Adverse Physical Health Outcomes and Healthcare Service Utilization in Siblings of Children With Cancer: A Systematic Review
Funding: H.C. is currently supported by funds from an Empowering Next-generation Researchers in Perinatal and Child Health (ENRICH) New Investigator Award, Rally Foundation for Childhood Cancer Research Young Investigator Award, Brain Tumor Foundation of Canada Feature Grant, Université de Montréal Faculty of Medicine Clinician–Scientist Award, Cole Foundation Transition Grant, and Fonds de Recherche du Québec—Santé.
ABSTRACT
Introduction
Siblings of children with cancer may be vulnerable to compromised long-term health. We aimed to describe the frequency (prevalence, incidence) of adverse physical health outcomes and healthcare service utilization among siblings of children with cancer and compare the risk of the above outcomes to siblings of children without cancer.
Methods
We searched Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, CINAHL, and Clarivate Web of Science through June 15, 2024. We included English and French-language studies, both with and without a healthy control population, that reported adverse physical health outcomes and/or healthcare service utilization outcomes among siblings of children with cancer. Studies focusing exclusively on mental health or quality of life were excluded. Abstracts were screened by two reviewers; full-text articles underwent data abstraction and risk of bias assessment. Results were synthesized descriptively.
Results
Of 26,570 studies screened, 44 were included. Heterogeneity was observed in all reported outcomes: mortality; cancer; organ system disease; overweight/obesity; pain; congenital anomalies; comorbidities; infections; amputations; adverse health behavior (smoking, alcohol consumption); infertility; healthcare service utilization (hospitalization, emergency department/urgent care visits, prescriptions). We detected a trend toward increased risk of cancer, hospitalizations, and prescription medication use compared to control siblings. Significant study heterogeneity rendered meta-analyses inappropriate.
Conclusions
Siblings of children with cancer are likely vulnerable to various adverse health outcomes. However, the published literature is widely heterogeneous regarding study design, populations, and outcomes measurements, limiting our comprehensive analysis of risk. Future research with homogenized methodology is needed to better quantify risk, which would inform targeted surveillance guidelines and interventions.
1 Introduction
1.1 Rationale
A childhood cancer diagnosis is a life-altering event for the entire family. Siblings of children with cancer are vulnerable to chronic stress [1], adverse physical responses such as pain and sleep impairment [2], and may be at increased risk of compromised long-term health and disproportionate healthcare utilization. Siblings of children with diverse chronic conditions have been reported to have an increased risk of adverse physical health diagnoses, healthcare service utilization, and prescription medication use [3, 4]. Given the life-threatening nature of a pediatric cancer diagnosis and the lengthy, intense treatment required for cure, siblings of children with cancer may also be at significant risk of adverse health outcomes. However, whether siblings of children with cancer experience disproportionately adverse health outcomes remains poorly understood.
1.2 Objectives
Extrapolating from the aforementioned work focused on siblings of children with chronic disease, we hypothesized that siblings of children with cancer are at an increased risk of adverse physical health outcomes and healthcare service utilization compared to siblings of children without cancer. We therefore conducted a systematic review of observational studies evaluating the frequency of adverse physical health conditions and healthcare service utilization among siblings of children with cancer, comparing their risks to siblings of healthy children when possible.
2 Methods
We undertook a systematic review and narrative synthesis evaluating the association between a child's cancer diagnosis and their sibling's physical health and healthcare service utilization. The protocol was established in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines [5] (Supplement) and was registered in the International Prospective Register of Systematic Reviews (CRD42023440951).
2.1 Search Strategy and Data Items
With the aid of a library scientist (PD), we searched Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, CINAHL, and Clarivate Web of Science through June 15, 2024 (search terms in Table S1). We identified additional studies by snowballing. We included English- and French-language observational studies, both with and without healthy control groups, that reported adverse physical health outcomes and/or healthcare service utilization among siblings of children with cancer. Specifically, outcomes included mortality, cancer, organ system-based adverse physical health outcomes, overweight/obesity, pain, congenital anomalies, comorbidities, infections, adverse health behaviors, and healthcare service utilization (Table 1).
