Exploring the Role of Chirality in Nucleic Acid Recognition
Corresponding Author
Daniele D'Alonzo
Dipartimento di Chimica Organica e Biochimica, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo, via Cinthia, 4, I-80126 Napoli, (phone/fax: +39 081 674119)
Dipartimento di Chimica Organica e Biochimica, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo, via Cinthia, 4, I-80126 Napoli, (phone/fax: +39 081 674119)Search for more papers by this authorAnnalisa Guaragna
Dipartimento di Chimica Organica e Biochimica, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo, via Cinthia, 4, I-80126 Napoli, (phone/fax: +39 081 674119)
Search for more papers by this authorGiovanni Palumbo
Dipartimento di Chimica Organica e Biochimica, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo, via Cinthia, 4, I-80126 Napoli, (phone/fax: +39 081 674119)
Search for more papers by this authorCorresponding Author
Daniele D'Alonzo
Dipartimento di Chimica Organica e Biochimica, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo, via Cinthia, 4, I-80126 Napoli, (phone/fax: +39 081 674119)
Dipartimento di Chimica Organica e Biochimica, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo, via Cinthia, 4, I-80126 Napoli, (phone/fax: +39 081 674119)Search for more papers by this authorAnnalisa Guaragna
Dipartimento di Chimica Organica e Biochimica, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo, via Cinthia, 4, I-80126 Napoli, (phone/fax: +39 081 674119)
Search for more papers by this authorGiovanni Palumbo
Dipartimento di Chimica Organica e Biochimica, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo, via Cinthia, 4, I-80126 Napoli, (phone/fax: +39 081 674119)
Search for more papers by this authorAbstract
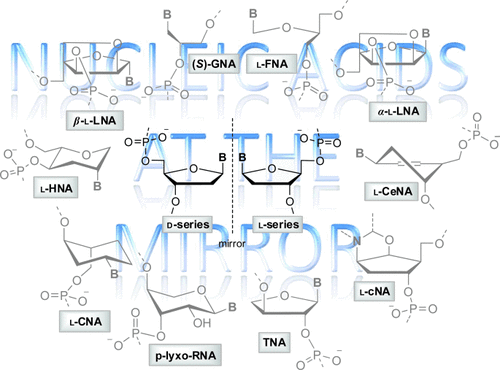
The study of the base-pairing properties of nucleic acids with sugar moieties in the backbone belonging to the L-series (β-L-DNA, β-L-RNA, and their analogs) are reviewed. The major structural factors underlying the formation of stable heterochiral complexes obtained by incorporation of modified nucleotides into natural duplexes, or by hybridization between homochiral strands of opposite sense of chirality are highlighted. In addition, the perspective use of L-nucleic acids as candidates for various therapeutic applications, or as tools for both synthetic biology and etiology-oriented investigations on the structure and stereochemistry of natural nucleic acids is discussed.
Graphical Abstract
References
- 1a C. F. Bennett, E. E. Swayze, Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259;
- 1b S. T. Crooke, ‘Antisense Drug Technology: Principles, Strategies, and Applications’, CRC Press, Boca Raton, 2008.
- 2 S. Buchini, C. J. Leumann, Curr. Opin. Chem. Biol. 2003, 7, 717.
- 3 M. Famulok, J. Med. Chem. 2009, 52, 6951; A. D. Keefe, S. Pai, A. Ellington, Nat. Rev. Drug Discovery 2010, 9, 537.
- 4 K. A. Whitehead, R. Langer, D. G. Anderson, Nat. Rev. Drug Discovery 2009, 8, 129; J. Kurreck, Angew. Chem., Int. Ed. 2009, 48, 1378.
- 5 R. Garzon, G. Marcucci, C. M. Croce, Nat. Rev. Drug Discovery 2010, 9, 775; M. Garofalo, C. M. Croce, Annu. Rev. Pharmacol. Toxicol. 2011, 51, 25.
- 6 M. Egli, P. S. Pallan, Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 281; M. Egli, P. S. Pallan, Chem. Biodiversity 2010, 7, 60.
- 7 D. H. Appella, Curr. Opin. Chem. Biol. 2009, 13, 687.
- 8 D. Loakes, P. Holliger, Mol. BioSyst. 2009, 5, 686.
- 9 J. Wengel, Org. Biomol. Chem. 2004, 2, 277.
- 10a A. Eschenmoser, Science 1999, 284, 2118;
- 10b A. Eschenmoser, Helv. Chim. Acta 2010, 93, 1439.
- 11 M.-O. Ebert, B. Jaun, Chem. Biodiversity 2010, 7, 2103.
- 12 P. Herdewijn, Chem. Biodiversity 2010, 7, 1.
- 13 B. Lebleu, H. M. Moulton, R. Abes, G. D. Ivanova, S. Abes, D. A. Stein, P. L. Iversen, A. A. Arzumanov, M. J. Gait, Adv. Drug Delivery Rev. 2008, 60, 517.
- 14 H. Kaur, B. R. Babu, S. Maiti, Chem. Rev. 2007, 107, 4672.
- 15 C. Zhou, J. Chattopadhyaya, Curr. Opin. Drug Discovery Dev. 2009, 12, 876.
- 16 C. J. Leumann, Chimia 2001, 55, 1041; C. J. Leumann, Chimia 2005, 59, 776.
- 17 S. Zhang, C. Switzer, J. C. Chaput, Chem. Biodiversity 2010, 7, 245.
- 18 E. Meggers, L. Zhang, Acc. Chem. Res. 2010, 43, 1092.
- 19 P. E. Nielsen, Chem. Biodiversity 2010, 7, 786.
