Concomitant TP53 mutations with response to crizotinib treatment in patients with ALK-rearranged non-small-cell lung cancer
Abstract
Background
TP53 mutations are the most prevalent mutations detected in non-small-cell lung cancer (NSCLC) and have been revealed as a negative prognostic biomarker of outcome. The impact of concomitant TP53 mutations in ALK-rearranged NSCLC remains uncertain.
Methods
Tumor samples from 64 ALK-rearranged NSCLC patients receiving crizotinib treatment were subjected to next-generation sequencing (NGS) to identify TP53 mutational status. The clinicopathologic features of the TP53 mutations and its impact on the effect of crizotinib treatment were analyzed.
Results
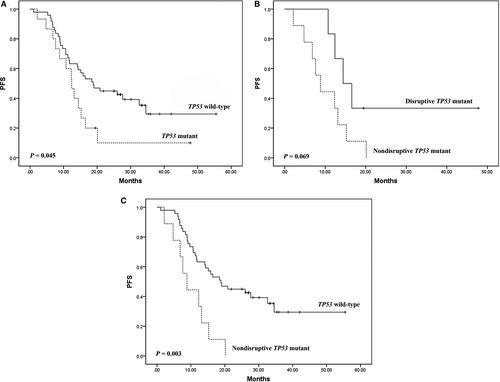
Among the 64 ALK-rearranged patients, 15 (23.4%) patients showed a TP53 mutation. Of these, six cases had disruptive mutations and nine with nondisruptive mutations. The objective response rate (ORR) and disease control rate (DCR) for TP53 mutated patients were both significantly lower compared with those for TP53 wild-type patients (p = 0.003 and 0.023, respectively). A significantly shorter progression-free survival (PFS) was found in TP53 mutated patients compared with TP53 wild-type patients (p = 0.045). Nondisruptive TP53 mutations were associated with a shorter PFS in comparison with disruptive TP53 mutations in ALK-rearranged patients (p = 0.069). When nondisruptive TP53 mutated patients were in comparison with TP53 wild-type patients, nondisruptive TP53 mutations were associated with a significant reduced PFS (p = 0.003).
Conclusions
TP53 mutations, especially nondisruptive mutations, negatively affected the response to crizotinib and correlated with shorter PFS in ALK-rearranged NSCLC patients.
1 INTRODUCTION
Recently, the landscape of treatments for non-small-cell lung cancer (NSCLC) has been changed by the development of molecular diagnosis and targeted therapies. Anaplastic lymphoma kinase (ALK) gene rearrangement defines a distinct molecular subtype of NSCLC and has been found in 3%-7% of all NSCLC patients.1-3 Crizotinib, a potent tyrosine kinase inhibitor (TKI) of ALK, ROS1 and MET, was the first molecule inhibitor targeting ALK to be widely used in the clinic.4 Two Phase III trials, PROFILE 1007 and PROFILE 1014 confirmed the benefit of crizotinib over cytotoxic chemotherapy in advanced ALK-rearranged NSCLC.5, 6
Despite excellent response rates and durable responses in some cases, most patients treated with ALK TKIs inevitably progress within 1-2 years due to the acquired resistance.5-10 Various mechanisms of acquired resistance to ALK TKI have been determined, which were summarized into two major classes: ALK-dependent resistance (ALK secondary resistance mutations or amplification) and ALK-independent resistance (activation of bypass tracks and lineage changes).11 In addition to acquired resistance, a small number (approximately 5%) of patients with ALK-rearranged NSCLC treated with first-line crizotinib have progressive disease as their best response due to intrinsic resistance.6, 12 However, mechanisms of intrinsic resistance are poorly understood, and this represents an important gap in the field of ALK TKI resistance.
Although TP53 mutations has been reported to be associated with inferior response to EGFR-TKIs and poor outcome in EGFR-mutated NSCLC patients, the association between TP53 mutations and the effect of crizotinib treatment in ALK-rearranged NSCLC patients was still uncertain. The aim of the present study was to investigate the clinicopathologic characteristics of TP53 mutation in ALK-rearranged NSCLC and its association with the effect of crizotinib in ALK-rearranged NSCLC patients.
2 MATERIALS AND METHODS
2.1 Patients and samples
We retrospectively analyzed 64 patients with ALK-rearranged NSCLC treated with crizotinib at Cancer Hospital, Chinese Academy of Medical Sciences (CAMS, Beijing, China) between January 2011 and December 2016. Epidemiologic and clinicopathologic data were collected, including age, sex, smoking history, histological type, Karnofsky physical score (KPS), pathological stage, previous treatment regimens, response to crizotinib and outcomes. This study was approved by our institutional review board and ethics committee of Cancer Hospital, CAMS.
