Two New Dolabrane Diterpenes from the Chinese Mangrove Ceriops tagal
Shu-Jun Ni
College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorJun Li
State Key Laboratory Basis of Xinjiang Indigenous Medicinal Plants Resource Utilization, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Urumqi, 830011 P. R. China
Key Laboratory of Plant Resources and Chemistry of Arid Zone, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Urumqi, 830011 P. R. China
Search for more papers by this authorMin-Yi Li
College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorShu-Jun Ni
College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorJun Li
State Key Laboratory Basis of Xinjiang Indigenous Medicinal Plants Resource Utilization, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Urumqi, 830011 P. R. China
Key Laboratory of Plant Resources and Chemistry of Arid Zone, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Urumqi, 830011 P. R. China
Search for more papers by this authorMin-Yi Li
College of Pharmacy, Jinan University, Guangzhou, 510632 P. R. China
Search for more papers by this authorAbstract
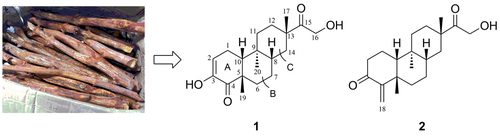
Two new dolabrane diterpenes, tagalenes J and K (1 and 2), together with eleven known analogues (3 – 13), were isolated from the ethanolic extract of the Chinese mangrove Ceriops tagal. The structures of these compounds were determined by extensive spectroscopic analysis, including 1D-, 2D-NMR and HR-ESI-MS, as well as the comparison with data in the literatures. Cytotoxicities of isolated compounds against MCF-7, SW480, HepG2, HeLa, PANC-1, and A2058 cancer cell lines were also evaluated. Compound 4 exhibited weak cytotoxic activity against SW480, HeLa, and PANC-1 cell lines with IC50 values of 27.7, 22.2, and 17.6 μm, respectively.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under https://doi.org/10.1002/cbdv.201700563.
| Filename | Description |
|---|---|
| cbdv201700563-sup-0001-SupInfo1.docxWord document, 3.5 MB | |
| cbdv201700563-sup-0002-SupInfo2.pdfPDF document, 2.5 MB |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. C. Fuentes, V. Castro, J. Jakupovic, R. Murillo, ‘Diterpenes and Other Components of Croton hirtus (Euphorbiaceae)’, Rev. Biol. Trop. 2004, 52, 269 – 285.
- 2L. Fang, A. Ito, H. B. Chai, Q. Mi, W. P. Jones, D. R. Madulid, M. B. Oliveros, Q. Gao, J. Orjala, N. R. Farnsworth, D. D. Soejarto, G. A. Cordell, S. M. Swanson, J. M. Pezzuto, A. D. Kinghorn, ‘Cytotoxic Constituents from the Stem Bark of Dichapetalum gelonioides Collected in the Philippines’, J. Nat. Prod. 2006, 69, 332 – 337.
- 3A. Kijjoa, ‘ Plant Secondary Metabolites with Immunomodulatory Activity’, in ‘ Natural Products in the New Millennium: Prospects and Industrial Application’, Springer Netherlands, 2002, pp. 299 – 309.
10.1007/978-94-015-9876-7_31 Google Scholar
- 4A. Kijjoa, M. A. Polónia, M. M. M. Pinto, T. Kitiratakarn, T. E. Gedris, W. Herz, ‘Dolabranes from Endospermum diadenum’, Phytochemistry 1994, 37, 197 – 200.
- 5A. Kijjoa, M. M. M. Pinto, C. Anantachok, T. E. Gedris, W. Herz, ‘Dolabranes from Endospermum diadenum’, Phytochemistry 1995, 40, 191 – 193.
- 6M. S. J. Nascimento, M. M. M. Pinto, L. M. Vieira, A. Kijjoa, A. M. S. Silva, B. H. Kroes, R. P. Labadie, ‘Anticomplementary Activity of Dolabranes from Endospermum diadenum’, Pharm. Pharmacol. Lett. 1998, 8, 93 – 96.
- 7S. M. Ansell, K. H. Pegel, D. A. H. Taylor, ‘Diterpenes from the Timber of Erythroxylum australe’, Phytochemistry 1993, 32, 937 – 943.