| Categories | Included outcomes |
|---|---|
| Adverse physical health outcomes | |
| Mortality | All-cause mortality, cancer-related mortality |
| Cancer | SMN, history of cancer, history of solid tumors |
| Organ system-based adverse physical health outcomes | |
| Neurological | Neurological conditions or impairments, hospitalization for nervous system conditions, neurocognitive deficits, sensory neuropathy, hypoesthesia, balance disorders, dysphagia or chewing difficulties, anosmia or ageusia, speech disorders, epilepsy |
| Cardiac and cerebrovascular | Cardiac, cerebrovascular, and cardiopulmonary conditions or impairments, hospitalization for circulatory disease, cardiac disease, ischemic heart disease, CHF and cardiomyopathy, cardiovascular risk factors, hypertension, blood pressure and heart medications, arrhythmia, stroke |
| Ophthalmologic | Eye and visual conditions or impairments, hospitalization for eye and mastoid conditions, cataracts, eye movement disorders, dry eye syndrome |
| Auditory | Ear conditions or impairment, hearing loss, tinnitus |
| Endocrine | Endocrine conditions or impairments, hospitalization for endocrine disease, hyper- or hypothyroidism, thyroid nodules or tumor, diabetes |
| Pulmonary | Any pulmonary conditions or impairments, hospitalization for respiratory conditions, asthma, dyspnea on exertion, drugs for respiratory disease, lung fibrosis, chronic cough, chest wall abnormalities |
| Bone/musculoskeletal | Musculoskeletal conditions or impairments, hospitalization for musculoskeletal conditions, osteoporosis and osteopenia, major joint replacement, scoliosis |
| Renal | Renal and urinary tract conditions or impairments, renal failure, repeated cystitis, repeated nephritis |
| Hepatic | Hepatic conditions or impairments |
| Genitourinary | Genitourinary conditions or impairments, hospitalization for genitourinary conditions |
| Digestive/gastrointestinal | Digestive/gastrointestinal conditions, hospitalization for digestive conditions, chronic constipation or diarrhea, gastro-esophageal reflux disease, problems with esophagus, frequent nausea |
| Overweight/obesity | Overweight, obesity |
| Pain and other symptoms | Pain, abnormal sensations, fatigue syndromes and chronic fatigue, headaches and migraines, stomach ache, use of prescribed pain medications, prolonged pain in arms, legs, back, prolonged pain in bones and joints |
| Amputations | Limb amputations |
| Infertility | Decreased fertility, consultation with reproductive specialist, ART |
| Congenital anomalies | Congenital anomalies/birth defects |
| Comorbidities | Comorbidities, any chronic CHC, clinically relevant validated outcomes (2 to 4) |
| Infections | HCV positivity |
| Adverse health behaviors | Smoking, excessive alcohol consumption |
| Healthcare service utilization | |
| Healthcare service utilization | Hospitalization, ED/urgent care visits, prescriptions |
- Abbreviations: ART, assisted reproductive treatment; CHC, chronic health conditions; CHF, congestive heart failure; ED, emergency department; HCV, hepatitis C virus; SMN, second malignant neoplasm.
2.2 Eligibility Criteria
We excluded studies which focused exclusively on mental health or quality of life measures, review articles, gray literature, studies which only included children with cancer and/or extended family members (e.g., grandparents), and if the reported health outcomes were not clinical diagnoses (e.g., nondiagnostic bloodwork, continuous anthropometric measurements).
2.3 Selection Process and Data Collection
All identified references were transferred into Covidence [6]. Duplicated studies were removed before screening. Two reviewers (LPS, ES) screened titles and abstracts, followed by full-text articles. Authors were contacted up to two times when data were incomplete or unclear. Data from included studies were reviewed and extracted using a predetermined data extraction tool (Table S2). Sample extractions were tested and validated by HC. Two reviewers (LPS, VS) extracted data independently.
2.4 Study Risk of Bias Assessment
Two reviewers (LPS, VS) assessed the risk of bias using the Joanna Briggs Institute (JBI) checklist for prevalence studies [7]. Accordingly, studies scoring 3 or lower were defined as low risk, 4–5 as moderate risk, and 5–6 as high risk of bias. Discrepancy was reviewed and resolved by consensus by HC. In case of participants' overlap between studies (e.g., Childhood Cancer Survivor Study (CCSS) publications), the largest and most recent study was included in the analysis for each outcome. We used a narrative review to summarize and report results by outcome type. Outcomes with two or more measures of risk were summarized in a forest plot using the software RStudio [8]. Results reporting a 95% confidence interval (CI) that did not cross the neutral value 1 were considered statistically significant.
3 Results
A total of 51,026 records were identified in the literature search, and 357 studies underwent full-text review. Forty-four studies were included in our review (Figure S1) and a total of 84,637 siblings and 102,377 controls were included (Table 2). The studies spanned 12 countries on three continents. All included studies were retrospective, with the exception of one prospective study [9]. Three (7%) studies were retrospective cohort studies [12, 49, 51], four (9%) were case–controls [9-12], and 37 (84%) were population-based registry studies [10, 11, 13-47]. Thirty-four (77%) were questionnaire/survey/interview-based [9-12, 14-21, 24-30, 32-40, 42-46, 48] whereas the remaining studies reported medical record and/or insurance data. The results summarized by outcomes are presented in Table S3.