2.2 Targeted next-generation sequencing
The tumor specimens of the 64 patients were formalin-fixed, paraffin-embedded tissues and were all enough for evaluating by NGS. All specimens were subjected to NGS of 56 cancer-related genes with use of a kit (Burning Rock Biotech, Guangzhou, China) according to our NGS protocol as previously reported.13
2.3 Clinical response evaluation
Oral crizotinib was administered at a dose of 250 mg twice daily continuously (28-day cycles) until progressive disease (PD) or unacceptable toxicity. Best clinical response to crizotinib treatment was classified on the basis of interval CT scans as complete response (CR), partial response (PR), stable disease (SD) or PD using standard Response Evaluation Criteria in Solid Tumors criteria version 1.1.14 Tumor assessments were performed independently by experienced radiologists every 6-8 weeks until RECIST-defined disease progression. The objective response rate (ORR) was defined as the sum of CR and PR The disease control rate (DCR) was calculated as the percentage of patients with CR, PR, and SD.
2.4 Statistical analysis
Progression-free survival (PFS) was calculated from the date of initiating crizotinib treatment to disease progression. Overall survival (OS) was defined as the time from first diagnosis of stage IIIB/IV or postoperative recurrent until death. The association between clinicopathologic characteristics, response and TP53 mutational status was tested by the Pearson's χ2 test or Fisher exact test, when appropriate. The survival curves were estimated using the Kaplan-Meier method, and differences in survival were tested by the log-rank test. Cox regression univariate analysis was used to generate survival hazard ratios and 95% confidence intervals. The data were analyzed using SPSS software (version 20.0 of SPSS, IBM, Armonk, NY, USA). Statistical significance was assumed at two-sided p value <0.05.
3 RESULTS
3.1 Patient characteristics
Clinicopathologic characteristics of patients, type of ALK fusion, crizotinib treatment line, type of response to crizotinib and TP53 status are shown in Table 1. The majority of patients were female (54.7%) and never smokers (76.6%). Almost all patients were diagnosed with adenocarcinoma (98.4%). Fifty-two patients (81.3%) harbored an EML4-ALK fusion whereas 12 patients (18.7%) harbored other fusion partners for ALK including PRKAR1A, KLC1, AFTPH and EPS15. Of cases with EML4-ALK fusion, the most common EML4-ALK variant was variant 3a/b (E6:A20, 46.2%), followed by variant 1 (E13:A20, 25.0%), variant 2 (E20:A20, 19.2%) and Other rare variants (E14:A20 and E18:A20, 9.6%).
| Characteristics | No. of patients (n = 64) |
|---|---|
| Sex | |
| Male | 29 (45.3%) |
| Female | 35 (54.7%) |
| Age (years) | |
| Median | 50 |
| Range | 24-82 |
| Smoking history | |
| Never | 49 (76.6%) |
| Current/Former | 15 (23.4%) |
| Histology | |
| Adenocarcinoma | 63 (98.4%) |
| Nonadenocarcinoma | 1 (1.6%) |
| Type of ALK fusion | |
| Non-EML4-ALK | 12 (18.7%) |
| EML4-ALK | 52 (81.3%) |
| Variant 1 | 13 (25.0%) |
| Variant 2 | 10 (19.2%) |
| Variant 3a/b | 24 (46.2%) |
| Other variants | 5 (9.6%) |
| KPS | |
| 70 to <90 | 34 (53.1%) |
| 90-100 | 30 (46.9%) |
| p Stage | |
| IIIB/IV | 60 (93.7%) |
| Postoperative recurrent | 4 (6.3%) |
| Crizotinib treatment line | |
| First | 33 (51.6%) |
| ≥Second | 31 (48.4%) |
| Response to crizotinib | |
| CR | 3 (4.7%) |
| PR | 43 (67.2%) |
| SD | 12 (18.7%) |
| PD | 6 (9.4%) |
| ORR | 46 (71.9%) |
| DCR | 58 (90.6%) |
| TP53 status | |
| Wild-type | 49 (76.6%) |
| Mutated | 15 (23.4%) |
| Exon 5 | 3 (20.0%) |
| Exon 6 | 7 (46.7%) |
| Exon 7 | 2 (13.3%) |
| Exon 8 | 3 (20.0%) |
| Disruptive/nondisruptive | |
| Disruptive | 6 (40.0%) |
| Nondisruptive | 9 (60.0%) |
- CR, complete response; DCR, disease control rate; KPS, Karnofsky physical score; PD, progressive disease; PR, partial response; ORR, objective response rate; SD, stable disease.
