Cuproplasia and cuproptosis, two sides of the coin
Abstract
Copper is an essential micronutrient in the human body, mainly acting as a crucial cofactor required for a wide range of physiological processes across nearly all cell types. Recent advances revealed that tumor cells seize copper to fulfill their rapid proliferation, metastasis, immune evasion, and so on by reprogramming the copper regulatory network, defined as cuproplasia. Thus, targeting copper chelation to reduce copper levels has been considered a rational tumor therapy strategy. However, overloaded copper ions could be toxic, which leads to the aggregation of lipoylated mitochondrial proteins and the depletion of iron-sulfur clusters, ultimately resulting in cell death, termed cuproptosis. Upon its discovery, cuproptosis has attracted great interest from oncologists, and targeting cuproptosis by copper ionophores exhibits as a potential anti-tumor therapy. In this review, we present the underlying mechanisms involved in cuproplasia and cuproptosis. Additionally, we sum up the chemicals targeting either cuproplasia or cuproptosis for cancer therapy. Further attention should be paid to distinguishing cancer patients who are suitable for targeting cuproplasia or cuproptosis.
List of abbreviations
-
- ACO-2
-
- aconitase-2
-
- α-KG
-
- α-ketoglutarate
-
- ALDH
-
- aldehyde dehydrogenase
-
- ALS
-
- amyotrophic lateral sclerosis
-
- ARE
-
- antioxidant response element
-
- ARID1A
-
- AT-rich interactive domain-containing protein 1A
-
- ATOX1
-
- antioxidant 1
-
- ATP
-
- adenosine 5'-triphosphate
-
- ATP7A/B
-
- ATPase 7A/B
-
- BBB
-
- blood-brain barrier
-
- CcO
-
- cytochrome c oxidase
-
- CCS
-
- copper chaperone for superoxide dismutase
-
- CDKN2A
-
- cyclin dependent kinase inhibitor 2A
-
- CDS
-
- coding sequence
-
- COA6
-
- cytochrome c oxidase assembly factor 6
-
- COX17
-
- cytochrome c oxidase copper chaperone 17
-
- CP
-
- ceruloplasmin
-
- CTR1
-
- copper transport protein 1
-
- Cu
-
- copper
-
- CuSO4
-
- copper sulfate
-
- Cys
-
- cysteine
-
- DBT
-
- Dihydrolipoamide Branched Chain Transacylase E2
-
- DLAT
-
- Dihydrolipoamide S-Acetyltransferase
-
- DLST
-
- Dihydrolipoamide S-Succinyltransferase
-
- DMT1
-
- divalent metal transporter 1
-
- DNMT1
-
- DNA methyltransferase 1
-
- DPEN
-
- D-penicillamine
-
- DSF
-
- disulfiram
-
- ECM
-
- extracellular matrix
-
- EGF
-
- epidermal growth factor
-
- EGFR
-
- epidermal growth factor receptor
-
- ES
-
- elesclomol
-
- ETC
-
- electron transfer chain
-
- FAK
-
- focal adhesion kinase
-
- FDA
-
- Food and Drug Administration
-
- FDX1
-
- ferredoxin 1
-
- FGF2
-
- fibroblast growth factor 2
-
- FIH-1
-
- factor inhibiting hypoxia-inducible factor-1
-
- GBM
-
- glioblastoma
-
- GCSH
-
- glycine cleavage system H protein
-
- GLS
-
- glutaminase
-
- GLUT1
-
- glucose importer 1
-
- GSH
-
- glutathione
-
- HCC
-
- hepatocellular carcinoma
-
- HGF
-
- hepatocyte growth factor
-
- HIF-1α
-
- hypoxia-inducible factor-1α
-
- H2O2
-
- hydrogen peroxide
-
- HRE
-
- hypoxia-responsive element
-
- IL
-
- interleukin
-
- IMS
-
- intermembrane space
-
- IRF7
-
- interferon regulatory factor 7
-
- LC-MS/MS
-
- liquid chromatography-tandem mass spectrometry
-
- LDH
-
- lactate dehydrogenase
-
- LIAS
-
- lipoic acid synthetase
-
- LOX
-
- lysyl oxidase
-
- MAPK
-
- mitogen-activated protein kinase
-
- MET
-
- mesenchymal to epithelial transition factor
-
- MRE
-
- metal response element
-
- MT
-
- metallothionein
-
- MT1-MMP
-
- membrane-type 1 matrix metalloproteinase
-
- MTF1
-
- metal regulatory transcription factor 1
-
- Nrf2
-
- NF-E2 related factor 2
-
- NSCLC
-
- non-small cell lung cancer
-
- ODD
-
- oxygen-dependent degradation domain
-
- PD-L1
-
- programmed cell death ligand 1
-
- PeBoW
-
- PES1-BOP1-WDR12
-
- PFS
-
- progression-free survival
-
- PHD
-
- HIF-prolyl hydroxylase
-
- PKM
-
- pyruvate kinase muscle isoform
-
- POLD1
-
- DNA polymerase delta 1
-
- pRB
-
- retinoblastoma protein
-
- PTM
-
- post-translational modification
-
- ROS
-
- reactive oxygen species
-
- SA
-
- serum albumin
-
- SCO1/2
-
- synthesis of cytochrome c oxidase 1/2
-
- SDHB
-
- succinate dehydrogenase complex iron sulfur subunit B
-
- SLC31A1
-
- solute carrier family 31 member 1
-
- SOD1
-
- superoxide dismutase 1
-
- STEAP
-
- six-transmembrane epithelial antigen of the prostate
-
- TCA
-
- tricarboxylic acid
-
- TEPA
-
- tetraethylenepentamine pentahydrochloride
-
- TETA
-
- trientine
-
- TGN
-
- trans-Golgi network
-
- TTM
-
- tetrathiomolybdate
-
- ULK1
-
- Unc51-like autophagy-activating kinase 1
-
- VEGF
-
- vascular endothelial growth factor
-
- ZMP
-
- zinc metalloproteinase
1 BACKGROUND
Copper (Cu) is a vital trace element in the human body, which plays a significant role in mitochondrial respiration, antioxidant defense, cell growth regulation, and synthesizing hormones and neurotransmitters. Specifically, Cu serves as a key cofactor for cytochrome c oxidase in the mitochondria's electron transport chain [1], facilitating cell respiration and adenosine 5'-triphosphate (ATP) production. While inadequate Cu levels hinder growth, excessive Cu can be cytotoxic, causing oxidative damage and cell death. As a result, Cu absorption, transport, and excretion are tightly monitored and controlled.
The regulation of Cu homeostasis in tumor cells is a crucial aspect of cancer research. Tumor cells reprogram Cu homeostasis by altering the expression and activation of biomacromolecules responsible for Cu transport and metabolism, leading to higher intracellular Cu levels essential for heightened energy requirements [2, 3]. Concurrently, Cu concentrations in the tumor tissues of patients with lung, breast, or stomach cancer are significantly higher than in normal tissues [4]. This phenomenon, known as ‘cuproplasia', highlights the reliance of tumor cells on Cu [5]. As a result, Cu chelator has emerged as a potential strategy for cancer treatment by reducing intracellular free Cu. In contrast, excessive Cu induces a unique form of cell death, termed cuproptosis, which has been identified recently [6]. Expanding on this concept of targeting cancers with Cu overload, there is a growing interest in Cu ionophores that facilitate the transport of Cu into cells and mitochondria.
This review offers a thorough summary of the correlation between Cu and tumors by delving into the intricate relationships and specific mechanisms that govern the mutual regulation between them. Furthermore, recent developments in tumor treatment focusing on addressing Cu deficiency and overload are discussed to support the idea that modulating Cu metabolism presents new potential avenues for tumor therapy.