| Source | Study design | Period of referencea | Pathology of the index child | Source of data | No. of siblings enrolled | Age of siblings (years) | Outcomes | Control group |
|---|---|---|---|---|---|---|---|---|
| Armstrong et al., USA, Canada, 2013 | Population-basedb cohort | 1970–1986 | All childhood cancer | Self-report | 3159 | Median [range]: 36.0 [7.1–62.6] | Cardiovascular risk factors | No |
| Auger et al., Canada, 2022 | Population-basedc cohort | 2006–2019 | All childhood cancer | ICD-3, hospital discharge | 1600 | NA | Hospitalization | Yes |
| Baker et al., USA, 2010 | Retrospective cohort | 1974–1998 | ALL and AML treated with hematopoietic cell transplantation | Diagnosis by healthcare provider | 319 | Median: 44 | Organ system impairments | No |
| Belle et al., Switzerland, 2018 | Population-basedd cohort | 2007–2013 | Leukemia | Self-reported BMI | 819 | Range [15–45] | Overweight | Yes |
| Belle et al., Switzerland, 2018 | Population-basedd cohort | 1976–2005 | ALL, NHL, and HL | Self-report | 564 | NA | Overweight | Yes |
| Birch et al., England, 1990 | Population-basede cohort | 1954–1987 | STS (including RMS, fibrosarcoma and fibrous histiocytoma) | ICD-O, Self-report | 411 | Median: 22 | Cancer | No |
| Bouwman et al., the Netherlands, 2024 | Population-basedf, cross-sectional | 1963–2001 | All childhood cancer | Self-report, ICD-3 | 906 | Mean [SD]: 33.7 [9.8] | Overweight, obesity, smoking, limb amputation, comorbidities | Yes |
| Bowers et al., USA, Canada, 2005 | Population-basedb cohort | 1970–1986 | Pediatric HL | Self-report | 3846 | Mean [SD]: 28.8 [9.3] | Strokes | No |
| Buchbinder et al., USA, Canada, 2015 | Population-basedb cohort | 1970–1986 | CCSS-specific cancerh | Self-report | 1974 | Median [range]: 38 [31–44] | Tobacco use | Yes |
| Byrne et al., USA, 1995 | Population-basedg cohort | 1946–1962 | Retinoblastoma | Self-report | 84 | NA | Cancer, birth defects | No |
| Chow et al., USA, Canada, 2014 | Population-basedb cohort | 1970–1986 | CCSS-specific cancerh | Self-report, ICD-9, ICD-10 | 4023 | Median [range]: 34 [3–63] | Congestive heart failure | No |
| Chow et al., USA, Canada, 2017 | Population-basedb cohort | 1970–1986 | Childhood ALL | Self-report, ICD-9, ICD-10 | 4.023 | NA | Ischemic heart disease, stroke | No |
| Claessens et al., the Netherlands, 2024 | Population-basedf, cross-sectional | 1963–2001 | All childhood cancer | Self-report | 185 | NA | Reproductive health | No |
| Del Risco Kollerud et al., Norway, 2018 | Population-basedi cohort | 1960–2001 | All solid childhood tumors, except lymphomas | ICD-7, ICD-8, Medical Birth Registry of Norway | NA | Mean [SD]: 26 [12] | Cancer | No |
| Desai et al., Canada, 2021 | Population-basedj cohort | 1988–2016 | All childhood cancer | Health administrative data | 7591 | Median [IQR]: 5 [0–10] | CHC, healthcare use | Yes |
| Dixon et al., USA, Canada, 2020 | Population-basedb cohort | 1970–1999 | Childhood ALL | Self-report | 5051 | Median [range]: 35.9 [3.1–68.9] | CHC | No |
| Dixon et al., USA, Canada, 2022 | Population-basedb cohort | 1970–1999 | Childhood ALL | Self-report | 4693 | Median [range]: 36.7 [18.0–68.9] | Health status | No |
| Ehrhardt et al., USA, Canada, 2019 | Population-basedb cohort | 1970–1999 | NHL treated with the LMB regimen | Self-report | 1029 | Median [range]: 27.9 [0.3–52.3] | CHC | No |
| Essig et al., USA, Canada, 2014 | Population-basedb cohort | 1970–1986 | Childhood ALL | Self-report | 2232 | NA | CHC | No |
| Feudtner et al., USA, 2021 | Retrospective cohort | 2015–2016 | All childhood cancer | ICD-10-CM, health insurance claims | 2072 | Mean [SD]: 12.1 [6.5] | Healthcare encounters, diagnoses, prescriptions | Yes |
| Friedman et al., USA, Canada, 2005 | Population-basedb cohort | 1970–1986 | CCSS-specific cancerh | Self-report, oncologist review | 26,193 | Mean [SD]: 26.6 [10.2] | Cancer | Yes |
| Hartley et al., UK, 1991 | Population-basede cohort | 1954–1988 | Ewing's tumor | Self-report, records and registrations | 124 | Median: 27 | Cancer | No |
| Hayek et al., USA, Canada, 2020 | Population-basedb cohort | 1970–199 | CCSS-specific cancerh | Self-report | 2097 | Mean [SD]: 24.5 [8.4] | Frailty | No |
| Infante-Rivard et al., Canada, 2001 | Population-basedk case–control | 1980–1993 | Childhood ALL | Self-report, ICD-9 | 491 | Mean: 5.0; Median [SD]: 3.96 [3.7]; IQR: 3.98 | Congenital anomalies | Yes |
| Infante-Rivard et al., Canada, 2003 | Population-basedk case–control | 1980–1998 | Childhood ALL | Self-report, ICD-9 | 792 | NA | Hematopoietic malignancies | Yes |