- 20 C. Thibaudeau, P. Acharya, J. Chattopadhyaya, ‘Stereoelectronic Effects in Nucleosides and Nucleotides and Their Structural Implications’, Uppsala University Press, Uppsala, 2005.
- 21 C. J. Leumann, Bioorg. Med. Chem. 2002, 10, 841.
- 22 E. Lescrinier, M. Froeyen, P. Herdewijn, Nucleic Acids Res. 2003, 31, 2975.
- 23 H. Urata, J. Pharm. Soc. Jpn. 1999, 119, 689.
- 24 R. Corradini, S. Sforza, T. Tedeschi, R. Marchelli, Chirality 2007, 19, 269.
- 25 A. Holy, Collect. Czech. Chem. Commun. 1972, 37, 4072.
- 26 D. D'Alonzo, A. Guaragna, G. Palumbo, Curr. Org. Chem. 2009, 13, 71.
- 27 W. Saenger, ‘Principles of Nucleic Acid Structure’, Springer Verlag, New York, 1984; S. Neidle, ‘Principles of Nucleic Acid Structure’, Elsevier, Amsterdam, 2008.
- 28 P. Belmont, J.-F. Constant, M. Demeunynck, Chem. Soc. Rev. 2001, 30, 70.
- 29 A. Ramaswamy, M. Froeyen, P. Herdewijn, A. Ceulemans, J. Am. Chem. Soc. 2010, 132, 587, and refs. cit. therein.
- 30 M. Egli, P. Lubini, P. S. Pallan, Chem. Soc. Rev. 2007, 36, 31.
- 31 P. S. Pallan, P. Lubini, M. Egli, Chem. Commun. 2007, 1447.
- 32 I. Schloenvogt, S. Pitsch, C. Lesueur, A. Eschenmoser, B. Jaun, R. M. Wolf, Helv. Chim. Acta 1996, 79, 2316.
- 33 A. Rich, S. Zhang, Nat. Rev. Genet. 2003, 4, 566.
- 34 R. D. Wells, Trends Biochem. Sci. 2007, 32, 271; J. Zhao, A. Bacolla, G. Wang, K. M. Vasquez, Cell. Mol. Life Sci. 2010, 67, 43.
- 35a I. Tazawa, S. Tazawa, L. M. Stempel, P. O. P. Ts'o, Biochemistry 1970, 9, 3499;
- 35b D. J. Anderson, R. J. Reischer, A. J. Taylor, W. J. Wecher, Nucleosides Nucleotides 1984, 3, 499;
- 35c C. A. A. van Boeckel, G. M. Visser, R. A. Hegstrom, J. H. van Boom, J. Mol. Evol. 1987, 25, 100.
- 36 H. Urata, K. Shinohara, E. Ogura, Y. Ueda, M. Akagi, J. Am. Chem. Soc. 1991, 113, 8174.
- 37 M. Doi, M. Inoue, K. Tomoo, T. Ishida, Y. Ueda, M. Akagi, H. Urata, J. Am. Chem. Soc. 1993, 115, 10432.
- 38 S. Bolik, M. Rübhausen, S. Binder, B. Schulz, M. Perbandt, N. Genov, V. Erdmann, S. Klussmann, C. Betzel, RNA 2007, 13, 1877.
- 39 G. W. Ashley, J. Am. Chem. Soc. 1992, 114, 9731.
- 40 M. J. Damha, P. A. Giannaris, P. Marfey, Biochemistry 1994, 33, 7877.
- 41 Y. Hashimoto, N. Iwanami, S. Fujimori, K. Shudo, J. Am. Chem. Soc. 1993, 115, 9883.
- 42 H. Urata, E. Ogura, K. Shinohara, Y. Ueda, M. Akagi, Nucleic Acids Res. 1992, 20, 3325.
- 43 E. Moyroud, E. Biala, P. Strazewski, Tetrahedron 2000, 56, 1475.
- 44 M. J. Damha, P. A. Giannaris, P. Marfey, L. S. Reid, Tetrahedron Lett. 1991, 32, 2573.
- 45 S. Klussmann, A. Nolte, R. Bald, V. A. Erdmann, J. P. Fürste, Nat. Biotechnol. 1996, 14, 1112.
- 46 H. Urata, C. Tamaki, M. Matsuno, S. Wada, M. Akagi, Biochem. Biophys. Res. Commun. 2009, 390, 192.
- 47 S. Fujimori, K. Shudo, Y. Hashimoto, J. Am. Chem. Soc. 1990, 112, 7436.
- 48 A. Garbesi, M. L. Capobianco, F. P. Colonna, L. Tondelli, F. Arcamone, G. Manzini, C. W. Hilbers, J. M. E. Aelen, M. J. J. Blommers, Nucleic Acids Res. 1993, 21, 4159.
- 49 A. Garbesi, M. L. Capobianco, F. P. Colonna, M. Maflini, D. Niccoiai, L. Tondelli, Nucleosides Nucleotides 1998, 17, 1275.
- 50 H. Urata, Y. Ueda, H. Suhara, E. Nishioka, M. Akagi, J. Am. Chem. Soc. 1993, 115, 9852.
- 51 M. J. J. Blommers, L. Tondelli, A. Garbesi, Biochemistry 1994, 33, 7886.
- 52 H. Urata, Y. Ueda, M. Akagi, Viva Orig. 2004, 31, 233.
- 53 H. Urata, M. Akagi, Tetrahedron Lett. 1996, 37, 5551.
- 54 C. El Amri, O. Mauffret, F. Santamaria, G. Tevanian, B. Rayner, S. Fermandjian, J. Biomol. Struct. Dyn. 2001, 19, 459.
- 55 S. Vichier-Guerre, F. Santamaria, B. Rayner, Tetrahedron Lett. 2000, 41, 2101.