3.2 TP53 mutation
Out of the 64 ALK-rearranged patients, 15 (23.4%) patients showed a TP53 mutation: 20.0% were on exon 5, 46.7% on exon 6, 13.3% on exon 7, and 20.0% on exon 8 (Table 1). According to a previous report about differentiation of TP53 mutations,15 we divided TP53 mutations into two types, disruptive and nondisruptive, and observed six disruptive mutations and nine nondisruptive mutations. It is worth noting that all disruptive mutations except one were located in exon 6, whereas nondisruptive mutations evenly distributed in the four exons (Supplementary Table S1). Supplementary Table S2 shows associations between TP53 mutations and clinical characteristics. Smoking was significantly associated with high frequency of TP53 mutations (p = 0.021). No statistically significant association was found between TP53 mutations and either sex, age, histology, KPS and tumor pathological stage.
3.3 TP53 status in relation to ALK fusion
We found no significant association between TP53 status and two types of ALK fusion (EML4-ALK and Non-EML4-ALK) and different EML4-ALK variants (Variant 1, Variant 2, Variant 3a/b) (Supplementary Tables S3 and S4). With regard to the distribution of TP53 gene mutations, TP53 exon 6 mutations and 8 mutations were both more common in patients with EML4-ALK fusion. In addition, TP53 disruptive mutations were more common in patients with EML4-ALK variant 2 (Supplementary Table S4).
3.4 Response, PFS, and TP53 mutations
ORR and DCR to crizotinib treatment in all 64 patients were 71.9% and 90.6%, respectively. The ORR and DCR for TP53 wild-type patients were both significantly higher compared with those for TP53 mutated patients (p = 0.003 and 0.023, respectively; Table 2). Patients with nondisruptive TP53 mutations showed lower ORR and DCR to crizotinib than patients with disruptive TP53 mutations, although this difference was not statistically significant (p = 0.136 and 0.103, respectively; Table 2).
| ORR | p value | DCR | p | |
|---|---|---|---|---|
| Type of ALK fusion (n = 64) | 0.726 | 0.312 | ||
| EML4-ALK | 38 (73.1%) | 48(92.3%) | ||
| Non-EML4-ALK | 8 (66.7%) | 10(83.3%) | ||
| EML4-ALK variants (n = 49) | 0.135 | 0.566 | ||
| Variant 1 | 8 (61.5%) | 12(92.3%) | ||
| Variant 2 | 9 (90.0%) | 10(100%) | ||
| Variant 3a/b | 19 (79.2%) | 21(87.5%) | ||
| Other EML4-ALK variants | 2 (40%) | 5(100%) | ||
| TP53 status (n = 64) | 0.003 | 0.023 | ||
| Mutated | 6 (40.0%) | 11(73.3%) | ||
| Wild-type | 40 (81.6%) | 47(95.9%) | ||
| TP53 mutation type (n = 15) | 0.136 | 0.103 | ||
| Disruptive | 4 (66.7%) | 6(100%) | ||
| Nondisruptive | 2 (22.2%) | 5(55.6%) | ||
| TP53 mutation site (n = 15) | 0.166 | 0.199 | ||
| Exon 5 | 0 | 1(33.3%) | ||
| Exon 6 | 4 (57.1%) | 6(85.7%) | ||
| Exon 7 | 0 | 1(50.0%) | ||
| Exon 8 | 2 (66.7%) | 3(100%) |
- DCR, disease control rate; ORR, objective response rate.
Overall, median PFS and OS was 15.5 months (2.1-55.5) and 48.8 months (11.8-not reached). PFS was significantly longer in TP53 wild-type patients than in TP53 mutated patients (HR = 1.96, 95% CI = 1.02-3.80, p = 0.045; Figure 1A). Disruptive TP53 mutations were associated with a longer PFS in comparison with nondisruptive TP53 mutation in those with ALK rearrangements (HR = 2.91, 95% CI = 0.88-9.63, p = 0.069; Figure 1B). When nondisruptive TP53 mutated patients were in comparison with TP5 wild-type patients, nondisruptive TP53 mutations were associated with a significant reduction in PFS (HR = 3.27, 95% CI = 1.51-7.08, p = 0.003; Figure 1C). We found no significant difference in OS according to TP53 mutation status.
4 DISCUSSION
In the current study, we explored the correlation between TP53 mutations and the outcome of ALK-rearranged NSCLC patients treated with crizotinib. Our data revealed that TP53 mutations were significantly associated with reduced response to crizotinib and inferior PFS. In particular, nondisruptive TP53 mutations represent a heterogeneous subgroup of ALK-rearranged NSCLC patients with inferior PFS. To our best knowledge, we investigate for the first time the association between nondisruptive TP53 mutations and survival in a case series of ALK-rearranged NSCLC patients.