| Landy et al., USA, Canada, 2013 | Population-basedb cohort | 1999–2003 | CCSS-specific cancerh | Hospital visits, body fat measures | 30 | NA | Overweight, obesity | No |
| Lown et al., USA, Canada, 2012 | Population-basedb cohort | 1970–1986 | All childhood cancer | Self-report | 3034 | Median [range]: 29 [18–56] | Risky and heavy drinking | Yes |
| Lu et al., USA, Canada, 2011 | Population-basedb cohort | 1970–1986 | CCSS-specific cancerh | Self-report | 3034 | NA | Pain conditions & prescriptions | No |
| Meacham et al., USA, Canada, 2009 | Population-basedb cohort | 1970–1986 | CCSS-specific cancerh | Self-report | 2936 | Mean [range]: 33.4 [9.6–58.4] | Diabetes mellitus | No |
| Molgaard-Hansen et al., Denmark, Finland, Iceland, Norway, Sweden, 2009 | Population-basedl cohort | 1984–2003 | Childhood AML | Self-report | 86 | NA | Healthcare encounters | No |
| Möttönen et al., Finland, 1995 | Prospective case–control | 1987–1989 | Childhood ALL | Self-report | 18 | Mean [range]: 10.6 [2.8–23.2] | Headache, stomach ache | Yes |
| Ng et al., USA, 2005 | Cross-sectional | 1969–1996 | Pediatric HL | FACIT-F, self-report | 224 | Median: 46 | Fatigue | No |
| Oeffinger et al., USA, Canada, 2003 | Population-basedb cohort | 1970–1986 | Childhood ALL | Self-reported BMI | 2516 | Mean [SD]: 29.0 [7.3] | Overweight, obesity | No |
| Oeffinger et al., USA, Canada, 2006 | Population-basedb cohort | 1970–1986 | CCSS-specific cancerh | Self-report | 3034 | Mean [range]: 29.2 [18.0–56.0] | CHC | No |
| Ou et al., USA, 2017 | Retrospective cohort | 1998–2013 | Childhood ALL | EDW, ICD-9 | 2032 | Mean [range]: 11.3 [5.1–24.3] | Hospitalization | Yes |
| Penson et al., the Netherlands, 2023 | Population-basedf cross-sectional | 1963–2001 | All childhood cancer | Self-report | 449 | Median [SD]: 36.8 [10.2] | Chronic fatigue | No |
| Rueegg et al., Switzerland, 2012 | Population-basedd cohort | 1976–2003 | All childhood cancer | Self-report | 534 | NA | Medical conditions limiting sports | No |
| Sherief et al., Egypt, 2019 | Cross-sectional | 2016–2018 | All childhood cancer | Blood sample, PCR | 274 | NA | HCV infection | No |
| Sláma et al., Switzerland, 2024 | Population-basedd cohort | 1976–2015 | Childhood LCH | Self-report | 999 | Median [IQR]: 25 [18–32] | CHC | No |
| Streefkerk et al., the Netherlands, 2024 | Population-basedf cohort | 1963–2001 | All childhood cancer | Self-report | 1066 | Median [range]: 31.9 [24.5–39.4] | Organ system impairments | No |
| Tacyildiz et al., Turkey, 2024 | Retrospective case–control | 1998–2019 | Pediatric HL | Self-report, review of patient charts | 56 |
Median: 19.81 ± 5.85 |
Organ system disease, health care utilization | No |
| Van der Plas et al., USA, Canada, 2024 | Population-basedb cross-sectional | 1970–1999 | Pediatric rhabdomyosarcoma | Self-report | 706 | Median [IQR]: 32 [27–38] | Pain, health perceptions | No |
| Winther et al., Denmark, Finland, Iceland, Norway, Sweden, 2001 | Population-basedm cohort | 1943–1993 | All childhood cancer | ICD-7 | 42,277 | NA | Cancer | No |
| Yu et al., Denmark, Sweden, 2017 | Population-basedn cohort | 1973–2008 | All childhood cancer | Death registers, ICD-8, ICD-9, ICD-10 | NA | Median [IQR]: 7.0 [3.3–12.1] | Mortality | Yes |
- Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMI, Body mass index; CCSS, Childhood cancer survivor study; CHC, chronic health conditions; CNS, central nervous system; EDW, enterprise data warehouse (electronic medical records); FACIT-F, Functional assessment of chronic illness therapy – Fatigue; HCV, Hepatitis C virus; HL, Hodgkin lymphoma; ICD, International classification of disease; IQR, interquartile range; LCH, Langerhans cell histiocytosis; LMB, Burkitt lymphoma; NA, data not available; NHL, non-Hodgkin lymphoma; PCR, polymerase chain reaction; RMS, rhabdomyosarcoma; SD, standard deviation; STS, soft tissue sarcoma.
- a Refers to the child's cancer diagnosis.
- b Childhood Cancer Survivor Study (CCSS).
- c Maintenance and Use of Data for the Study of Hospital Clientele data set, Quebec, Canada.
- d Swiss Childhood Cancer Survivor Study (SCCSS).
- e Manchester Children's Tumor Registry.
- f Dutch Childhood Cancer Survivor Study-Late Effects After Childhood Cancer (DCCSS-LATER).
- g National Cancer Institute and Five-Center Study (the Connecticut Tumor Registry, a group of hospitals in California, the Universities of Iowa and Kansas, and MD Anderson Cancer Center, Houston).
- h CCSS-specific cancers include leukemia, CNS tumors, HL, NHL, renal tumors, neuroblastomas, STS, bone tumors.
- i Norwegian Family Based Life Course Study (the Cancer Registry, the Medical Birth Registry, Statistics Norway).