- 8S. M. Ansell, K. H. Pegel, D. A. H. Taylor, ‘Diterpenes from the Timber of 20 Erythroxylum Species’, Phytochemistry 1993, 32, 953 – 959.
- 9S. M. Ansell, K. H. Pegel, D. A. H. Taylor, ‘Diterpenes from the Timber of Erythroxylum pictum’, Phytochemistry 1993, 32, 945 – 952.
- 10M. S. Abdel-Kader, A. A. Omar, N. A. Abdel-Salam, F. R. Stermitz, ‘Erythroxan Diterpenes from Fagonia species’, Phytochemistry 1994, 36, 1431 – 1433.
- 11H. C. Krebs, H. Duddeck, S. Malik, W. Beil, P. Rasoanaivo, M. Andrianarijaona, ‘Chemical Composition and Antitumor Activities from Givotia madagascariensis’, ChemInform 2004, 35, 58 – 62.
10.1002/chin.200424160 Google Scholar
- 12F. Bohlmann, L. Hartono, J. Jakupovic, ‘A Diterpene Related to Erythroxydiol from Helichrysum refluxum’, Phytochemistry 1985, 24, 611 – 612.
- 13X.-F. Cheng, Z.-L. Chen, ‘Three New Diterpenoids from Mallotus apelta Muell.Arg’, J. Asian Nat. Prod. Res. 1999, 1, 319 – 325.
- 14M. H. Grace, Y. Jin, G. R. Wilson, R. M. Coates, ‘Structures, Biogenetic Relationships, and Cytotoxicity of Pimarane-derived Diterpenes from Petalostigma pubescens’, Phytochemistry 2006, 67, 1708 – 1715.
- 15Y. Kitahara, A. Yoshikoshi, ‘The Structure of Dolabradiene’, Tetrahedron Lett. 1964, 5, 1755 – 1761.
10.1016/S0040-4039(01)89504-6 Google Scholar
- 16J. Wu, Q. Xiao, J. Xu, M.-Y. Li, J.-Y. Pan, M.-H. Yang, ‘Natural Products from True Mangrove Flora: Source, Chemistry and Bioactivities’, Nat. Prod. Rep. 2008, 25, 955 – 981.
- 17Q. F. Guo, L. Chen, M. A. Jing-Wei, Y. Zhang, ‘Advances on Chemical Constituents and Pharmacological Activities of Ceriops genus’, Chin. J. Chin. Mater. Med. 2016, 15, 1 – 6.
- 18R. P. Rastogi, B. N. Mehrotra, S. Sinha, P. Pant, R. Seth, ‘ Compendium of Indian Medicinal Plants’, Central Drug Research Institute, Publications & Information Directrate, 1998.
- 19S.-J. Ni, J. Li, M.-Y. Li, ‘Two New Phenylpropanoids from the Chinese Mangrove Ceriops tagal’, Nat. Prod. Res. 2017, 1 – 6.
- 20Y. Peng, S.-J. Ni, J. Li, M.-Y. Li, ‘Three New Dolabrane Diterpenes from the Chinese Mangrove Plant of Ceriops tagal’, Phytochem. Lett. 2017, 21, 38 – 41.
- 21Y. Zhang, Z. Deng, T. Gao, P. Proksch, W. Lin, ‘Tagalsins A – H, Dolabrane-type Diterpenes from the Mangrove Plant, Ceriops tagal’, Phytochemistry 2005, 66, 1465 – 1471.
- 22Y. Chen, W.-J. Wang, J. Wu, ‘Two New Dolabranes from the Chinese Mangrove Ceriops tagal’, J. Asian Nat. Prod. Res. 2016, 18, 41 – 45.
- 23Y. Peng, M.-Y. Li, ‘Diterpenes from Hainan Mangrove, Ceriops tagal’, Nat. Prod. Res. Dev. 2016, 28, 1870 – 1874.
- 24W.-M. Hu, M.-Y. Li, J. Li, Q. Xiao, G. Feng, J. Wu, ‘Dolabranes from the Chinese Mangrove, Ceriops tagal’, J. Nat. Prod. 2010, 73, 1701 – 1705.
- 25X. Wu, H. Liao, H. Lu, C. Zhang, ‘A New Dolabrane Dinorditerpene from Ceriops tagal’, OALib 2016, 3, 1 – 6.