Mutations of TP53 gene are the most prevalent mutations detected in lung cancer and often coexist with driver mutations. TP53 mutations are present in almost half of NSCLC patients and the incidence of TP53 mutations is higher in lung squamous cell carcinomas than that in lung adenocarcinomas, with mutation rates between 20% and 40% in the latter.16-18 In line with prior studies, we found that TP53 mutations occurred in 23.4% of ALK-rearranged NSCLC patients in our case series. In terms of a previous study, it is the direct mutagenic action on DNA the means by which smoking causes lung cancer and the TP53 gene is one of the most frequent targets of tobacco smoking-related DNA mutations.19 The findings of our study showed that smoking was significantly associated with high frequency of TP53 mutations, consistent with several studies in which TP53 mutations more frequently occurred in patients with smoking-associated cancer (26%-71%) compared with patients who never smoked (8%-47%).20-22
As a pivotal tumor suppressor, p53 regulates a series of cell activities to protect against cancer. In response to various types of cellular stress, TP53 gene is activated, resulting in the accumulation of p53 protein, which is implicated in cell-cycle arrest, DNA repair, senescence, apoptosis, metabolism, aging, and differentiation.23 The total biological function of these processes is to prevent the transformation of a normal cell into a cancerous cell.14 Therefore, the transforming potential of oncogenes can be accelerated by loss of p53 function which mainly originates from TP53 mutations. TP53 mutation is one of the most widely investigated prognostic biomarker in patients with NSCLC. In unselected NSCLCs, prognostic impact of TP53 mutations on survival remains controversial.24-28 In EGFR-mutated NSCLCs, previous studies have suggested that TP53 mutations were not only associated with poor response to EGFR TKIs, but also correlated with shorter survival in these patients.29-32 However, the results of aforementioned studies only partly reached statistical significance. In ALK-rearranged NSCLCs, Kron et al performed a detailed analysis of concomitant mutations and revealed that the existence of concomitant TP53 mutations was an adverse prognostic factor for PFS to ALK TKIs and a negative predictor for OS.33 In the present study, we found that the occurrence of ALK rearrangement with concomitant TP53 mutations negatively affected the response to crizotinib and correlated with shorter PFS, which is consistent with previous findings.
Moreover, we divide TP53 mutations into two types, disruptive and nondisruptive, based on the degree of disorder of p53 protein structure predicted from the crystal structure of the p53–DNA complexes.15 The results of our study demonstrated that nondisruptive TP53 mutations represent a heterogeneous subgroup of ALK-rearranged NSCLC patients with inferior PFS. When nondisruptive TP53 mutated patients were in comparison with TP53 wild-type patients, nondisruptive TP53 mutations were associated with a significant reduction in PFS (p = 0.003). Although a correlation between nondisruptive TP53 mutations and worse outcomes has been reported in EGFR-mutated lung NSCLCs,34 this study first reported the negative prognostic role of nondisruptive TP53 mutations in ALK-rearranged NSCLCs treated with crizotinib. The mechanism underlying the negative prognostic role of nondisruptive TP53 mutations has not been fully elucidated. Prior experimental evidence revealed that nondisruptive mutations caused partial loss of p53 functions, whereas, of note, the retained functional properties of p53 protein were often associated with gain-of-function (GOF) activities that exerted by abrogating the function of p53-related proteins p63/p73 and modulating transcriptional output.15, 35-38 Mutant p53 GOF activities can render some cell types increased tumorigenicity, motility, and growth rate.39, 40 Moreover, increased metastasis and invasiveness and decreased sensitivity to chemotherapeutic drugs are features of mutant p53 GOF activities, which have been demonstrated in cell models.41, 42
There are some limitations in the current study. First, it was a retrospective, single institutional study and therefore patient selection bias was inevitable. Second, due to a relatively small cohort, the results cannot be regarded as definitive. A prospective study with a larger sample size of ALK-rearranged NSCLCs with TP53 mutations is warranted in the future.
Conclusively, our results highlighted the negative prognostic role of TP53 mutations in ALK-rearranged NSCLC patients treated with crizotinib. Moreover, nondisruptive TP53 mutations seem to represent a heterogeneous subgroup of ALK-rearranged NSCLC patients with inferior PFS.
ACKNOWLEDGMENTS
The study was supported by the grant 2016YFC0905400 from the National Key R&D Program of China and Beijing Municipal Science & Technology Commission No. LC2015A13.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.