2 CU TRANSPORT, METABOLISM AND HOMEOSTASIS
As an essential nutrient, Cu is absorbed from the diet primarily in the duodenum and small intestine through the gastrointestinal tract [7]. Generally, divalent Cu (Cu(II)) is reduced to monovalent Cu (Cu(I)) by cell-surface metalloreductases, such as the six-transmembrane epithelial antigen of the prostate (STEAP) [8]. Then Cu(I) is captured and transported into intestinal cells via copper transport protein 1 (CTR1), also known as solute carrier family 31 member 1 (SLC31A1), in an energy-independent manner [9]. While CTR1 is crucial for Cu absorption, studies have indicated that divalent metal transporter 1 (DMT1) can also facilitate Cu transport into cells as a compensatory mechanism when CTR1 is absent [10]. Inside the cytoplasm, Cu can be directed to the trans-Golgi network (TGN) via the chaperone antioxidant 1 (ATOX1). ATOX1 binds to Cu(I) and transfers it to ATPases in the Golgi network for Cu recycling or efflux [11]. To date, ATP7A and ATP7B are the only two ATPases reported [12]. These ATPases have the capability to detect and respond to changes in intracellular Cu levels. While the Cu levels reach the threshold, ATP7A or ATP7B translocates to the appropriate plasma membrane domains (basolateral or apical) to excrete excessive Cu from the cell. If necessary, ATPases are transported back to the TGN for Cu recycling [13]. ATP7A and ATP7B exhibit distinct expression patterns in specific organs. ATP7A is predominantly found in tissues such as the placenta as well as the blood-brain barrier (BBB) while ATP7B is primarily expressed in the liver [14]. Like intestinal epithelial cells, tumor cells absorb Cu via STEAP and CTR1, and excrete Cu from the cells through ATP7A or ATP7B (Figure 1A).
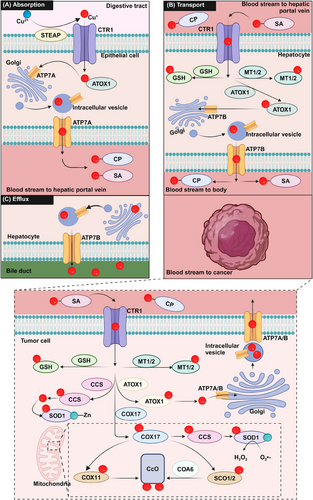
The distribution of dietary Cu from the small intestine to the body involves several steps [15]. Once Cu is transported into the blood, serum chaperone proteins, including albumin and transcuprein assist in moving Cu along the portal system to the liver [16], where hepatocytes take up Cu via CTR1. The liver serves as the primary storage and excretion organ for Cu [17]. After Cu leaves the liver, it travels through the systemic circulation to reach target tissues and organs by binding to carrier proteins such as ceruloplasmin (CP) and serum albumin (SA) [18-20]. In humans, the normal blood Cu concentrations are generally between 11.0-22.0 µmol/L; approximately 75% of Cu is bound to CP in a non-exchangeable manner and 25% is bound to SA in an exchangeable form [21]. (Figure 1A).
After Cu enters the cell through CTR1, the metal moves to various cellular compartments that regulate specific biological processes. In the cytoplasm, Cu chaperone for superoxide dismutase (CCS) directly interacts with Cu and transports it to copper/zinc superoxide dismutase 1 (SOD1) to detoxify reactive oxygen species (ROS) [22]. However, studies have demonstrated that when human SOD1 is expressed in mammalian cells in the absence of CCS, the SOD1 enzyme retains a certain level of activity [23]. This CCS-independent activation of mammalian SOD1 is associated with glutathione, specifically its reduced form [24]. Both CCS and SOD1 are involved in scavenging superoxides in the cytoplasm, and their inactivation can result in cell death [25]. Another important Cu-chaperone protein in the cytoplasm is ATOX1, which helps maintain stable intracellular Cu levels by transporting Cu to the TGN for recycling or excretion via ATP7A or ATP7B.
Other than these proteins delivering Cu to specific chambers, a group of proteins function as chelators to store Cu and prevent excessive free Cu from harming cells. Metallothionein (MT) and glutathione (GSH) are key natural intracellular chelators that bind excess Cu to reduce its toxicity [26]. As a negative feedback, excess Cu induces the expression of MT-1 and MT-2 by activating the metal response element (MRE)-binding transcription factor-1 (MTF-1)-MRE and NF-E2 related factor 2 - antioxidant response element (Nrf2-ARE) pathways [27]. As the major cellular defense response against oxidative stress, GSH is vital to eliminate reactive oxygen species (ROS) generated from excess Cu [28].
Apart from cytoplasmic Cu-chaperone proteins, Cu chaperones are also present within the mitochondria. These chaperones play a crucial role in facilitating the use of Cu by cytochrome c oxidase (CcO) during oxidative phosphorylation. The mitochondrial intermembrane space (IMS) houses cytochrome c oxidase copper chaperone 17 (COX17), which is responsible for transporting cytoplasmic Cu into the IMS [28]. Once in the IMS, Cu bound to COX17 is transferred to cytochrome c oxidase 1 (SCO1) and SCO2 [29]. These metal partners, SCO1 and SCO2, play a critical role in the assembly of COX by facilitating the transfer of Cu to the COX2 subunit or the COX1 subunit through COX11, and any mutation of SCOs leads to a deficiency of Cu in mitochondria [29, 30]. Additionally, cytochrome c oxidase assembly factor 6 (COA6) functions as a thiol-reductase, playing a crucial role in the evolutionary conservation of respiratory complex IV biogenesis, and its deficiency can lead to susceptibility to mitochondrial disease [31]. In conjunction with factors other than Cu, this action facilitates Cu binding [32] (Figure 1B).
Regulated excretion is important to maintain Cu balance, as about 85% of dietary Cu absorbed by the intestine should be excreted from the body. The primary route of Cu excretion is through the hepatobiliary pathway across the bile canalicular membrane of hepatocytes, with less than 5% excreted via renal excretion unless it exceeds the renal tubular reabsorption capacity [15]. The excess Cu is eliminated from the body by metabolizing in hepatocytes and incorporating into bile through ATP7B [15]. Consequently, the liver plays a crucial role in both storing and excreting Cu (Figure 1C).
In summary, Cu homeostasis is fine-tuned by Cu absorption, transport, storage, and excretion network, and disruption of this homeostasis can significantly affect cell fate, potentially leading to disease development. For details on the regulation of Cu homeostasis, please refer to the reference review [33].
3 REGULATION OF CU HOMEOSTASIS IN TUMOR
3.1 Interactions between tumors and Cu
Malignant tumors enhance the accumulation of intracellular Cu by modifying Cu-related proteins such as CTR1 and ATP7B in response to various stresses like chemotherapy [34] and hypoxia [35]. These elevated intracellular Cu levels subsequently foster malignant tumors' environmental acclimatization and growth. The role of Cu in tumor development has been a long-standing concern, with numerous studies showing that malignancies require higher levels of Cu than normal tissues [36]. Elevated Cu concentration has been observed in the tissues or serum of patients with various types of tumors, including liver, breast, colon, prostate, and thyroid tumors [37-40]. It is widely recognized that Cu is critical in promoting tumorigenesis through various mechanisms. Cu-dependent cell growth and proliferation were referred to as ‘cuproplasia' [5]. Here, we outline how tumor cells upregulate intracellular Cu ion levels to promote tumor survival, growth, and metastasis (Figure 2).