- 56 S. Vichier-Guerre, F. Morvan, G. Fulcrand, B. Rayner, Tetrahedron Lett. 1997, 38, 93.
- 57 I. Cherrak, O. Mauffret, F. Santamaria, A. Hocquet, M. Ghomi, B. Rayner, S. Fermandjian, Nucleic Acids Res. 2003, 31, 6986.
- 58 H. Urata, M. Go, N. Ohmoto, K. Minoura, M. Akagi, Chem. Commun. 2002, 544.
- 59 H. Urata, H. Hara, Y. Hirata, N. Ohmoto, M. Akagi, Tetrahedron: Asymmetry 2005, 16, 2908.
- 60 H. Urata, H. Shimizu, H. Hiroaki, D. Kohda, M. Akagi, Biochem. Biophys. Res. Commun. 2003, 309, 79.
- 61 H. Urata, H. Shimizu, M. Akagi, Nucleosides, Nucleotides Nucleic Acids 2006, 25, 359.
- 62 M. Egli, S. Portmann, N. Usman, Biochemistry 1996, 35, 8489.
- 63 H. Urata, H. Miyagoshi, H. Kakuya, H. Tokumoto, T. Kawahata, T. Otake, M. Akagi, Chem. Pharm. Bull. 1998, 46, 458; H. Urata, H. Miyagoshi, T. Yumoto, M. Akagi, J. Chem. Soc., Perkin Trans. 1 1999, 13, 1833.
- 64 G. S. Bisacchi, S. T. Chao, C. Bachard, J. P. Daris, S. Innaimo, G. A. Jacobs, O. Kocy, P. Lapointe, A. Martel, Z. Merchant, W. A. Slusarchyk, J. E. Sundeen, M. G. Young, R. Colonno, R. Zahler, Bioorg. Med. Chem. Lett. 1997, 7, 127.
- 65 H. Urata, H. Miyagoshi, T. Kumashiro, K. Mori, K. Shoji, M. Akagi, J. Am. Chem. Soc. 2001, 123, 4845.
- 66 H. R. Drew, R. M. Wing, T. Takano, C. Broka, S. Tanaka, K. Itakura, R. E. Dickerson, Proc. Natl. Acad. Sci. U.S.A. 1981, 78, 2179.
- 67 H. Urata, H. Miyagoshi, T. Kumashiro, T. Yumoto, K. Mori, K. Shoji, K. Gohda, M. Akagi, Org. Biomol. Chem. 2004, 2, 183.
- 68 S. Obika, S. M. A. Rahman, A. Fujisaka, Y. Kawada, T. Baba, T. Imanishi, Heterocycles 2010, 81, 1347.
- 69 J. Wengel, Acc. Chem. Res. 1999, 32, 301.
- 70 M. Meldgaard, J. Wengel, J. Chem. Soc., Perkin Trans. 1 2000, 3539.
- 71 L. Kværnø, J. Wengel, Chem. Commun. 2001, 1419.
- 72 S. Obika, D. Nanbu, Y. Hari, K. Morio, Y. In, T. Ishida, T. Imanishi, Tetrahedron Lett. 1997, 38, 8735; S. Obika, D. Nanbu, Y. Hari, J.-i. Andoh, K.-i. Morio, T. Doi, T. Imanishi, Tetrahedron Lett. 1998, 39, 5401.
- 73 S. K. Singh, P. Nielsen, A. A. Koshkin, J. Wengel, Chem. Commun. 1998, 455; A. A. Koshkin, S. K. Singh, P. Nielsen, V. K. Rajwanshi, R. Kumar, M. Meldgaard, C. E. Olsen, J. Wengel, Tetrahedron 1998, 54, 3607.
- 74 M. Petersen, J. Wengel, Trends Biotechnol. 2003, 21, 74.
- 75 B. Vester, J. Wengel, Biochemistry 2004, 43, 13233.
- 76 R. N. Veedu, J. Wengel, RNA Biol. 2009, 6, 321.
- 77 R. N. Veedu, J. Wengel, Chem. Biodiversity 2010, 7, 536.
- 78 N. K. Christensen, T. Bryld, M. D. Sørensen, K. Arar, J. Wengel, P. Nielsen, Chem. Commun. 2004, 282.
- 79 F. Morvan, F. Debart, J.-J. Vasseur, Chem. Biodiversity 2010, 7, 494.
- 80 W. H. Gmeiner, K. E. Rao, B. Rayner, J. J. Vasseur, F. Morvan, J. L. Imbach, J. W. Lown, Biochemistry 1990, 29, 10329.
- 81
P. Nielsen, N. K. Christensen, J. K. Dalskov, Chem.–Eur. J. 2002, 8, 712.
10.1002/1521-3765(20020201)8:3<712::AID-CHEM712>3.0.CO;2-0 CAS PubMed Web of Science® Google Scholar
- 82 V. K. Rajwanshi, A. E. Hakansson, R. Kumar, J. Wengel, Chem. Commun. 1999, 2073.
- 83 J. T. Nielsen, P. C. Stein, M. Petersen, Nucleic Acids Res. 2003, 31, 5858.
- 84 M. D. Sørensen, L. Kværnø, T. Bryld, A. E. Håkansson, B. Verbeure, G. Gaubert, P. Herdewijn, J. Wengel, J. Am. Chem. Soc. 2002, 124, 2164.
- 85 N. Kumar, K. E. Nielsen, S. Maiti, M. Petersen, J. Am. Chem. Soc. 2006, 128, 14.
- 86 B. Vester, L. B. Lundberg, M. D. Sørensen, B. R. Babu, S. Douthwaite, J. Wengel, J. Am. Chem. Soc. 2002, 124, 13682.
