Synthesis and Antifungal Activities of Trichodermin Derivatives as Fungicides on Rice
Abstract
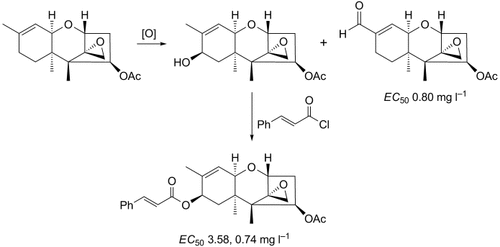
Twenty new trichodermin derivatives, 2a–5, containing alkoxy, acyloxy, and Br groups in 4-, 8-, 9-, 10- and 16-positions were synthesized and characterized. The antifungal activities of the new compounds against rice false smut (Ustilaginoidea virens), rice sheath blight (Rhizoctonia solani), and rice blast (Magnaporthe grisea) were evaluated. The results of bioassays indicated that the antifungal activities were particularly susceptible to changes at 4-, 8-, and 16-positions, but low to changes at 9- and 10-positions. Most of these target compounds exhibited good antifungal activities at the concentration of 50 mg l−1. Compound 4 (9-formyltrichodermin; EC50 0.80 mg l−1) with an CHO group at 9-position displayed nearly the same level of antifungal activity against Ustilaginoidea virens as the commercial fungicide prochloraz (EC50 0.82 mg l−1), while compound 3f ((8R)-8-{[(E)-3-phenylprop-2-enoyl]oxy}trichodermin; EC50 3.58 and 0.74 mg l−1) with a cinnamyloxy group at C(8) exhibited much higher antifungal activities against Rhizoctonia solani and Magnaporthe grisea than the commercial fungicides prochloraz (EC50 0.96 mg l−1) and propiconazole (EC50 5.92 mg l−1), respectively. These data reveal that compounds 3f and 4 possess high antifungal activities and may serve as lead compounds for the development of fungicides in the future.