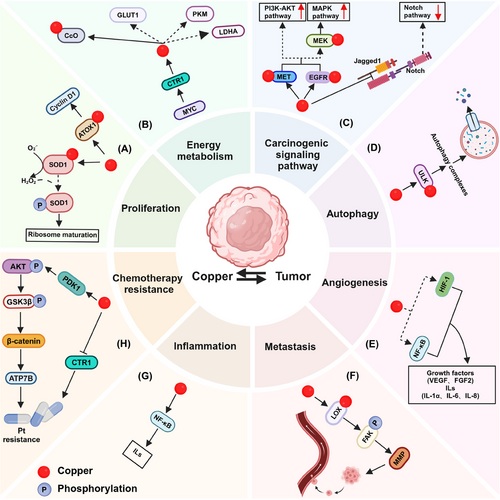
3.1.1 Tumor proliferation
The cell cycle comprises a series of precisely regulated stages that ensure the timely division and multiplication of cells. The cell cycle is primarily classified into four phases: G1 (growth phase 1), S (synthesis phase), G2 (growth phase 2), and M (mitosis phase). Cyclin D1 is a crucial cell cycle protein responsible for regulating the transition from the G1 phase to the S phase. In normal cells, the expression level of Cyclin D1 is tightly regulated. However, Cyclin D1 activity in tumor cells is frequently elevated, resulting in dysregulated cell cycle control and uncontrolled cell proliferation [41]. Interestingly, the expression of Cyclin D1 and probably other cell cycle-regulated proteins are regulated by Cu-related proteins. ATOX1 functions as a transcription factor in a Cu-dependent manner in tumors by facilitating Cu transport into the nucleus through its conserved C-terminal KKTGK motif and N-terminal Cu-binding site. Specifically, ATOX1 is transported into the nucleus, where it binds to the -535 to -530 (5'-GAAAGA-3') region of the Cyclin D1 promoter, increasing the expression of Cyclin D1 [42]. Taken together, ATOX1 translocates to the nucleus, binds to DNA, and activates transcription of Cyclin D1, thereby promoting tumor proliferation (Figure 2A).
SOD1, a key cuproenzyme, is crucial for oncogene-driven cell proliferation, and inhibiting SOD1 has the potential to hinder the growth of tumor cells by triggering multiple cell death pathways [43]. As a primary cytoplasmic superoxide dismutase, SOD1 is responsible for dismutating superoxide and converting superoxide radicals into H2O2 with the assistance of Cu. For instance, SOD1 rapidly translocated to the nucleus as H2O2 levels rise. Mechanistically, H2O2 enhanced the binding of SOD1 to Dun1/Cds1 kinase, leading to the phosphorylation of SOD1 at the S60 and S99 sites, which facilitates the nuclear localization of SOD1 [44]. In non-small cell lung cancer (NSCLC) driven by mutated K-RAS, nuclear-localized SOD1 plays a critical role in ribosome biogenesis and cell proliferation. Through liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, BOP1 and WDR12 were identified to interact with nuclear SOD1 [45]. These two proteins are part of the PeBoW (PES1-BOP1-WDR12) heterotrimeric complex, which plays a crucial role in pre-rRNA processing and the maturation of the 60S ribosomal subunit [46, 47]. Therefore, SOD1 interacts with the PeBoW complex and plays a crucial role in regulating its assembly, which is essential for the maturation of pre-60S ribosomal subunits and cell proliferation (Figure 2A).
3.1.2 Energy metabolism
One key aspect is the fulfillment of higher energy requirements by tumor cells, which necessitate increased mitochondrial function to support their rapid growth [48]. A plausible explanation is that Cu performs as a cofactor of mitochondrial CcO, an enzyme essential for mitochondrial respiration and ATP synthesis, which is required for rapidly dividing cells to achieve a high level of energy metabolism [2] (Figure 2B).
In accordance with recent research, the expression of CTR1 is higher in tumor tissues compared to non-tumor tissues, facilitating increased Cu uptake and proliferation in various tumor types such as gastric and liver malignancies [49]. C-MYC, a crucial regulator of cell proliferation, tumor invasion, and metastasis, binds to a particular region of the CTR1 promoter, which governs the transcription of the CTR1. Consequently, the levels of CTR1 and c-MYC proteins progressively increase in liver tissues from NAFLD-cirrhosis to hepatocellular carcinoma (HCC) patients [3]. Additionally, Davis et al. indicated that knocking out the CTR1 in HCC cells could drastically hinder the proliferation and metabolism of tumor cells by blocking the hypoxia-mediated increase in glucose importer 1 (GLUT1), pyruvate kinase muscle isoform (PKM) or lactate dehydrogenase A (LDHA) transcripts [35] (Figure 2B). Consequently, the inhibition of c-MYC may impede the development of liver tumors by downregulating CTR1 mRNA, reducing cytoplasmic Cu levels and tumor energy metabolism.
3.1.3 Carcinogenic signaling pathway
The activation of the carcinogenic signaling pathway is also facilitated by Cu, which regulates multiple signaling pathways by interacting with key molecules. Cu has the capacity to directly bind to and stimulate epidermal growth factor receptor (EGFR) and mesenchymal to epithelial transition factor (MET) signaling even in the absence of their respective ligands, epidermal growth factor (EGF) and hepatocyte growth factor (HGF) [50]. Consequently, Cu can initiate receptor tyrosine kinase-mediated signal transduction in a ligand-independent manner, which in turn encourages tumorigenesis (Figure 2C). Mutations in BRAF kinases, particularly the Val 600→Glu (V600E) mutation, activate the mitogen-activated protein kinase (MAPK) pathway, which plays a significant role in tumorigenesis and disease recurrence in melanoma, colorectal tumor (CRC), thyroid tumor, and leukemia [51-53]. BRAF(V600E) phosphorylates and activates MEK1/2 kinases, subsequently stimulating ERK1/2 kinases, leading to the stimulation of ERK1/2 kinases and triggering a signal cascade that drives malignancy. The elevated cytoplasmic Cu via up-regulated CTR1 could directly interact and activate MEK1/2 to enhance the activation of ERK1/2 [54]. Conversely, reducing CTR1 levels or mutating the MEK1/2-Cu interaction sites H188, M230, H239, or M187 inhibit BRAF(V600E)-driven signaling and ERK1/2 phosphorylation, thus hindering oncogenesis [54, 55]. Blockhuys S et al. utilized immunohistochemical techniques to show that ATOX1 protein levels were elevated in breast tumor tissues compared to normal tissues. Interestingly, except for the HER2+ subtype, higher ATOX1 intensity was observed in the other three breast tumor subtypes. Among these subtypes, the luminal A subtype, characterized by the lowest proliferation rate, exhibited the highest frequency of low ATOX1 levels [56]. Furthermore, another study revealed significant overexpression of ATOX1 in breast tumor and melanoma tissues compared to normal tissues. Deletion of ATOX1 or inhibition with the small molecule DCAC50 suppressed BRAF(V600E)-dependent melanoma proliferation by reducing ERK1/2 phosphorylation [57]. Cu chaperone protein CCS is predominantly expressed in the cytoplasm of breast tumors. A reduction in CCS levels can inhibit the phosphorylation and activation of ERK1/2, mediated by the accumulation of ROS, thereby preventing the proliferation and migration of breast cancer cells [58]. Mechanistically, CCS selectively interacts with Cu and facilitates its transfer to MEK1 [59]. This transfer enhances the Cu-dependent kinase activity of MEK1, amplifying the RAF-MEK-ERK signaling cascade and promoting tumor proliferation.
The notch signaling pathway, an evolutionally conserved pathway, regulates tissue development and homeostasis, playing a double-edged role in the occurrence and development of tumors [60, 61]. Notch ligand Jagged 1 and adhesion molecule E-cadherin are key cell surface proteins associated with the invasion and metastasis of prostate tumors. One study demonstrated that Cu significantly contributes to the invasion of prostate cancer, suggesting that the selective post-translational activation of zinc metalloproteinase (ZMP)-mediated protein shedding plays a crucial role in this process (Figure 2C). Mechanistically, Cu can stimulate the ZMP-mediated proteolysis of Jagged 1 and E-cadherin, thereby enhancing the invasion of prostate tumor cells [62].