- 87
V. K. Rajwanshi, A. E. Håkansson, M. D. Sørensen, S. Pitsch, S. K. Singh, R. Kumar, P. Nielsen, J. Wengel, Angew. Chem., Int. Ed. 2000, 39, 1656.
10.1002/(SICI)1521-3773(20000502)39:9<1656::AID-ANIE1656>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 88 IUPAC-IUB Joint Commission on Biochemical Nomenclature, Pure Appl. Chem. 1983, 55, 1273.
- 89 M. Petersen, K. Bondensgaard, J. Wengel, J. P. Jacobsen, J. Am. Chem. Soc. 2002, 124, 5974.
- 90
K. M. E. Nielsen, M. Petersen, A. E. Håkansson, J. Wengel, J. P. Jacobsen, Chem.–Eur. J. 2002, 8, 3001.
10.1002/1521-3765(20020703)8:13<3001::AID-CHEM3001>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 91 M. Petersen, A. E. Håkansson, J. Wengel, J. P. Jacobsen, J. Am. Chem. Soc. 2001, 123, 7431.
- 92 M. Frieden, S. M. Christensen, N. D. Mikkelsen, C. Rosenbohm, C. A. Thrue, M. Westergaard, H. F. Hansen, H. Ørum, T. Koch, Nucleic Acids Res. 2003, 31, 6365.
- 93 B. R. Babu, Raunak, M. D. Sørensen, P. J. Hrdlicka, S. Trikha, A. K. Prasad, V. S. Parmar, J. Wengel, Pure Appl. Chem. 2005, 77, 319.
- 94 A. S. Madsen, P. J. Hrdlicka, T. S. Kumar, J. Wengel, Carbohydr. Res. 2006, 341, 1398.
- 95 Q. Li, F. Yuan, C. Zhou, O. Plashkevych, J. Chattopadhyaya, J. Org. Chem. 2010, 75, 6122.
- 96 T. S. Kumar, A. S. Madsen, J. Wengel, P. J. Hrdlicka, J. Org. Chem. 2006, 71, 4188.
- 97 Y. S. Latha, N. Yathindra, Biopolymers 1992, 32, 249.
- 98 L. Keinicke, M. D. Sørensen, J. Wengel, Bioorg. Med. Chem. Lett. 2002, 12, 593.
- 99 G. Gaubert, B. R. Babu, S. Vogel, T. Bryld, B. Vester, J. Wengel, Tetrahedron 2006, 62, 2278.
- 100 T. S. Kumar, M. E. Østergaard, P. K. Sharma, P. Nielsen, J. Wengel, P. J. Hrdlicka, Bioorg. Med. Chem. Lett. 2009, 19, 2396.
- 101 P. J. Hrdlicka, T. S. Kumar, J. Wengel, Chem. Commun. 2005, 4279.
- 102 T. S. Kumar, J. Wengel, P. J. Hrdlicka, ChemBioChem 2007, 8, 1122.
- 103 T. S. Kumar, A. S. Madsen, M. E. Østergaard, S. P. Sau, J. Wengel, P. J. Hrdlicka, J. Org. Chem. 2009, 74, 1070.
- 104 I. Van Daele, N. Bomholt, V. V. Filichev, S. Van Calenbergh, E. B. Pedersen, ChemBioChem 2008, 9, 791.
- 105 S. P. Sau, T. S. Kumar, P. J. Hrdlicka, Org. Biomol. Chem. 2010, 8, 2028.
- 106 A. Garbesi, F. Hamy, M. Maffini, G. Albrecht, T. Klimkait, Nucleic Acids Res. 1998, 26, 2886.
- 107 H. Urata, T. Kumashiro, T. Otake, T. Kawahata, M. Akagi, Nucleic Acids Res. Suppl. 2002, 2, 163; H. Urata, T. Kumashiro, T. Kawahata, T. Otake, M. Akagi, Biochem. Biophys. Res. Commun. 2004, 313, 55.
- 108 S. Negi, M. Dhanasekaran, T. Hirata, H. Urata, Y. Sugiura, Chirality 2006, 18, 254.
- 109 H. Urata, Y. Ueda, Y. Usami, M. Akagi, J. Am. Chem. Soc. 1993, 115, 7135.
- 110 C. Dose, D. Ho, H. E. Gaub, P. B. Dervan, C. H. Albrecht, Angew. Chem., Int. Ed. 2007, 46, 8384.
- 111 Y. Kim, C. J. Yang, W. Tan, Nucleic Acids Res. 2007, 35, 7279.
- 112 N. C. Hauser, R. Martinez, A. Jacob, S. Rupp, J. D. Hoheisel, S. Matysiak, Nucleic Acids Res. 2006, 34, 5101; G. Hayashi, M. Hagihara, A. Kobori, K. Nakatani, ChemBioChem 2007, 8, 169; G. Hayashi, M. Hagihara, K. Nakatani, J. Biotechnol. 2008, 135, 157.
- 113 C. Lin, Y. Ke, Z. Li, J. H. Wang, Y. Liu, H. Yan, Nano Lett. 2009, 9, 433.
- 114 D. Eulberg, S. Klussman, ChemBioChem 2003, 4, 979.
- 115 S. Klussmann, in ‘ Highlights in Bioorganic Chemistry: Methods and Applications’, Eds. C. Schmuck, H. Wennemers, Wiley-VCH, Weinheim, 2005, p. 248.
- 116 L. J. Sooter, A. D. Ellington, Chem. Biol. 2002, 9, 857.
- 117 S. A. Funke, D. Willbold, Mol. BioSyst. 2009, 5, 783.