- j Pediatric Oncology Group of Ontario's Networked Information System (POGONIS).
- k Tertiary care centers designated by government policy to treat and hospitalize children with cancer in the province of Québec, Canada.
- l Nordic Society of Pediatric Hematology and Oncology (NOPHO)-AML-84, -88, and -93 trials.
- m National central population registries—cancer registration.
- n Linked national registers in Denmark and Sweden.
Fourteen (32%) studies included a control group: four studies [9, 10, 13, 41] included data from siblings of children without cancer; nine studies [3, 11, 14, 17, 23, 28, 42, 43, 49] included normative or population data; and one study included both [15]. Eighteen (41%) publications included overlapping sibling cohorts from the CCSS [18, 20, 24-28, 30-33, 35, 40, 42-45, 50]. Four (9%) publications were based on the Swiss Childhood Cancer Survivor Study (SCCSS) [14, 15, 37, 38] and four (9%) were based on the Dutch Childhood Cancer Survivor Study—Late effects after childhood cancer (DCCS-LATER) [6, 17, 21, 36, 39]. The representative publication chosen for each outcome is identified in Table S3. Overall, 13 (30%) studies [3, 9, 10, 13, 16, 23, 28, 29, 41-43, 47] were focused on siblings or family members of children with cancer as the study population, whereas 29 (66%) studies [11, 12, 17-22, 24-27, 30-40, 44-46, 48, 49, 51] focused on children with cancer as the study population and siblings were treated as their control group.
The risk of bias assessment resulted in 38 (86%) studies [3, 10, 11, 13-18, 20-28, 30-33, 35-44, 46-49, 51, 52] rated as low risk, five (11%) studies [12, 19, 29, 34, 45] as moderate risk, and one (2%) study [9] at high risk (Table S4).
3.1 Mortality
Two studies [23, 41] focused on mortality among siblings of children with cancer. One study [23] reported no significant difference in all-cause mortality incidence between siblings and controls (hazard ratio (HR), 0.73; 95% CI, 0.44–1.3) [23]. The second study [41] reported a greater risk of cancer-related mortality compared to siblings of healthy children (mortality rate ratio, 2.83; 95% CI, 1.41–5.67) [41].
3.2 Cancer
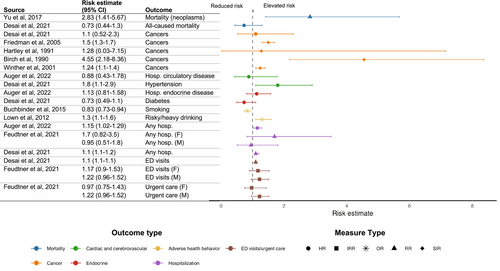
Of the 13 studies focused on cancer development among siblings of children with cancer, seven [11, 12, 19, 27, 30, 39, 51] reported measures of frequency and six [16, 22, 23, 28, 29, 47] reported measures of risk. Prevalence of cancer in siblings ranged from 0% (0/56) [12] to 5% (3/60) [19]. Compared to controls, two studies found no statistically significant risk of cancer (HR, 1.1; 95% CI, 0.52–2.3 [23] and standardized incidence ratio (SIR), 1.28; 95% CI, 0.03–7.15 [29]). In contrast, three studies reported an increased cancer risk in siblings compared to controls (SIR, 1.5; 95% CI, 1.3–1.7 [28], SIR, 4.55; 95% CI, 2.18–8.36 [16], and SIR, 1.24; 95% CI, 1.1–1.4 [47] respectively) (Figure 1). A fourth study [22] reported an increased risk of both retinoblastoma (HR, 6.11; 95% CI, 3.09–12.05) and hepatoblastoma (HR, 5.85; 95% CI, 1.70–20.18) in siblings of children with solid tumors compared to controls. After excluding for probable hereditary cancer syndromes, the risk of hepatoblastoma remained significantly elevated (HR, 1.73; 95% CI, 1.07–2.78), whereas the risk of retinoblastoma was still increased, yet not statistically significant (HR, 2.33; 95% CI, 0.88–6.46) [22].
3.3 Chronic Organ System Diseases
3.3.1 Neurological
Eight studies [13, 26, 27, 30, 37-39, 51] evaluated neurological outcomes in siblings. Prevalence of neurological impairment was reported in seven studies [26, 27, 30, 37-39, 51] ranging from 0.3% (2/534) [37] to 24.5% (78/319) [51]. There was an increased risk of hospitalization for nervous system conditions in siblings compared to controls (HR, 1.57; 95% CI, 1.05–2.35) [13]. Detailed neurological outcomes are reported in Table S3.
3.3.2 Cardiac and Cerebrovascular
Overall, 16 studies [12, 13, 18, 20, 23, 25-27, 30, 34, 37-39, 45, 48, 51] reported data on cardiac and cerebrovascular outcomes. Fourteen studies [6, 12, 18, 20, 25-27, 30, 34, 37, 38, 45, 48, 51] reported measures of frequency of cardiac outcomes.
3.3.3 Hypertension
The prevalence of hypertension in siblings of children with cancer ranged from 3% (raw data not available) [38] to 19.1% (61/319) [51]. One study showed that, compared to controls, siblings were at increased risk of hypertension (HR, 1.8; 95% CI, 1.1–2.9) [23].