3.1.4 Autophagy
Facilitating autophagy is a crucial process in which cells take advantage of lysosomal degradation to remove damaged or unnecessary components, providing basic blocks and energy for cell recycling [63]. Autophagy is considered to be a self-defense mechanism of the body and plays a dual role in tumor development. In the early stages, autophagy can help prevent carcinogenesis by suppressing cell necrosis and inflammation [64]. However, in later stages, autophagy can support the energy needs of tumor cells for survival and growth [65]. Upon comparison with the Cu-binding sequences in MEK1, it was observed that the autophagy kinases Unc51-like autophagy-activating kinase 1 (ULK1) and ULK2 share substantial sequence around the amino acids H188, M230, and H239, which are essential for Cu binding. The interaction between Cu and ULK1/2, akin to MEK1, facilitates autophagosome formation, thereby promoting the survival and proliferation of KRASG12D driving lung adenocarcinomas [66] (Figure 2D).
3.1.5 Angiogenesis
Inducing angiogenesis is a hallmark of tumors, which helps tumors receive nutrients and oxygen while releasing waste and carbon dioxide [67]. Exposure of endothelial cells to 100 µmol/L Cu significantly enhances migration and proliferation, while endothelial cell migration and proliferation are inhibited when the Cu transporter CTR1 is silenced [68]. Mechanistically, Cu chelation-induced Cu deficiency primarily inhibits the level and transcriptional activity of NF-κB, leading to the suppression of NF-κB-mediated pro-angiogenic factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF2), interleukin(IL)-1α, IL-6, and IL-8 [69] (Figure 2E). Furthermore, tetraethylenepentamine pentahydrochloride (TEPA)-induced Cu chelation activates factor inhibiting hypoxia-inducible factor-1 (FIH-1), leading to the hydroxylation of hypoxia-inducible factor-1α (HIF-1α) and subsequent inhibition of its interaction with p300 and hypoxia-responsive element (HRE) without affecting the expression or stability of HIF-1, thereby inhibiting the expression of HIF-1-mediated VEGF expression. However, in the presence of 25 µmol/L copper sulfate (CuSO4), the effect of TEPA was suppressed [70]. Nevertheless, the precise mechanisms by which Cu activates NF-κB and how Cu regulates FIH-1 remain unclear and warrant further investigation.
3.1.6 Metastasis
Metastasis is a unique cell biological behavior of malignant tumors and is the primary cause of death in advanced tumor patients [71]. The lysyl oxidase (LOX) family, a Cu-dependent metalloenzyme in the extracellular matrix, plays a crucial role in premetastatic niche formation [72]. Although the mechanism by which Cu is transported to LOX remains unclear, the Cu transporter ATP7A plays a vital role in the regular enzymatic activity of LOX. In an orthotopic mouse breast tumor model, silencing ATP7A inhibited LOX activity in the 4T1 cells, leading to the loss of LOX-dependent metastatic mechanisms [73]. Mechanistically, elevated expression of LOX promotes phosphorylation of the non-receptor tyrosine kinase focal adhesion kinase (FAK) at Y576, significantly enhancing tumor cell proliferation and migration. FAK is critical in facilitating tumor cell migration and invasion by modulating interactions between cells and the extracellular matrix (ECM). This process is mediated by membrane-type 1 matrix metalloproteinase (MT1-MMP). The interaction between MT1-MMP and FAK enhances matrix degradation at focal adhesions (Figure 2F). Mechanistically, FAK forms a complex with p130Cas and indirectly interacts with MT1-MMP, which is phosphorylated at the Y573 site of its cytoplasmic tail by Src kinase. This phosphorylation promotes the degradation of the focally adherent ECM, thereby contributing to tumor invasion and metastasis [74]. As a result, LOX promotes the migration and aggressiveness of osteosarcoma cells in vitro and produces more lung metastasis in vivo [75, 76].
3.1.7 Inflammation
Inflammation is a complex physiological process essential for clearing pathogens and repairing damaged tissues, triggered by the body in response to harmful stimulation. However, unchecked chronic inflammation, primarily driven by macrophages, can cause tissue damage and tumorigenesis [77]. Inflammatory macrophages can produce hydrogen peroxide when a Cu signal is present in the mitochondria, facilitating NADH oxidation to generate NAD+. This process ensures the synthesis of α-ketoglutarate (α-KG) and acetyl-CoA, initiating the transcriptional regulation of epigenetic factors such as KDMs and KATs and ultimately enhancing the expression of inflammatory genes like interleukins, thereby promoting inflammation, tumor proliferation and metastasis [78]. Furthermore, the inflammatory cytokine IL-17 promotes Cu uptake by stimulating the metalloreductase STEAP4, thereby facilitating the progression of colon tumors. Specifically, IL-17 enhances Cu uptake by increasing the mRNA levels of STEAP4, which sustains IL-17-induced NF-κB activation and contributes to tumor progression and metastasis [79] (Figure 2G).
3.1.8 Chemotherapy resistance
The accumulation of platinum-based anti-tumor drugs within cells is essential for their therapeutic efficacy. A reduction in the influx of platinum or an increase in its efflux leads to decreased intracellular levels of these drugs, which indicates platinum resistance. CTR1 plays a crucial in the entry of cisplatin (a platinum based anti-tumor drug) into cells [80]. Like Cu(I), cisplatin interacts with the methionine-rich motif located in the extracellular domain of CTR1, bringing cisplatin into the cells via endocytosis [81, 82]. It has been observed that lower levels of CTR1 correlate with increased resistance to cisplatin in tumors, while higher expression of CTR1 is associated with enhanced sensitivity to cisplatin [83]. Studies have demonstrated that exposure to excessive Cu causes the CTR1 protein to undergo endocytosis and subsequent degradation [84, 85]. Consequently, treating cells with Cu results in diminished cisplatin uptake and enhanced drug resistance [80]. In contrast, the addition of the Cu chelator bathocuproine disulphonate produces the opposite effect [86]. Moreover, some studies have elucidated potential mechanistic explanations for how Cu-related signaling regulates the efflux of platinum-based drugs, which contributes to chemotherapeutic resistance. It was reported that elevated levels of intracellular Cu ions bind to PDK1, enhancing its interaction with AKT and subsequently activating the Wnt/β-catenin pathway. Specifically, the β-catenin/TCF4 transcription complex directly interacts with the ATP7B promoter region (−1250 to −1000 bp upstream of the transcription start site), accelerating the transcription of ATP7B [87] (Figure 2H). Up-regulated ATP7A or ATP7B was found to enhance the delivery of Cu to members of the LOX family within the Golgi compartments and platinum efflux, ultimately contributing to platinum resistance in ovarian tumor cells [73, 88].
In conclusion, tumor cells enhance intracellular Cu levels by modifying proteins associated with Cu homeostasis. This elevated Cu facilitates tumor growth and adaptation to the host environment, creating a positive feedback loop that exacerbates tumor progression. Consequently, reducing Cu ion levels to disrupt this feedback loop may represent a promising strategy for tumor treatment.
3.2 Cuproptosis
Previous research has demonstrated that an excess of metal ions can result in cell death through specific pathways. In 2012, Dixon SJ et al. first identified a type of iron-dependent programmed cell death called ferroptosis, characterized by the excessive build-up of lipid peroxides and reactive oxygen species (ROS) mediated by iron-dependent fenton reaction [89]. Similarly, the relationship between Cu and cell death has been a focus of investigation for several decades [90]. However, the precise mechanism by which Cu leads to cell death remains poorly understood. Historically, researchers have associated Cu-induced cell death with apoptosis [91], autophagy [92], or ferroptosis [93]. Remarkably, it was not until March 2022 that Tsvetkov et al. elucidated the precise mechanism of Cu-induced cell death, coining the term cuproptosis to describe this unique form of cell death, which was a milestone in the research field of Cu-mediated cell death [94].