- 118 R. C. Milton, S. C. Milton, S. B. Kent, Science 1992, 256, 1445; T. N. M. Schumacher, L. M. Mayr, D. L. Minor Jr., M. A. Milhollen, M. W. Burgess, P. S. Kim, Science 1996, 271, 1854.
- 119 C. Tuerk, L. Gold, Science 1990, 249, 505; D. L. Robertson, G. F. Joyce, Nature 1990, 344, 467; D. Ellington, J. W. Szostak, Nature 1990, 346, 818.
- 120 D. Eulberg, K. Buchner, C. Maasch, S. Klussmann, Nucleic Acids Res. 2005, 33, e45.
- 121 F. Jarosch, K. Buchner, S. Klussmann, Nucleic Acids Res. 2006, 34, e86.
- 122 A. Vater, S. Klussmann, Curr. Opin. Drug Discovery Dev. 2003, 6, 253.
- 123 H. Ulrich, Handb. Exp. Pharmacol. 2006, 173, 305.
- 124 A. Nolte, S. Klussmann, R. Bald, V. A. Erdmann, J. P. Fürste, Nat. Biotechnol. 1996, 14, 1116.
- 125 W. G. Purschke, F. Radtke, F. Kleinjung, S. Klussmann, Nucleic Acids Res. 2003, 31, 3027.
- 126 V. Ninichuk, S. Clauss, O. Kulkarni, H. Schmid, S. Segerer, E. Radomska, D. Eulberg, K. Buchner, N. Selve, S. Klussmann, H.-J. Anders, Am. J. Pathol. 2008, 172, 628.
- 127 S. Clauss, O. Gross, O. Kulkarni, A. Avila-Ferrufino, E. Radomska, S. Segerer, D. Eulberg, S. Klussmann, H.-J. Anders, J. Pathol. 2009, 218, 40; O. Kulkarni, D. Eulberg, N. Selve, S. Zöllner, R. Allam, R. D. Pawar, S. Pfeiffer, S. Segerer, S. Klussmann, H.-J. Anders, J. Pharmacol. Exp. Ther. 2009, 328, 371.
- 128 F. Ylera, R. Lurz, V. A. Erdmann, J. P. Fürste, Biochem. Biophys. Res. Commun. 2002, 290, 1583.
- 129 K. P. Williams, X.-H. Liu, T. N. M. Schumacher, H. Y. Lin, D. A. Ausiello, P. S. Kim, D. P. Bartel, Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 11285.
- 130 W. G. Purschke, D. Eulberg, K. Buchner, S. Vonhoff, S. Klussmann, Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 5173.
- 131 S. Leva, A. Lichte, J. Burmeister, P. Muhn, B. Jahnke, D. Fesser, J. Erfurth, P. Burgstaller, S. Klussmann, Chem. Biol. 2002, 9, 351.
- 132 B. Wlotzka, S. Leva, B. Eschgfäller, J. Burmeister, F. Kleinjung, C. Kaduk, P. Muhn, H. Hess-Stumpp, S. Klussmann, Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 8898.
- 133 S. Helmling, C. Maasch, D. Eulberg, K. Buchner, W. Schröder, C. Lange, S. Vonhoff, B. Wlotzka, M. H. Tschöp, S. Rosewicz, S. Klussmann, Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 13174; P. Kobelt, S. Helmling, A. Stengel, B. Wlotzka, V. Andresen, B. F. Klapp, B. Wiedenmann, S. Klussmann, H. Mönnikes, Gut 2006, 55, 788; C. Becskei, K. U. Bilik, S. Klussmann, F. Jarosch, T. A. Lutz, T. Riediger, J. Neuroendocrinol. 2008, 20, 85.
- 134 K. U. Bilik, E. Ergüven, S. Klussmann, F. Jarosch, P. Y. Wielinga, T. A. Lutz, T. Riediger, Neuroreport 2007, 18, 1855.
- 135 D. Faulhammer, B. Eschgfäller, S. Stark, P. Burgstaller, W. Englberger, J. Erfurth, F. Kleinjung, J. Rupp, S. Dan Vulcu, W. Schröder, S. Vonhoff, H. Nawrath, C. Gillen, S. Klussmann, RNA 2004, 10, 516.
- 136 A. Vater, F. Jarosch, K. Buchner, S. Klussmann, Nucleic Acids Res. 2003, 31, e130.
- 137 T. Denekas, M. Tröltzsch, A. Vater, S. Klussmann, K. Messlinger, Br. J. Pharmacol. 2006, 148, 536.
- 138 S. Helmling, E. Moyroud, W. Schroeder, I. Roehl, F. Kleinjung, S. Stark, G. Bahrenberg, C. Gillen, S. Klussmann, S. Vonhoff, Nucleosides, Nucleotides Nucleic Acids 2003, 22, 1035.
- 139 T. Schoetzau, J. Langner, E. Moyroud, I. Roehl, S. Vonhoff, S. Klussmann, Bioconjugate Chem. 2003, 14, 919.
- 140 K. Robeyns, P. Herdewijn, L. Van Meervelt, J. Am. Chem. Soc. 2008, 130, 1979.
- 141
D. D'Alonzo, A. Guaragna, A. Van Aerschot, P. Herdewijn, G. Palumbo, Tetrahedron Lett. 2008, 49, 6068.
10.1016/j.tetlet.2008.07.159 Google Scholar
- 142 C. Frauendorf, F. Hausch, I. Röhl, A. Lichte, S. Vonhoff, S. Klussmann, Nucleic Acids Res. 2003, 31, e34.
- 143 B. Kuhnast, S. Klussmann, F. Hinnen, R. Boisgard, B. Rousseau, J. P. Fürste, B. Tavitian, F. Dollé, J. Labelled Compd. Radiopharm. 2003, 46, 1205.
