (1 H, s), 6.14 (1 H, s), 6.3–6.4 (3 H, m), 7.6–7.8 (4 H, m), 8.0–8.1 (4 H, m). 13C-NMR (75.5 MHz, CDCl3): 37.0 (CH), 55.9 (MeO), 110.4 (quinone-C3), 114.6 (phenyl-C5), 119.6 (phenyl-C2), 122.7 (phenyl-C6), 126.3, 126.5, 127.2, 129.6, 131.0, 132.7, 133.2, 135.0 (aryl carbons), 145.5 (phenyl-C4), 154.8 (quinone-C−OH), 181.3 (CO), 184.7 (CO). MS: 482 (5) [M+⋅], 308 (47), 280 (100), 265 (22), 249 (27), 237 (25), 209 (17), 174 (87), 146 (23), 105 (97), 76 (42). HR-ESI-MS: calc. for C28H19O8, [M+H]+, 483.10744; found 483.10706.
2,2′-[(Furan-2-yl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1k). Compound 1k was prepared analogously to 1c from 2-hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), furfural (57 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) in AcOH (6 mL). Yield: 80 mg (0.19 mmol, 38 %). Yellow solid. M.p. 118–120 °C. ATR-IR: 3329, 1647, 1592, 1580, 1501, 1460, 1363, 1336, 1261, 1212, 1160, 1142, 1092, 1074, 1043, 1011, 970, 883, 869, 819, 797, 761, 722. 1H-NMR (300 MHz, CDCl3): 6.09 (1 H, d, J=3.3), 6.20 (1 H, s), 6.2–6.3 (1 H, m), 7.3–7.4 (1 H, m), 7.6–7.8 (4 H, m), 8.0–8.1 (4 H, m), 8.2–8.3 (2 H, br. s). 13C-NMR (75.5 MHz, CDCl3): 31.8 (CH), 107.2 (furyl-C3), 110.5 (quinone-C3), 120.7, 126.4, 127.2, 129.6, 132.6, 133.3, 135.0 (aryl carbons), 141.8 (furyl-C5), 150.5 (furyl-C2), 154.8 (C−OH), 181.2 (CO), 184.3 (CO). MS: 426 (43) [M+⋅], 224 (42), 174 (100), 146 (22), 105 (78), 77 (23). HR-ESI-MS: calc. for C25H15O7, [M+H]+, 427.08123; found 427.08009.
2,2′-[(Furan-3-yl)methylene]bis(3-hydroxynaphthalene-1,4-dione) (1l). Compound 1l was prepared analogously to 1c from 2-hydroxy-1,4-naphthoquinone (174 mg, 1.00 mmol), furyl-3-aldehyde (57 mg, 0.5 mmol) and a catalytic amount of β-alanine (15 mg) in AcOH (6 mL). Yield: 100 mg (0.24 mmol, 48 %). Yellow solid. M.p. 167–170 °C. ATR-IR: 3328, 1646, 1592, 1579, 1502, 1460, 1362, 1336, 1264, 1219, 1158, 1071, 1044, 1024, 967, 920, 891, 872, 793, 756, 739, 722, 682, 658. 1H-NMR (300 MHz, CDCl3): 6.0–6.1 (1 H, m), 6.3–6.4 (1 H, m), 7.3–7.4 (1 H, m), 7.6–7.8 (4 H, m), 8.0–8.1 (4 H, m). 13C-NMR (75.5 MHz, CDCl3): 28.9 (CH), 111.5 (quinone-C3), 121.9, 122.2, 126.4, 127.1, 129.6, 132.6, 133.3, 135.0 (aryl carbons), 140.2 (furyl-C2), 143.0 (furyl-C5), 154.9 (C−OH), 181.2 (CO), 184.8 (CO). MS: 426 (14) [M+⋅], 252 (21), 224 (66), 196 (57), 174 (100), 146 (24), 139 (27), 105 (96), 76 (42). HR-ESI-MS: calc. for C25H15O7, [M+H]+, 427.08123; found 427.08056.
Toxoplasma gondii Culture Conditions
Serial passages of the cell lines Vero (ATCC® CCL81™, USA) were used for the cultivation of T. gondii tachyzoites of the RH strains (a gift from Dr. Saeed El-Ashram, State Key Laboratory for Agrobiotechnology, China Agricultural University, Beijing, China). Vero cells were cultured by using RPMI 1640 medium (Sigma, USA) with heat-inactivated 10 % fetal bovine serum (FBS, Invitrogen, USA) in a humidified 5 % CO2 atmosphere at 37 °C. 96-Well plates (5×103 cells/well in 200 μL RPMI 1640 medium) were used for the cultivation of the Vero cells and then incubated at 37 °C and 5 % CO2 for one day, followed by removal of medium and washing the cells with phosphate buffered saline (PBS). Then, RPMI 1640 medium with 2 % FBS containing tachyzoites (RH strains) of T. gondii at a ratio of 5 (parasite): 1 (Vero cells) was added. After incubation at 37 °C and 5 % CO2 for 5 h, cells were washed with PBS and then overlaid with medium containing tested compounds or atovaquone (50, 25, 12.5, 6.25, 3.13, 1.65, and 0.75 μg mL−1).