Cuproptosis relies on excessive Cu delivered to mitochondria through Cu ionophores like elesclomol, which cannot be rescued by inhibitors of apoptosis, necrosis, or ferroptosis, except for Cu chelators (tetrathiomolybdate, etc.) [94]. In addition, this form of cell death primarily affects mitochondria, leading to oxidative damage to the membrane and reduced enzyme function in the TCA cycle [95]. Cu ionophores, as inducers of cuproptosis, transport Cu(II) into the mitochondria, where it is converted into the more cytotoxic Cu(I) by ferredoxin 1 (adrenodoxin; FDX1), a member of the ferredoxin family containing the Fe-S cluster in mitochondria. FDX1 plays a crucial role in the cellular stress caused by mitochondrial proteins during cuproptosis. Aside from its role in Cu(I) conversion during cuproptosis, FDX1 also facilitates the lipoylation of mitochondrial proteins by directly interacting with lipoic acid synthetase (LIAS) and enhancing its binding to the lipoyl carrier protein, glycine cleavage system H protein (GCSH) [96, 97]. Lipoylation is a highly conserved post-translational modification (PTM) of proteins that occurs in various organisms, from bacteria to humans, affecting specific lysine residues in four enzymes that are part of metabolic complexes regulating carbon entry into the TCA cycle [98]. These four enzymes include Dihydrolipoamide S-Acetyltransferase (DLAT), Dihydrolipoamide S-Succinyltransferase (DLST), Dihydrolipoamide Branched Chain Transacylase E2 (DBT) and Glycine Cleavage System Protein H (GCSH). Among them, DLAT and DLST are significant components of PDH and KDH complexes, respectively, where lipoylation is crucial for their functions [98]. However, overloaded Cu(I) in mitochondria can directly bind to lipoylated proteins, facilitating the aggregation of lipoylated proteins to promote cuproptosis [94]. Notably, DLAT aggregation in such a Cu-dependent manner is one of the most crucial markers for detecting cuproptosis at present. Increased Cu(I) levels in mitochondria not only promote lipoylated proteins to aggregate but also weaken the Fe-S cluster proteins, including FDX1, LIAS, aconitase-2 (ACO-2), succinate dehydrogenase complex iron sulfur subunit B (SDHB), and DNA polymerase delta 1 (POLD1) [94]. Taken together, overloaded Cu(II) is delivered into mitochondria via Cu ionophores, which is further converted to Cu(I) by FDX1, promoting lipoylated protein aggregation and Fe-S protein turnover to trigger proteotoxic stress, eventually resulting in cell death termed cuproptosis (Figure 3). In normoxic conditions, blockage of the electron transfer chain (ETC) can mitigate cuproptosis. This implies that elevated activity in the TCA cycle and protein lipoylation can increase vulnerability to cuproptosis by promoting the aggregation of lipoylated mitochondrial proteins and decreasing Fe-S cluster content [94]. Hence, shifting the metabolism of tumor cells from aerobic glycolysis to the TCA cycle could increase their sensitivity to cuproptosis.
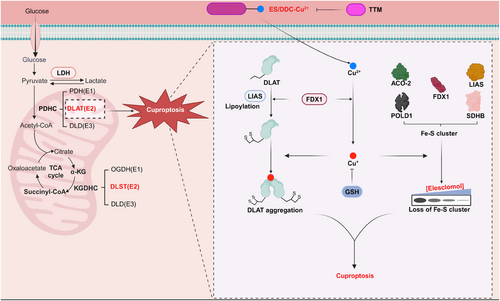
Other than FDX1, using CRISPR-Cas9 knockout screens, Tsvetkov et al. also defined genes involved in the lipoic acid pathway (LIAS and LIPT1) and genes encoding the pyruvate dehydrogenase complex components (DLAT, DLD, PDHA1, and PDHB) are necessary for cuproptosis [94]. In addition to these genes positively linked to cuproptosis, sensitivity to cuproptosis has been negatively linked to cyclin-dependent kinase inhibitor 2A (CDKN2A), glutaminase (GLS), and metal regulatory transcription factor 1 (MTF1) [94].
Following the initial discovery of cuproptosis, recent studies have investigated its regulatory mechanisms in tumor cells, primarily focusing on the protein lipoylation pathway and Cu efflux. For example, the lactylation of METTL16 at K226, an atypical methyltransferase, enhanced the stability of FDX1 mRNA by increasing methylation at site 602A in the coding sequence (CDS) region of FDX1. This modification significantly elevated mitochondrial protein lipoylation levels and cuproptosis in vitro and in vivo [99]. In addition, the ferroptosis inducers sorafenib and erastin can promote cuproptosis in primary liver cancers by enhancing the aggregation of Cu-dependent lipoylated proteins in vitro and in vivo. Mechanistically, both sorafenib and erastin upregulate protein lipoylation by inhibiting the degradation of FDX1, a process mediated by the mitochondrial matrix-associated protease AFG3L2. Additionally, these compounds reduce intracellular Cu chelation by inhibiting the synthesis of GSH through the blockade of cystine import [100]. Moreover, the deficiency of AT-rich interactive domain-containing protein 1A (ARID1A) suppressed glycolysis by downregulating PKM transcription, which in turn enhanced the TCA cycle in liver tumors, ultimately sensitizing cuproptosis in a FDX1-dependent manner [101]. Furthermore, a recent study demonstrated that the β-catenin/TCF4 transcriptional complex directly binds to the ATP7B promoter, leading to its increased expression. The upregulation of ATP7B facilitates the efflux of Cu ions, thereby reducing intracellular Cu levels and inhibiting cuproptosis [87].
To summarize, cuproptosis is thought to interact with components of the TCA cycle in mitochondria rather than the ETC or ATP generation, and it requires a highly conserved protein post-translational modification known as lipoylation. However, there has been less emphasis on understanding the mechanism of reducing the Fe-S cluster. We speculate that exploring the role of Fe-S cluster proteins in cuproptosis will be a valuable contribution to this research area. Furthermore, we contend that utilizing Cu-induced cell death as a therapeutic strategy for tumor eradication could be a potent approach.
4 TARGETING CU HOMEOSTASIS FOR TUMOR THERAPIES
Cu, necessary for the host's regular bodily functions, can generate disease and even death once its equilibrium is upset. Recent evidence highlights that Cu plays an important role in the carcinogenesis and metastasis of tumor cells. Tumor cells reprogram the Cu metabolism regulation network to increase the intracellular Cu levels, fulfilling the tumor's requirement. Consequently, it renders tumor cells more susceptible to Cu-targeted strategies and has sparked interest in developing potential Cu-dependent antitumor drugs to combat tumors. Researchers are now focusing on identifying new Cu-dependent pathways to develop therapeutic approaches that exploit the vulnerability of tumor cells to Cu. Herein, we provide an overview of the two primary strategies currently used to target Cu in tumor treatment: Cu chelators to block cuproplasia and Cu ionophores to induce Cu-dependent cell death, such as cuproptosis (Figure 4).