3.3.4 Stroke
The prevalence of stroke in siblings of children with cancer ranged from 0% (0/999) [38] to 0.5% (27/5051) [25]. One study reported cumulative incidence for stroke (cumulative incidence, 1.1%; 95% CI, 0.4%–1.7%) [45].
3.3.5 Cardiac Disease
The prevalence of congestive heart failure (CHF) ranged from 0% (0/999) [38] to 0.3% (1/319) [20, 25, 51]. Two studies reported measures of cumulative incidence for ischemic heart disease (cumulative incidence, 1.2%; 95% CI, 0.4–1.7) [45] and congestive heart failure (cumulative incidence, 0.3%; 95% CI, 0.1%–0.5%) [20], respectively. One study [13] assessed the hospitalization rate for circulatory disease, reporting no excess risk (HR, 0.88; 95% CI, 0.43–1.78).
3.3.6 Endocrine
Fourteen studies [11-13, 23, 25-27, 30, 33, 38, 39, 44, 48, 51] reported data on endocrine outcomes in siblings of children with cancer. The prevalence of diabetes ranged from 1% (raw data not available) [38] to 3.1% (10/319 and 21/792) [11, 51]. No significant increased risk of diabetes was reported for siblings of children with cancer compared to matched controls (HR, 0.73; 95% CI, 0.49–1.1) in one study [23]. No significant risk of hospitalization for endocrine disease in siblings were reported in one study (HR, 1.13; 95% CI, 0.81–1.58) (Figure 1) [13]. Detailed endocrine outcomes are reported in Table S2.
3.3.7 Pulmonary
Eight studies [11-13, 30, 34, 38, 39, 50] evaluated pulmonary outcomes in siblings of children with cancer. The prevalence of asthma was 22.1% (153/792) in siblings compared to 18.5% (127/707) in matched controls [11]. There was an increased risk of hospitalization for respiratory disease (HR, 1.18; 95% CI, 1.01–1.40) [13] associated with having a sibling with cancer compared to controls.
3.4 Overweight/Obesity
Overall, six studies [15, 17, 31, 33, 35, 38] evaluated overweight status in siblings and all reported prevalence which ranged from 20% (149/725) [15] to 31.1% (913/2936) [33]. Two studies [15, 17] reported data from controls (20% (149/725) in siblings vs. 24% (2285/9591) in controls [15], and 27.4% (raw data not available) in siblings vs. 28.4% (raw data not available) in controls [17]). Ten studies [15, 17, 25-27, 30, 31, 33, 35, 44] reported the prevalence of obesity among siblings of children with cancer, ranging from 4% (34/725) [15] to 28.5% (593/2097) [30]. Two studies reported data from matched controls (4% (34/725) vs. 6% (603/9591) for controls [15], and 9.4% (raw data not available) vs. 9.3% (raw data not available) for controls [17], relative measures not provided). Among the studies which reported frequency measures of overweight/obesity in siblings of children with cancer and controls, no study included a measure of risk.
3.5 Healthcare Service Utilization
3.5.1 Hospitalizations and Emergency Department/Urgent Care Visits
Five studies [3, 13, 23, 34, 49] evaluated data on hospitalization in siblings of children diagnosed with cancer. In one study, the prevalence of any hospitalization among siblings was 40% (34/86) [34]. Another study reported a higher rate of hospitalization in siblings (rate per 100 person-years, 2.69; 95% CI, 1.01–24.3) compared to controls (rate per 100 person-years, 1.87; 95% CI, 1.13–3.09) [49]. Two studies [13, 23] found an increased risk of hospitalization associated with having a sibling with cancer (respectively RR, 1.15; 95% CI, 1.02–1.29 [13] and IRR, 1.1; 95% CI, 1.1–1.2 [23]) (Figure 1). The risk of hospitalization was significantly greater for nervous system (HR, 1.57; 95% CI, 1.05–2.35), respiratory (HR, 1.18; 95% CI, 1.01–1.40), digestive (HR, 1.38; 95% CI, 1.09–1.74), and skin (HR, 1.60; 95% CI, 1.13–2.28) disorders in siblings [13]. Children who had a sibling with cancer were at risk of hospitalization for conditions such as pneumonia, inflammatory bowel disease, and other morbidities [13]. One study [3] assessed sisters and brothers independently and found a trend toward increased risk of hospitalization (respectively IRR, 1.7; 95% CI, 0.82–3.5, and 0.95; 95% CI, 0.51–1.8) [3]. Sibling cases were also more likely than controls to receive preventive healthcare, such as undergoing periodic health checkups (OR, 1.1; 95% CI, 1.0–1.1) and receiving influenza vaccinations (OR, 1.5; 95% CI, 1.4–1.6) [23].
Two studies [3, 23] examined rates of emergency department (ED) and urgent care visits in siblings of children with cancer. One study reported that, compared to controls, siblings had higher rates of both low and high acuity ED visits (RR, 1.1; 95% CI, 1.1–1.2) [23]. Another study did not find a higher incidence in sisters and brothers separately (respectively IRR, 1.17; 95% CI, 0.9–1.53, and 1.22; 95% CI, 0.96–1.52) [3], compared to controls (Figure 1).