- 144 R. Boisgard, B. Kuhnast, S. Vonhoff, C. Younes, F. Hinnen, J.-M. Verbavatz, B. Rousseau, J. P. Fürste, B. Wlotzka, F. Dollé, S. Klussmann, B. Tavitian, Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 470.
- 145 J. Schlesinger, I. Koezle, R. Bergmann, S. Tamburini, C. Bolzati, F. Tisato, B. Noll, S. Klussmann, S. Vonhoff, F. Wuest, H.-J. Pietzsch, J. Steinbach, Bioconjugate Chem. 2008, 19, 928.
- 146 M. Klussmann, H. Iwamura, S. P. Mathew, D. H. Wells Jr., U. Pandya, A. Armstrong, D. G. Blackmond, Nature 2006, 441, 621; D. G. Blackmond, Cold Spring Harbor Perspect. Biol. 2010, 2, a002147.
- 147 J. R. Cronin, S. Pizzarello, Science 1997, 275, 951; S. Pizzarello, E. Shock, Cold Spring Harbor Perspect. Biol. 2010, 2, a002105.
- 148 S. Pizzarello, A. L. Weber, Science 2004, 303, 1151.
- 149 A. B. Northrup, D. W. C. MacMillan, Science 2004, 305, 1752.
- 150 R. Breslow, Z.-L. Cheng, Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 5723.
- 151 M. W. Powner, B. Gerland, J. D. Sutherland, Nature 2009, 459, 239; J. D. Sutherland, Cold Spring Harbor Perspect. Biol. 2010, 2, a005439.
- 152 L. E. Orgel, Nature 1992, 358, 203.
- 153 S. S. Mansy, J. P. Schrum, M. Krishnamurthy, S. Tobé, D. A. Treco, J. W. Szostak, Nature 2008, 454, 122.
- 154 C. Böhler, P. E. Nielsen, L. E. Orgel, Nature 1995, 376, 578.
- 155 J. P. Ferris, A. R. Hill Jr., R. Liu, L. E. Orgel, Nature 1996, 381, 59; W. Huang, J. P. Ferris, Chem. Commun. 2003, 1458.
- 156 W. Gilbert, Nature 1986, 319, 618.
- 157 G. F. Joyce, G. M. Visser, C. A. A. van Boeckel, J. H. van Boom, L. E. Orgel, J. van Westrenen, Nature 1984, 310, 602.
- 158 I. A. Kozlov, S. Pitsch, L. E. Orgel, Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 13448.
- 159 J. G. Schmidt, P. E. Nielsen, L. E. Orgel, J. Am. Chem. Soc. 1997, 119, 1494.
- 160 I. A. Kozlov, P. K. Politis, S. Pitsch, P. Herdewijn, L. E. Orgel, J. Am. Chem. Soc. 1999, 121, 1108.
- 161 P. C. Joshi, S. Pitsch, J. P. Ferris, Chem. Commun. 2000, 2497; P. C. Joshi, S. Pitsch, J. P. Ferris, Orig. Life Evol. Biosphere 2007, 37, 3; D. C. Mathew, Z. Luthey-Schulten, Orig. Life Evol. Biosphere 2010, 40, 303.
- 162 G. F. Joyce, Nature 1989, 338, 217.
- 163 H. Urata, R. Sasaki, H. Morita, M. Kusumoto, Y. Ogawa, K. Mitsuda, M. Akagi, Chem. Commun. 2005, 2578.
- 164 J. P. Schrum, T. F. Zhu, J. W. Szostak, Cold Spring Harbor Perspect. Biol. 2010, 2, a002212.
- 165 U. J. Meierhenrich, J.-J. Filippi, C. Meinert, P. Vierling, J. P. Dworkin, Angew. Chem., Int. Ed. 2010, 49, 3738.
- 166 Y. Brudno, D. R. Liu, Chem. Biol. 2009, 16, 265.
- 167 P. Herdewijn, P. Marlière, Chem. Biodiversity 2009, 6, 791.
- 168 A. W. Schwartz, L. E. Orgel, Science 1985, 228, 585.
- 169 H. Asanuma, T. Toda, K. Murayama, X. Liang, H. Kashida, J. Am. Chem. Soc. 2010, 132, 14702.
- 170 L. E. Orgel, Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99.
- 171 G. F. Joyce, A. W. Schwartz, S. L. Miller, L. E. Orgel, Proc. Natl. Acad. Sci. U.S.A. 1987, 84, 4398.
- 172 K. C. Schneider, S. A. Benner, J. Am. Chem. Soc. 1990, 112, 453.
- 173 Y. Merle, E. Bonneil, L. Merle, J. Sági, A. Szemzö, Int. J. Biol. Macromol. 1995, 17, 239.
- 174 J. Chaput, C. Switzer, J. Mol. Evol. 2000, 51, 464.
- 175 B. D. Heuberger, C. Switzer, J. Am. Chem. Soc. 2008, 130, 412.
- 176 K.-U. Schöning, P. Scholz, S. Guntha, X. Wu, R. Krishnamurthy, A. Eschenmoser, Science 2000, 290, 1347.
- 177 A. Eschenmoser, Orig. Life Evol. Biosphere 2004, 34, 277.
- 178 L. Zhang, A. Peritz, E. Meggers, J. Am. Chem. Soc. 2005, 127, 4174.
- 179 A. Eschenmoser, E. Loewenthal, Chem. Soc. Rev. 1992, 21, 1.
- 180 J. Kofoed, M. Machuqueiro, J.-L. Reymond, T. Darbre, Chem. Commun. 2004, 1540.