4.1 Anti-tumor strategy of Cu depletion
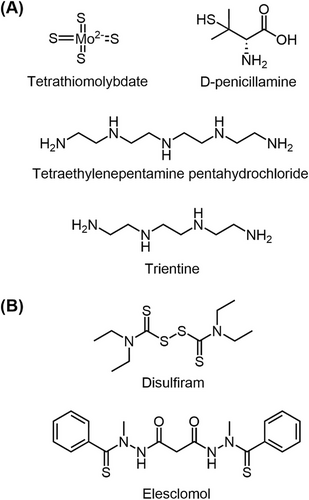
Due to the importance of elevated Cu levels in tumor development, an increasing number of studies have focused on Cu chelators as potential anti-tumor agents. These chelators work by reducing intracellular Cu levels in tumor cells (Figure 4A). Taking advantage of Cu chelators, such as penicillamine, trientine, or tetrathiomolybdate (TTM), can deplete bioavailable Cu, leading to a reduction in tumor vessel diameter, inhibition of endothelial cell proliferation (as indicated by decreased Ki67 expression) and a decrease in ICAM/CD54 expression [102]. Therefore, depleting Cu levels shows promise as a therapeutic strategy for treating tumors. In addition to in vitro studies, the antitumor activity of Cu-chelating agents such as penicillamine, trientine, and TTM has also been explored in animal models and clinical trials, demonstrating promising antitumor potential (Table 1).
| Agents | Action | Condition and phase | Results |
|---|---|---|---|
| TTM | Chelator | Mesothelioma (phase II) [103] | TTM demonstrates antiangiogenic effects in malignant pleural mesothelioma and exhibits minimal toxicity, showing comparable efficacy to previous multimodal trials. |
| TEPA | Chelator | Preclinical phase [104] | TEPA promotes the ubiquitin-mediated degradation of PD-L1, increases the number of tumor-infiltrating CD8+ T cells and natural killer cells, slows tumor growth, and improves survival in mice, underscoring its potential as an anti-tumor immune enhancer. |
| DPEN | Chelator | Preclinical phase [105] |
The combination of diet and penicillamine treatment led to the development of smaller and relatively avascular tumors in the brains of rabbits. However, this treatment did not effectively inhibit tumor growth, the vascularization of femoral VX2 carcinoma in the thigh muscle, or the metastases to the lung. |
| TETA | Chelator | Preclinical phase [106] | Given that TETA has been utilized in clinical practice without any severe side effects, particularly in comparison to penicillamine, it may represent a promising new strategy for treating hepatocellular carcinoma. |
| DSF | Ionophore | Glioblastoma (phase I) [107] | DSF can be safely combined with temozolomide, although it may induce reversible neurological toxicities. |
| ES | Ionophore | Melanoma (phase II/III) [108, 109] | In a phase II trial, the addition of ES to paclitaxel doubled the median PFS and decreased the likelihood of disease progression or death compared to paclitaxel alone. However, the combination of ES and paclitaxel did not achieve the PFS endpoint in a phase III trial. |
- Abbreviations: DPEN, D-penicillamine; DSF, disulfiram; ES, elesclomol; PD-L1, programmed cell death ligand 1; TEPA, tetraethylenepentamine pentahydrochloride; TETA, trientine; TTM, tetrathiomolybdate.
TTM, a Cu chelator, hinders Cu-dependent proliferation in tumors by reducing intracellular Cu levels without causing significant harm to normal tissues [110]. TTM inhibits the Cu-dependent mitochondrial complex IV activity, resulting in reduced mitochondrial respiration and increased oxygen availability. This increase in oxygen levels is necessary for activating HIF-prolyl hydroxylase (PHD), facilitating the interaction between PHD and the oxygen-dependent degradation domains (ODDs) of HIF-1α. This interaction leads to the hydroxylation of HIF-1α, which triggers the degradation of HIF-1α through the E3 ubiquitin ligase von Hippel-Lindau (VHL)-dependent ubiquitination-proteasome pathway. This degradation process ultimately hinders the transactivation of HIF-1α target genes such as PDK1, GLUT1, and VEGF, thereby inhibiting angiogenesis [111]. Apart from working as a Cu chelator, an in vitro investigation revealed that TTM limits cell proliferation by suppressing ATP7A expression upregulated by mutant KRASG12D in CRC, with the exact mechanism needing further elucidation [112]. Animal models have further confirmed the anti-angiogenic and anti-tumor effects of TTM, leading to its exploration as a potential anti-tumor drug in clinical trials. In both breast tumor xenograft in nude mice and Her2/neu tumor-prone transgenic mice, TTM effectively suppresses tumor growth and angiogenesis by depleting Cu, thus preventing the NF-κB-mediated transcription of proangiogenic factors like VEGF, IL-1α, and IL-8 [69, 113]. In a RIP1-Tag2 transgenic mouse model of pancreatic neuroendocrine tumors, Seiko Ishida et al. demonstrated that TTM-induced reduction in systemic Cu levels led to decreased proliferation and development of pancreatic tumors in vivo [114]. In TTM-treated Her2/neu and FVB mice, the median time for tumor development was delayed nearly one-fold compared to the control group. By observing mammary gland composition and architecture after TTM treatment, this study illustrated that TTM induced breast hypoplastic remodeling by weakening the epithelial duct branch system and reducing breast microvascular density, ultimately impeding breast tumor growth [115]. Combining the above findings, we conclude that TTM more or less achieves its antitumor effect on reducing Cu coenzyme activity. A phase II clinical trial elucidated that TTM delayed tumor progression in patients with stage I and II malignant mesothelioma due to its antiangiogenic effect while showing no noticeable impact in stage III patients [103]. Another phase II trial found that while TTM was well tolerated in advanced kidney tumor treatment and maintained Cu depletion, certain pro-angiogenic factors like IL-6, IL-8, and VEGF associated with TTM may be linked to Cu consumption rather than disease stability. These clinical studies suggest a potential for combining TTM with other antitumor therapies [116].
TEPA, a chelating agent for Cu ions, has been found to reduce programmed cell death ligand 1 (PD-L1) mRNA levels and enhance ubiquitin-mediated degradation by blocking the phosphorylation of STAT3 and EGFR in an experimental tumor model. This mechanism results in elevated levels of CD8+ T cells and natural killer cells, ultimately inhibiting tumor growth and enhancing survival rates in mice [104]. Due to possible Cu binding to EGFR and activating EGFR signaling in a ligand-independent manner, we speculate that TEPA might function to block EGFR activation by reducing Cu levels.
D-penicillamine (DPEN), a well-established Cu chelator for treating Wilson's disease [117], is capable of counteracting tumor development by depleting Cu. DPEN is an amino acid derivative characterized by the presence of a sulfhydryl group (-SH), which exhibits strong metal ion chelating properties. The sulfhydryl group of DPEN forms a coordination bond with Cu(II), forming a complex. During this interaction, DPEN facilitates the reduction of Cu(II) to Cu(I). This reduction is crucial for Cu excretion, as Cu(I) is less soluble and has a greater propensity to form insoluble chelates [118]. In addition, DPEN can enhance oxidative stress by catalyzing H2O2 production through Cu chelation, selectively targeting tumor cells, and improving responses to radiation and carboplatin treatment [119]. In a rabbit brain tumor model with large vascularized VX2 carcinomas, utilizing a Cu-depleted diet and penicillamine treatment led to decreased endothelial cell turnover, reduced microvascular density in brain tumors, and suppression of tumor volume. However, the efficacy of this treatment was influenced by regional variations in vascular endothelial responsiveness, Cu distribution, and Cu enzyme activity [105].
Trientine (TETA) is an orally active Cu(II)-selective Cu chelator that is considered to be better tolerated than penicillamine in treating Wilson's disease [120, 121]. Research by Yoshii J et al. suggests that trientine exhibits more substantial anti-tumor effects as opposed to penicillamine, particularly in suppressing tumor growth by inhibiting angiogenesis and promoting apoptosis in a mouse hepatocellular carcinoma xenograft model [106]. Through molecular analysis techniques such as RT-PCR and ELISA, it was demonstrated that trientine could reduce the level of angiogenic factor IL-8, leading to tumor regression [122]. Furthermore, trientine shows promise in overcoming chemotherapy resistance, as indicated in a pilot trial involving carboplatin and trientine for platinum-resistant high-grade epithelial ovarian tumors [123]. While trientine has demonstrated beneficial effects in various contexts, further investigation is needed to understand its potential off-target effects fully. For instance, a study revealed that trientine can inhibit hepatosteatosis and induce autophagy by modulating SAT1 activity without necessarily affecting Cu levels in tissues and organs [124].