After incubation at 37 °C and 5 % CO2 for 72 h, the cells were stained with 1 % toluidine blue after washing with PBS and fixation in 10 % formalin. The cells were examined under an inverted photomicroscope (MCD-400, Leica, Japan) to determine the infection index (number of infected cells from 200 tested cells) of T. gondii.13, 14
Leishmania major Promastigotes and Amastigotes
Promastigotes of L. major were isolated from a Saudi male patient in February 2016 and maintained at 26 °C in Schneider's Drosophila medium (Invitrogen, USA) supplemented with 10 % heat inactivated FBS (Invitrogen, USA) and antibiotics in a tissue culture flask with weekly transfers. Promastigotes were cryopreserved in liquid nitrogen at concentrations of 3×106 parasite/ml. The virulence of L. major parasites was maintained by passing in female BALB/c mice by injecting hind footpads with 1×106 stationary-phase promastigotes. After 8 weeks, L. major amastigotes were isolated from mice. Isolated amastigotes were transformed to promastigote forms by culturing at 26 °C in Schneider's medium supplemented with 10 % FBS and antibiotics. For infection, amastigote-derived promastigotes with less than five in vitro passages were used. Male and female BALB/c mice were obtained from Pharmaceutical College, King Saud University, Kingdom of Saudi Arabia, and maintained in specific pathogen-free facilities.
In order to evaluate the activity of tested compounds against L. major promastigotes, promastigotes from logarithmic-phase cultured in phenol red-free RPMI 1640 medium (Invitrogen, USA) with 10 % FBS were suspended on 96-wells plates to yield 106 cells mL−1 (200 μL/well) after hemocytometer counting. Compounds were added to obtain the final concentrations (50, 25, 12.5, 6.25, 3.13, 1.65, and 0.75 μg mL−1). Negative control wells containing cultures with DMSO (1 %) and without compound and positive control wells containing cultures with decreasing concentration of amphotericin B (reference compound, 50, 25, 12.5, 6.25, 3.13, 1.65, and 0.75 μg mL−1) were used. Plates were incubated at 26 °C for 72 h to evaluate the antiproliferative effect. The number of viable promastigotes were assessed by colorimetric method using the tetrazolium salt colorimetric assay (MTT). It measures the reduction of the MTT component into an insoluble formazan product. This colored product was solubilized by adding detergent solution to lyse the cells. The samples were analyzed by using a microplate absorbance spectrophotometer (xMark, Bio-Rad, USA) at 570 nm.14
In order to evaluate the activity of tested compounds against amastigotes in macrophages, peritoneal macrophages from female BALB/c mice (6–8 weeks of age) were collected by aspiration, then 5×104 cells/well were seeded on 96-wells plates in RPMI 1640 medium with 10 % FBS for 4 h at 37 °C in 5 % CO2 atmosphere to promote cell adhesion. Medium was discarded and washed with PBS. 200 μL of solution containing L. major promastigotes (at a ratio of 10 promastigotes: 1 macrophage in RPMI 1640 medium with 10 % FBS) were added per well. Plates were incubated for 24 h at 37 °C in humidified 5 % CO2 atmosphere to allow infection and amastigote differentiation. Then, the infected macrophages were washed three times with PBS to remove the free promastigotes and overlaid with fresh RPMI 1640 medium containing compounds at final concentrations (50, 25, 12.5, 6.25, 3.13, 1.65, and 0.75 μg mL−1) and cells were incubated at 37 °C in humidified 5 % CO2 atmosphere for 72 h. Negative control containing cultures with DMSO (1 %) and without compounds and positive control wells containing cultures with decreasing concentration of amphotericin B (reference compound, 50, 25, 12.5, 6.25, 3.13, 1.65, and 0.75 μg mL−1) were used. The percentage of infected macrophages was evaluated microscopically after removing medium, washing, fixation, Giemsa staining.14
Trypanosoma Cell Line and Culture Conditions
Cells of the T. b. brucei bloodstream trypomastigote cell lines Lister 427 were maintained in HMI-9 medium, pH 7.5, supplemented with 10 % FBS in a humidified 5 % CO2 atmosphere at 37 °C.15
Alamar Blue (AB) Assay
The AB assay was used to identify viable cells after treatment with drug candidates.16-19 This assay was based on the irreversible reaction of the blue dye resazurin and NADH to pink resofurin in intact cells. T. b. brucei cells (8000/well) were seeded on 96-well microplates, treated with the tested compounds (dissolved in DMSO) and incubated for 72 h (5 % CO2, 95 % humidity, 37 °C). 10 μL of the AB reagent (500 mm resazurin sodium salt in PBS) were added and incubated for further 4 h at 37 °C. The fluorescence (extinction at 544 nm, emission at 590 nm) was measured on an Omega Fluostar (BMG Labtech) fluorescence plate reader.
Cytotoxicity Assay
MTT assay was carried out for cytotoxicity evaluation of compounds. Briefly, Vero cells were cultured in 96-well plates (5×103 cells/well/200 μL) for 24 h in RPMI 1640 medium with 10 % FBS and 5 % CO2 at 37 °C. Cells were washed with PBS and treated with tested compounds for 72 h at varying concentrations (50, 25, 12.5, 6.25, 3.13, 1.65, and 0.75 μg mL−1) in medium with 10 % FBS. As negative control, cells were treated with medium only. Thereafter, the supernatant was removed and 50 μL RPMI 1640 medium containing 14 μL MTT (5 mg mL−1) were added and incubated for 4 h. After that, the supernatant was removed and 150 μL DMSO were added in order to dissolve the formazan. Microplate absorbance spectrophotometer was applied for colorimetric analysis (λ=540 nm). Cytotoxic effects were expressed by IC50 values (concentration that caused a 50 % reduction in viable cells).14, 20
Author Contribution Statement
B. B. prepared the tested compounds and wrote the article. I. S. N., T. K. and J. J. carried out the antiparasitic assays. W. S. K., K. E. and R. S. provided the material, supervised the work and proofread the article.
Acknowledgements
The authors gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research on the material support for this research under the number cosao-2018-1-14-s-3859 during the academic year 1439 AH/2018 AD.