- 181 A. B. Northrup, I. K. Mangion, F. Hettche, D. W. C. MacMillan, Angew. Chem., Int. Ed. 2004, 43, 2152; A. Córdova, I. Ibrahem, J. Casas, H. Sundén, M. Engqvist, E. Reyes, Chem.–Eur. J. 2005, 11, 4772.
- 182 L. Burroughs, M. E. Vale, J. A. R. Gilks, H. Forintos, C. J. Hayes, P. A. Clarke, Chem. Commun. 2010, 46, 4776.
- 183 A. L. Weber, S. Pizzarello, Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 12713.
- 184 J. B. Lambert, S. A. Gurusamy-Thangavelu, K. Ma, Science 2010, 327, 984.
- 185 H.-J. Kim, S. A. Benner, Science 2010, 329, 902.
- 186 G. Cooper, N. Kimmich, W. Belisle, J. Sarinana, K. Brabham, L. Garrel, Nature 2001, 414, 879.
- 187
L. Orgel, Science 2000, 290, 1306;
P. Herdewijn, Angew. Chem., Int. Ed. 2001, 40, 2249;
10.1002/1521-3773(20010618)40:12<2249::AID-ANIE2249>3.0.CO;2-I CAS PubMed Web of Science® Google ScholarG. F. Joyce, Nature 2002, 418, 214.
- 188 K.-U. Schöning, P. Scholz, X. Wu, S. Guntha, G. Delgado, R. Krishnamurthy, A. Eschenmoser, Helv. Chim. Acta 2002, 85, 4111.
- 189 X. Wu, S. Guntha, M. Ferencic, R. Krishnamurthy, A. Eschenmoser, Org. Lett. 2002, 4, 1279; M. Ferencic, G. Reddy, X. Wu, S. Guntha, J. Nandy, R. Krishnamurthy, A. Eschenmoser, Chem. Biodiversity 2004, 1, 939.
- 190 M. K. Schlegel, A. E. Peritz, K. Kittigowittana, L. L. Zhang, E. Meggers, ChemBioChem 2007, 8, 927.
- 191 Y.-W. Yang, S. Zhang, E. O. McCullum, J. C. Chaput, J. Mol. Evol. 2007, 65, 289.
- 192 C. J. Wilds, Z. Wawrzak, R. Krishnamurthy, A. Eschenmoser, M. Egli, J. Am. Chem. Soc. 2002, 124, 13716.
- 193 P. S. Pallan, C. J. Wilds, Z. Wawrzak, R. Krishnamurthy, A. Eschenmoser, M. Egli, Angew. Chem., Int. Ed. 2003, 42, 5893.
- 194 M.-O. Ebert, C. Mang, R. Krishnamurthy, A. Eschenmoser, B. Jaun, J. Am. Chem. Soc. 2008, 130, 15105.
- 195 M. K. Schlegel, X. L. Xie, L. L. Zhang, E. Meggers, Angew. Chem., Int. Ed. 2009, 48, 960.
- 196 M. K. Schlegel, L.-O. Essen, E. Meggers, Chem. Commun. 2010, 46, 1094.
- 197 R. Declercq, A. Van Aerschot, R. J. Read, P. Herdewijn, L. Van Meervelt, J. Am. Chem. Soc. 2002, 124, 928.
- 198 M. Egli, P. S. Pallan, R. Pattanayek, C. J. Wilds, P. Lubini, G. Minasov, M. Dobler, C. J. Leumann, A. Eschenmoser, J. Am. Chem. Soc. 2006, 128, 10847.
- 199 P. S. Pallan, P. Lubini, M. Bolli, M. Egli, Nucleic Acids Res. 2007, 35, 6611.
- 200 B. D. Heuberger, C. Switzer, Org. Lett. 2006, 8, 5809.
- 201 J. C. Chaput, J. K. Ichida, J. W. Szostak, J. Am. Chem. Soc. 2003, 125, 856.
- 202 J. C. Chaput, J. W. Szostak, J. Am. Chem. Soc. 2003, 125, 9274; V. Kempeneers, K. Vastmans, J. Rozenski, P. Herdewijn, Nucleic Acids Res. 2003, 31, 6221; A. Horhota, K. Zou, J. K. Ichida, B. Yu, L. W. McLaughlin, J. W. Szostak, J. C. Chaput, J. Am. Chem. Soc. 2005, 127, 7427; J. K. Ichida, A. Horhota, K. Zou, L. W. McLaughlin, J. W. Szostak, Nucleic Acids Res. 2005, 33, 5219.
- 203 M. Renders, G. Emmerechts, J. Rozenski, M. Krecmerová, A. Holý, P. Herdewijn, Angew. Chem., Int. Ed. 2007, 46, 2501.
- 204 C.-H. Tsai, J. Chen, J. W. Szostak, Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 14598.
- 205 A. T. Horhota, J. W. Szostak, L. W. McLaughlin, Org. Lett. 2006, 8, 5345.
- 206 J. J. Chen, C.-H. Tsai, X. Cai, A. T. Horhota, L. W. McLaughlin, J. W. Szostak, PLoS ONE 2009, 4, e4949.
- 207 D. Zhou, I. M. Lagoja, J. Rozenski, R. Busson, A. Van Aerschot, P. Herdewijn, ChemBioChem 2005, 6, 2298; D. Zhou, M. Froeyen, J. Rozenski, A. Van Aerschot, P. Herdewijn, Chem. Biodiversity 2007, 4, 740.
- 208 J. J. Chen, X. Cai, J. W. Szostak, J. Am. Chem. Soc. 2009, 131, 2119.
- 209 P. Herdewijn, Biochim. Biophys. Acta, Gene Struct. Expr. 1999, 1489, 167.