In conclusion, targeting Cu-related tumor-specific vulnerabilities with small molecule Cu chelators is anticipated to enhance the effectiveness of existing tumor treatments. Notably, we also need to be clear that this field is still evolving, and specific challenges must be addressed. Existing Cu-related medications lack selectivity and may disrupt the body's Cu balance when used long-term, resulting in potential adverse effects for patients undergoing treatment.
4.2 Anti-tumor strategy of Cu overload
The elucidation of the cuproptosis mechanism has paved the way for future pharmaceutical advancements. Cu ionophores, also known as cuproptosis-related drugs, manufacture cuproptosis by increasing intracellular Cu levels through Cu translocation, offering a potential therapeutic avenue for tumor treatment (Figure 4B). Medications such as disulfiram (DSF) and elesclomol (ES) have garnered significant interest due to their ability to elevate cellular Cu levels, and they are currently undergoing clinical trials (Table 1).
DSF, a Food and Drug Administration (FDA)-approved drug with a long history of treating alcoholism, boasts favorable pharmacokinetic properties and a strong safety profile. Drug repurposing is a promising strategy to address the challenges in tumor therapy. Over the past decade, emerging evidence indicates that DSF grants great antitumor potential in various preclinical models, such as prostate tumors, breast tumors, and lymphoid malignant tumors [125-127]. The key to the anti-tumor effect of DSF lies in its ability to target the tumor stem cell marker aldehyde dehydrogenase (ALDH) to inhibit tumor stem cells and suppress proteasome activity in tumor cells through the formation of complexes with metal ions [128]. In addition, it is important to highlight that extracellular Cu is essential for the cytotoxicity of DSF, with the addition of Cu(II) significantly enhancing DSF-induced cell death. Mechanistically, Cu(II) undergoes a complex extracellular redox reaction with DSF to produce the potent Cu(deDTC)2 complex (also known as CuET), a reaction specific to Cu(II) and not seen with other metals such as Fe(II or III), Mn(III), and Zn(II). This Cu(deDTC)2 complex exhibits increased efficacy against melanoma and is proposed to be the primary agent responsible for DSF-induced toxicity [129]. In current studies, DSF, acting as a Cu transporter in conjunction with Cu ions (DSF-Cu), has been shown to induce various forms of cell death, such as apoptosis, ferroptosis, and cuproptosis [130, 131]. Furthermore, DSF has been identified as a DNMT inhibitor, suppressing the expression and activity of DNA methyltransferase 1 (DNMT1) and reducing methylation of the interferon regulatory factor 7 (IRF7) promoter region. This resulted in an increased expression of IRF7, leading to more significant binding of IRF7 to the PD-L1 promoter and an elevation in PD-L1 mRNA levels. Ultimately, this enhanced the immune response to anti-PD-1 antibodies in a breast tumor mouse model derived from a 4T1 cell line [132]. Recent studies have demonstrated that DSF functions as a Cu ionophore, facilitating the entry of Cu into cells. This process leads to the aggregation of lipoylated mitochondrial proteins and a reduction in the levels of Fe-S cluster proteins. Consequently, CuET induces cuproptosis by disrupting the mitochondrial TCA cycle in tumor cells [94]. Clinical studies have also demonstrated the anti-tumor and chemo-sensitizing effects of DSF-Cu on glioblastoma (GBM), likely attributed to its high blood-brain barrier permeability [133]. A phase I clinical trial revealed that the combination of DSF and temozolomide is not only safe but also enhances progression-free survival in GBM patients [107]. Furthermore, a nationwide survey indicated that tumor patients who continued taking DSF post-diagnosis had a lower risk of tumor-related mortality [134]. With well-established pharmacokinetics and documented safety, DSF presents itself as a promising ‘old’ drug with significant potential for expedited development as a new tumor treatment. Though the selectivity and the underlying mechanism of how DSF delivers or increases the mitochondrial Cu levels remain undefined, we think the anti-tumor effect of DSF relies on the induction of cuproptosis, at least partially.
Elesclomol (also known as STA-4783) is a well-researched cuproptosis inducer that has shown promise in enhancing the antitumor effects of taxanes. This compound, a bis (thiohydrazide) amide, forms a 1:1 complex with Cu(II) and has various mechanisms for killing tumor cells [135]. Elesclomol specifically binds to extracellular Cu(II) and enters cells as elesclomol-Cu(II), which then transports Cu(II) to mitochondria. By repeatedly redistributing Cu from the cell exterior to mitochondria upon dissociation from the complex, elesclomol leads to Cu accumulation in mitochondria. Notably, compared to other Cu transporters like DSF, elesclomol demonstrates superior selectivity for mitochondria [136]. Over the past decade, elesclomol-Cu(II) therapy has shown significant advancements. Previous research suggested that treatment with elesclomol-Cu(II) induces Cu-related apoptosis by increasing ROS levels beyond the cell's tolerance threshold in tumors [136, 137]. Since 2015, new mechanisms of tumor cell death induced by elesclomol-Cu(II) have been reported, including G1 cell cycle arrest, DNA double-strand breaks, and the degradation of ATP7A in CRC [138, 139]. In 2019, the identification of FDX1 through genome-wide CRISPR-Cas9 screening revealed it as a key gene linked to the effectiveness of elesclomol-Cu(II) therapy under proteolysis stress. Further analyses showed that FDX1 is a direct target of elesclomol-Cu(II), leading to a distinct form of Cu-dependent cell death [140]. Herein, FDX1, the gene most linked to elesclomol sensitivity, encodes a protein that directly binds elesclomol-Cu(II) through its α2/α3 helices and β5 strand, thereby inhibiting the formation of Fe-S clusters [140]. Additionally, research by Tsvetkov et al. highlighted that tumors with Hi-Mito disorder, favoring oxidative phosphorylation over glycolysis, exhibit resistance to proteasome inhibitors but are more susceptible to cuproptosis induced by elesclomol-Cu(II) [94]. Furthermore, studies have demonstrated that ferroptosis inducers sorafenib and erastin upregulate protein lipoylation by inhibiting the degradation of the FDX1 protein, a process mediated by the mitochondrial matrix-associated protease AFG3L2 [100]. Additionally, these compounds reduce intracellular Cu chelation by inhibiting cystine import, which leads to decreased synthesis of GSH and consequently promotes elesclomol-Cu(II)-induced cuproptosis in liver tumor [100]. In addition to its role in treating liver tumor, another study reported that the SIRT2-specific inhibitor AGK2 activates METTL16 by enhancing the lactylation of its K229 site. This activation results in an increase in m6A accumulation of FDX1 mRNA and its stability, thereby promoting elesclomol-Cu(II)-mediated cuproptosis in gastric tumor in vitro and in vivo [99]. Although elesclomol-Cu(II) has been studied for its potent tumoricidal effects, research has shown that intracellular Cu binds to PDK1, enhancing its interaction with AKT. This interaction activates the Wnt/β-catenin pathway and promotes ATP7B transcription, thereby demonstrating a capacity to resist cuproptosis. Consequently, combining the Wnt inhibitor LF3 with elesclomol-Cu(II) also shows significant potential [87]. Several clinical trials have evaluated the combination of paclitaxel and elesclomol, particularly in advanced melanoma cases. A phase I clinical trial demonstrated that the toxicity profile of elesclomol with paclitaxel was well-tolerated and comparable to that of paclitaxel alone [141]. In a phase II randomized, controlled, double-blind trial for stage IV metastatic melanoma, the addition of elesclomol to paclitaxel doubled the median progression-free survival (PFS) and reduced the likelihood of disease progression or death by 41.7% compared to paclitaxel alone [108]. Although the PFS endpoints were not met in a randomized, double-blind phase III trial of elesclomol in conjunction with paclitaxel in patients with chemotherapy-naive advanced melanoma, elesclomol demonstrated a high tumor-suppressive effect in patients with low lactate dehydrogenase (LDH) levels [109]. Low levels of LDH imply a mitochondrial hyperdynamic condition, which aligns with the observation that proteins involved in oxidative phosphorylation with elevated levels of lipoylation are more sensitive to elesclomol [94]. However, further clinical trials are needed to investigate the potential of using Cu ionophores to treat malignancies with active TCA cycles. In summary, combining Cu ionophores with targeted therapeutic agents can combat tumors with high mitochondrial metabolism levels, particularly those in a dynamic state of oxidative phosphorylation. The use of LDH as a predictor and prognostic indicator can guide this treatment effectively. In addition to utilizing LDH to assess tumor sensitivity to cuproptosis, another study demonstrated that in SF3B1-mutated leukemias, ABCB7 is mis-spliced and downregulated, leading to increased susceptibility to Cu ionophores. Furthermore, ABCB7 overexpression was shown to partially rescue the cuproptosis associated with the SF3B1 mutation, thereby suggesting that SF3B1 mutations could serve as a biomarker for future therapies based on Cu ionophores [142]. Overall, these findings suggest that Cu ionophores may be a promising strategy for inhibiting tumors with specific metabolic characteristics.