3.5.2 Prescriptions
Two studies [3, 34] reported prescription use in siblings of children with cancer. One study [34] showed a prevalence of medications of 9% (8/86) in siblings. Another study evaluated incidence rates of all prescriptions (expect mental health prescriptions) and an excessive rate of prescriptions was found in siblings, sisters, and brothers separately (respectively IRR, 1.97; 95% CI, 1.63–2.39, and 1.95; 95% CI, 1.61–2.35) compared to controls [3] (Figure 1).
Additional results are outlined in the online supplement (Appendix S1).
4 Discussion
We conducted the first comprehensive systematic review examining adverse physical health outcomes and healthcare service utilization in siblings of children diagnosed with pediatric cancer to elucidate the health vulnerabilities of this population. Our systematic review highlighted potential associations between sibling status and an increased prevalence of cancer, organ system impairment, and high healthcare service utilization. Specifically, in studies comparing siblings to controls, we found trends suggesting increased risks of cancer [28, 29], cancer-related mortality [41], hypertension [23], and excessive alcohol consumption [43]. Although some studies reported that siblings were at higher risk of hospitalizations [13, 23], ED visits [23], and prescription medication use [3], siblings were also more likely to seek preventive healthcare [23] compared to controls.
Prior work focused on siblings of children with chronic [4] and/or life-threatening [3] conditions reported increased risks of various adverse health outcomes (traumatic brain injury, overweightness, mortality, healthcare encounters, and medications) [3, 4], supporting our hypothesis that sibling status may confer an increased risk to compromised physical health. Our findings align with prior research that documented an elevated risk of adverse health behaviors and cardiovascular disease [1], as well as a higher likelihood of healthcare prevention behavior [53] in siblings of children with cancer.
Our review identified major gaps in sibling research while providing a framework upon which future sibling-directed work will be based. Few studies treated the siblings as the population of interest, as the majority of published studies were focused on cancer patients themselves. For instance, most CCSS reports included data on siblings as a control group. As such, these studies centered around outcomes which were selected as relevant to pediatric cancer survivors and were generally biased to highlight siblings as the healthier group compared to cancer survivors. As mentioned in Long et al. [1], studies with a priori sibling aims and selected outcomes relevant to siblings were more likely to report statistically and clinically significant findings for siblings. Moreover, CCSS studies which reported siblings' outcomes tended to compare siblings to nonmatched control groups (the control groups were matched to the childhood cancer survivors and not the siblings), limiting robust risk estimates for siblings. Similarly, most studies did not account for the heterogeneity of the sibling sample in their results (results were not adjusted for sibling characteristics), which further diminished their internal validity [1]. Sibling groups tended to have comparatively lower participation rates (particularly in CCSS studies), hindering the external validity of results and study power.
Given that the majority of studies were centered on the children with cancer and not the siblings, often the available sibling data was simplified or failed to include relevant confounding variables. The studies often did not account for socioeconomic status, attained sibling age, cancer-related factors, parental health, or genetic susceptibility [54]. Siblings within families with high socioeconomic means have been found to be more likely to use healthcare services, whereas advanced age has been described as a predictor of adverse health behaviors [54]. Determining whether cancer-related factors (e.g., index child's cancer diagnosis, treatment course, prognosis) significantly impact the sibling's health requires the availability of granular sibling data [55, 56]. For example, a life-threatening pediatric cancer diagnosis has been associated with greater use of healthcare services in siblings [3]. Conversely, siblings of children with skin cancer were more likely to practice skin cancer prevention [53], raising the question of potential protective factors among siblings.
Family-related exposures may also influence the physical health of siblings. A cancer diagnosis in a family member deeply disrupts family roles and may limit parental support for the siblings [57]. Siblings can be particularly vulnerable during cancer treatment as a result of parental absence, loss of family routine, frequent transitions between home and hospital, and overall lack of stability [57, 58]. The impact of parental health on sibling health has been poorly studied but likely influences sibling health and/or healthcare service utilization. For example, parental somatization has been found to be a predictor of siblings' symptoms and could result in reporting of sibling health status to a specialist [54]. Shared environmental exposures and genetic susceptibilities are known to be associated with the development of chronic health conditions within family settings and may represent important confounding factors that were largely absent from the published literature on siblings of children with cancer. Modifiable factors such as smoking (including second-hand cigarette smoke), alcohol consumption, exercise, and nutrition are known contributors to chronic conditions of the cardiovascular system, as well as cancer and metabolic disorders [59, 60]. Other contributors to chronic diseases include radiation exposure, residential location (e.g., near a nuclear facility), and prolonged contact with organic pollutants (e.g., pesticides, industrial, agricultural chemicals), volatile organic compounds (e.g., solvent, fuels), and plastics [59, 60]. For instance, pesticide exposures have been linked to the development of several cancers, including brain, prostate, kidney, non-Hodgkin lymphoma, and leukemia [61]. Periods of exposure are critical, with elevated risks associated with exposure during prenatal and postnatal periods, as well as with parental occupational exposures [61]. Additionally, only a few included studies [16, 19, 22, 23, 28, 29, 47] considered genetic susceptibility in their findings. Overall, both environmental and genetic factors could account for the development of cancer and other chronic diseases in families [47]. Deciphering the impact of these potential confounding factors on health outcomes in siblings is essential and will require further investigation. Our systematic review therefore highlighted the persistent knowledge gaps surrounding research on siblings of children with cancer, providing direction and opportunities for future work.