- 210 L. Kerremans, G. Schepers, J. Rozenski, R. Busson, A. Van Aerschot, P. Herdewijn, Org. Lett. 2001, 3, 4129.
- 211 D. Sišak, L. B. McCusker, G. Zandomeneghi, B. Meier, D. Bläser, R. Boese, W. B. Schweizer, R. Gilmour, J. D. Dunitz, Angew. Chem., Int. Ed. 2010, 49, 4503.
- 212 A. Eschenmoser, Tetrahedron 2007, 63, 12821.
- 213 M. Beier, F. Reck, T. Wagner, R. Krishnamurthy, A. Eschenmoser, Science 1999, 283, 699.
- 214 N. Hall, Chem. Commun. 2004, 1247.
- 215 A. Eschenmoser, Chimia 2005, 59, 836.
- 216 S. Pitsch, S. Wendeborn, R. Krishnamurthy, A. Holzner, M. Minton, M. Bolli, C. Miculca, N. Windhab, R. Micura, M. Stanek, B. Jaun, A. Eschenmoser, Helv. Chim. Acta 2003, 86, 4270.
- 217
R. Micura, R. Kudick, S. Pitsch, A. Eschenmoser, Angew. Chem., Int. Ed. 1999, 38, 680.
10.1002/(SICI)1521-3773(19990301)38:5<680::AID-ANIE680>3.0.CO;2-C CAS Web of Science® Google Scholar
- 218 O. Jungmann, H. Wippo, M. Stanek, H. K. Huynh, R. Krishnamurthy, A. Eschenmoser, Org. Lett. 1999, 1, 1527.
- 219 M. Bolli, R. Micura, A. Eschenmoser, Chem. Biol. 1997, 4, 309.
- 220 H. Wippo, F. Reck, R. Kudick, M. Ramaseshan, G. Ceulemans, M. Bolli, R. Krishnamurthy, A. Eschenmoser, Bioorg. Med. Chem. 2001, 9, 2411.
- 221 E. Lescrinier, R. Esnouf, J. Schraml, R. Busson, H. A. Heus, C. W. Hilbers, P. Herdewijn, Chem. Biol. 2000, 7, 719.
- 222a A. Van Aerschot, I. Verheggen, C. Hendrix, P. Herdewijn, Angew. Chem., Int. Ed. 1995, 34, 1338;
- 222b C. Hendrix, H. Rosemeyer, I. Verheggen, F. Seela, A. Van Aerschot, P. Herdewijn, Chem.–Eur. J. 1997, 3, 110;
- 222c C. Hendrix, H. Rosemeyer, B. De Bouvere, A. Van Aerschot, F. Seela, P. Herdewijn, Chem.–Eur. J. 1997, 3, 1513.
- 223 I. A. Kozlov, P. K. Politis, A. Van Aerschot, R. Busson, P. Herdewijn, L. E. Orgel, J. Am. Chem. Soc. 1999, 121, 2653; I. A. Kozlov, B. De Bouvere, A. Van Aerschot, P. Herdewijn, L. E. Orgel, J. Am. Chem. Soc. 1999, 121, 5856.
- 224 P. Herdewijn, Antiviral Res. 2006, 71, 317.
- 225
B. Allart, K. Khan, H. Rosemeyer, G. Schepers, C. Hendrix, K. Rothenbacher, F. Seela, A. Van Aerschot, P. Herdewijn, Chem.–Eur. J. 1999, 5, 2424.
10.1002/(SICI)1521-3765(19990802)5:8<2424::AID-CHEM2424>3.0.CO;2-W CAS Web of Science® Google Scholar
- 226 J. Wang, B. Verbeure, I. Luyten, E. Lescrinier, M. Froeyen, C. Hendrix, H. Rosemeyer, F. Seela, A. Van Aerschot, P. Herdewijn, J. Am. Chem. Soc. 2000, 122, 8595.
- 227 P. Gu, G. Schepers, C. Griebel, J. Rozenski, H.-J. Gais, P. Herdewijn, A. Van Aerschot, Nucleosides, Nucleotides Nucleic Acids 2005, 24, 993.
- 228
Y. Maurinsh, H. Rosemeyer, R. Esnouf, A. Medvedovici, J. Wang, G. Ceulemans, E. Lescrinier, C. Hendrix, R. Busson, P. Sandra, F. Seela, A. Van Aerschot, P. Herdewijn, Chem.–Eur. J. 1999, 5, 2139.
10.1002/(SICI)1521-3765(19990702)5:7<2139::AID-CHEM2139>3.0.CO;2-K CAS Web of Science® Google Scholar
- 229 D. D'Alonzo, A. Van Aerschot, A. Guaragna, G. Palumbo, G. Schepers, S. Capone, J. Rozenski, P. Herdewijn, Chem.–Eur. J. 2009, 15, 10121.
- 230 I. Verheggen, A. Van Aerschot, S. Toppet, R. Snoeck, G. Janssens, J. Balzarini, E. De Clercq, P. Herdewijn, J. Med. Chem. 1993, 36, 2033.
- 231 M. Froeyen, F. Morvan, J.-J. Vasseur, P. Nielsen, A. Van Aerschot, H. Rosemeyer, P. Herdewijn, Chem. Biodiversity 2007, 4, 803.
- 232 R. Krishnamurthy, S. Pitsch, M. Minton, C. Miculka, N. Windhab, A. Eschenmoser, Angew. Chem., Int. Ed. 1996, 35, 1537.
- 233 D. D'Alonzo, A. Guaragna, A. Van Aerschot, P. Herdewijn, G. Palumbo, J. Org. Chem. 2010, 75, 6402.