Traditional Cu ionophores are associated with various off-target effects, such as excessive Cu could lead to neurological side effects including seizures and brain circuit disturbances [143], leading to the emergence of nanomaterials designed for specific Cu-induced cell death in tumor tissues. Cancer stem cells (CSCs) typically reside in the hypoxic tumor microenvironment (TME) of triple-negative breast cancer (TNBC), where the expression of the cuproptosis-related protein FDX1 is inhibited, thereby diminishing the anti-cancer efficacy of cuproptosis. In response to this challenge, Xiao C et al. developed CuET@PHF, an active targeting cuproptosis-based nanomedicine that responds to reactive oxygen species (ROS). This formulation employs polydopamine and hydroxyethyl starch to stabilize CuET nanocrystals, effectively eradicating CSCs. The photothermal effect of CuET@PHF is capable of mitigating tumor hypoxia, which enhances both cuproptosis and immunogenic cell death in 4T1 CSCs. Consequently, the combination of CuET@PHF with mild photothermal therapy not only significantly inhibits tumor growth but also effectively prevents tumor recurrence and distant metastasis by targeting CSCs and bolstering anti-tumor immune responses [144].
While the potential of Cu overload as a cancer treatment strategy is promising, it is crucial to acknowledge that elevated Cu levels can lead to various side effects. Additionally, the poor water solubility of ES-Cu might be another hindrance to the efficacy of Cu overload-based anti-tumor therapies [145]. Therefore, further efforts need to pay more attention to the specificity and solubility of Cu ionophores in tumors, employing a nanoparticle delivery system could be a rational solution. Moreover, both Cu ionophores and chelators have shown potential in tumor therapy; therefore, determining the optimal treatment conditions, such as classified biomarkers and combined therapeutics, remains an emergent challenge in utilizing these medications in the future.
5 CONCLUSIONS
The role of Cu in tumors is multifaceted, akin to two sides of a coin. On the one hand, Cu is a vital cofactor for numerous enzymes that activate oncogenic signaling, fostering tumor growth and progression. On the other hand, an excess of Cu could be toxic, which facilitates the aggregation of lipoylated mitochondrial proteins and impedes iron-sulfur cluster proteins, culminating in cell demise. Thus, tumor cells finetune Cu metabolism to reach a comfort zone. For example, tumors enhance CTR1 expression to fulfill their elevated Cu demands [2, 49], while increased cytoplasmic CCS transports Cu to MEK1, enhancing MEK1 kinase activity [55]. KRAS-mutated cells counteract intracellular Cu overload toxicity by elevating ATP7A expression [112]. Though numerous studies have documented the interaction between Cu and tumor cells, limited efforts are focused on the relationship between Cu and the tumor microenvironment, especially in tumor immunity. Hence, investigating the involvement of Cu in tumor microenvironment holds significant promise for future research endeavors. Furthermore, as a newly identified programmed cell death, various unresolved issues persist in cuproptosis. For instance, while existing research predominantly focuses on the lipoylation of mitochondrial proteins in Cu-induced cell death, the molecular intricacies of iron-sulfur cluster protein reduction, another pivotal aspect, remain inadequately understood. In addition, the integration and regulation of intrinsic Cu transporters and exporters in cuproptosis need further exploration. Moreover, given the central role of the TCA cycle in energy metabolism and the connection of lipoylation to lipid metabolism, it is plausible to consider that, apart from glucose metabolism, other pathways like amino acid and lipid metabolism may influence the susceptibility to Cu-induced cell death. While some genes associated with Cuproptosis have been identified through research, additional experimental validation and mechanistic investigations are required to elucidate the precise link between these genes and Cu-induced cell death. Lastly, most of the current explored mechanisms of cuproptosis are largely based on cell culture data, more in vivo evaluations are urgently needed in the future.
So far, depleting and supplementing Cu to toxic levels have been considered potential strategies for targeting tumors. Clinical trials have explored using Cu chelators and Cu ionophores to modulate intracellular Cu levels in tumor cells, either by reducing Cu content essential for survival or promoting Cu-induced cell death, thereby inhibiting tumor progression. The clinical efficacy and safety of these approaches have garnered interest from researchers, with larger-scale trials and new designs guided by biomarkers needed to evaluate their effectiveness and safety further. While targeting Cu has shown promising advancements in tumor therapy, several challenges still need to be addressed, paving the way for future research in this field. One key issue is the lack of sensitive biological markers for targeted Cu ion therapy. For instance, LDH expression and activity could potentially serve as indicators for detecting sensitivity to Cu-induced cell death. Low LDH levels in patients possibly indicate a need for clinical use of Cu ion transporters [109]. More importantly, new evidence and biomarkers identification are needed to distinguish those patients who are suitable for Cu chelation, and others for Cu overload. Moreover, the essential role of Cu in the human body can be disrupted during systemic anti-tumor therapies targeting Cu ions, a concern for clinicians. To address this issue, researchers have explored local delivery systems or Cu-related nanoparticles for targeting tumors, aiming to enhance treatment specificity and reduce off-target effects. These systems include liver-specific Cu delivery through targeted ionophore metal supplementation and liposomal nanoformulations for delivering Cu(II) complexes to tumor site, as well as strategies to reduce Cu levels using mitochondria-targeted, Cu-depleting nanoparticles [146-148]. By shifting from systemic to local treatments, these studies offer promising avenues for improving therapeutic outcomes while minimizing unintended consequences. Nevertheless, with the upcoming discoveries on the crosstalk of Cu and tumors, we believe that targeting Cu-based therapies, especially synergistic strategies, would be a rational solution to precise tumor therapies.
AUTHOR CONTRIBUTIONS
Lifeng Feng and Hongchuan Jin provided the conception of this review article, supervised and revised the writing, and reviewed the manuscript before submission. Kaizhong Lu made contributions to the conception, searched the references and wrote the article. Chandra Sugiarto Wijaya and Qinghua Yao contributed to the interpretation of the article and the figures. All authors read and approved the final manuscript.
ACKNOWLEDGMENT
The authors have nothing to report. This study was supported by the Key Project of the Natural Science Foundation of Zhejiang Province (No. LZ24H160006 and LZ20H290001).
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.
Not applicable.