This review has several limitations. Although we reported a trend toward increased risk of multiple outcomes among siblings of children with cancer compared to controls, important study heterogeneity rendered study comparisons difficult and meta-analyses inappropriate. Firstly, we found significant heterogeneity in the populations of interest (relating to both the child with cancer and their siblings). Most studies did not account for the specific cancer diagnosis, stage of the disease, treatment of the index child, and time since diagnosis of the index child. For the siblings, there was heterogeneity regarding their age distribution, length of follow-up, and timing of outcome assessment. Among the included studies, we also identified varying study designs including population-based, cross-sectional, and case–control studies. Each study design may infer unique methodological biases, further supporting our decision to omit meta-analyses.
Importantly, there was noteworthy heterogeneity in the study outcome measurements and definitions. Data sources included medical records, administrative databases, national health registries, and self-reported outcomes collected from interviews and surveys. In our systematic review, 72% of the studies included self-reported data in siblings of children with cancer. Self-reported data may introduce information biases such as response, reference, and recall biases [62, 63]. The patient's ability to accurately report the occurrence of health outcomes requires validated questionnaires [62]. Self-reported data may not reliably estimate health conditions compared to other unbiased data sources such as health administrative data [62]. Future work should leverage alternative methods for establishing health outcomes in our population. Additionally, health outcomes were defined differently across studies, introducing outcome-reporting bias. The selective reporting of heterogeneous outcomes among siblings of children with cancer was a driving factor behind the omission of meta-analyses in our systematic review [64].
Another limitation of the published literature is that few studies compared the sibling population to matched controls, which prevented comparative measurements and likely contributed to the conflicting results in the literature. Few studies provided longitudinal measurements of outcome assessment and included short follow-up periods for siblings; most studies were cross-sectional and retrospective, restricting the long-term assessment of the impact of childhood cancer on the siblings. The scarcity of normative data for our population hindered the analysis of association. Given the heterogeneous nature of the available data, attempting to extract normative data for calculating association estimates was found to be ineffective. Therefore, risk could not be determined for studies which did not include a matched control population.
Lastly, stratified analyses to determine the impact of specific factors (e.g., sibling attained age, index child's cancer type) on the health outcomes of siblings could not be conducted, as the majority of studies reported pooled sibling data and accessing the primary data was not possible.
Overall, the important heterogeneity in the published sibling literature contributed to the lack of clearly defined health risks in this population. Although these limitations represented barriers to performing meta-analyses, our extensive and robust literature review provides a solid foundation for future sibling-directed work. Future research using matched population controls is needed to establish valid comparisons for determining the risks of organ system impairment and healthcare service utilization among siblings of children with cancer. Prospective, longitudinal sibling studies would allow for the characterization of risk and predictive factors, and inference of causal relationships. Specifically, identifying at-risk subgroups of siblings of children with cancer is imperative for developing informed, preventative recommendations and surveillance strategies. This study therefore lays the groundwork for further investigation to improve healthcare directives and clinical guidelines for the long-term care of siblings and their families.
5 Conclusion
In this systematic review, we identified that siblings of children with cancer may have an increased risk of developing adverse physical health diagnoses and healthcare service utilization. We encountered important study heterogeneity, specifically surrounding populations of interest, study designs, and outcomes of interest, which prevented meta-analyses. Despite these limitations, the current review underscores the pressing need for further research to improve our understanding of the impact of a pediatric cancer diagnosis on the siblings' long-term health. Future studies with standardized methodologies would allow for data pooling and a comprehensive analysis of outcome risk development among siblings of children with cancer. A robust understanding of the physical health risks associated with having a sibling with cancer would inform surveillance guidelines and support the development of targeted interventions to mitigate risks.
Author Contributions
Victorine Sirveaux: conceptualization (equal), data curation (lead), formal analysis (lead), investigation (lead), project administration (equal), supervision (equal), visualization (lead), writing – original draft preparation (lead), writing – review and editing (lead). Lily Puterman-Salzman: conceptualization (equal), data curation (equal), investigation (equal), project administration (equal), supervision (equal), writing – original draft preparation (supporting), writing – review and editing (supporting). Yue Qian Zhang: data curation (equal), investigation (equal), writing – review and editing (supporting). Eleni Sotirakos: data curation (equal), investigation (supporting), writing – review and editing (supporting). Philippe Dodin: investigating (supporting), methodology (lead), writing – review and editing (supporting). Guillaume Dumas: methodology (supporting), writing – review and editing (supporting). Eyal Cohen: methodology (supporting), writing – review and editing (supporting). Nadia Roumeliotis: methodology (supporting), writing – review and editing (supporting). Petros Pechlivanoglou: methodology (supporting), writing – review and editing (supporting). Hallie Coltin: conceptualization (lead), funding acquisition (lead), investigation (supporting), methodology (equal), project administration (supporting), supervision (lead), writing – original draft preparation (supporting), writing – review and editing (equal).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. H.C. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.