Efficacy and safety of first-line sintilimab plus anlotinib versus chemotherapy for metastatic non-small cell lung cancer: a phase II, open-label, randomized controlled trial
Tianqing Chu and Hua Zhong contributed equally to this work.
Abstract
Background
The prognosis for non-small cell lung cancer (NSCLC) patients treated with standard platinum-based chemotherapy was suboptimal, with safety concerns. Following encouraging results from a preliminary phase I study, this phase II trial investigated the efficacy and safety of first-line sintilimab and anlotinib in metastatic NSCLC.
Methods
In this open-label, randomized controlled trial (NCT04124731), metastatic NSCLC without epithelial growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), or proto-oncogene tyrosine-protein kinase ROS (ROS1) mutations, and previous treatments for metastatic disease were enrolled. Participants were randomly assigned in a 1:1 ratio to either sintilimab (200 mg every 3 weeks) plus anlotinib (12 mg D1-14 every 3 weeks) or a standard platinum-based chemotherapy regimen. Patients in the chemotherapy group were permitted to switch to sintilimab after disease progression. The primary endpoint was the objective response rate (ORR).
Results
From November 2019 to March 2023, 99 patients were randomized into the sintilimab plus anlotinib group (n = 49) and the chemotherapy group (n = 50). The ORR was significantly higher in the sintilimab plus anlotinib group (44.9%; 95% confidence interval [CI] = 30.7%-59.8%) compared to the chemotherapy group (18.0%; 95% CI = 8.6%-31.4%, P = 0.003). Progression-free survival (PFS) was also notably longer (median: 14.4 vs. 5.6 months; hazard ratio [HR] = 0.39; 95% CI = 0.23-0.67; P < 0.001). The 24-month overall survival rate was 58.4% (95% CI = 40.4%-72.6%) and 43.2% (95% CI = 26.0%-59.2%), respectively. The rate of grade 3 or higher treatment-related adverse events was lower in the sintilimab plus anlotinib group (28.0%) than in the chemotherapy group (49.0%), especially for the hematological toxicities.
Conclusion
First-line sintilimab plus anlotinib showed improved ORR and PFS, alongside a superior safety profile, compared to the standard platinum-based chemotherapy for metastatic NSCLC patients.
List of Abbreviations
-
- AE
-
- adverse event
-
- ALK
-
- anaplastic lymphoma kinase
-
- CI
-
- confidence interval
-
- CR
-
- complete response
-
- CTCAE
-
- Common Terminology Criteria for Adverse Event
-
- DCR
-
- disease control rate
-
- DoR
-
- duration of response
-
- ECOG PS
-
- Eastern Cooperative Oncology Group performance status
-
- EGFR
-
- epithelial growth factor receptor
-
- FAS
-
- full analysis set
-
- HR
-
- hazard ratio
-
- irAE
-
- immune-related adverse event
-
- iRECIST
-
- immune Response Evaluation Criteria in Solid Tumors
-
- NCI
-
- National Cancer Institute
-
- NR
-
- not reached
-
- NSCLC
-
- non-small cell lung cancer
-
- ORR
-
- objective response rate
-
- OS
-
- overall survival
-
- PFS
-
- progression-free survival
-
- PD
-
- progressive disease
-
- PD-1
-
- programmed death 1
-
- PD-L1
-
- programmed death-ligand 1
-
- PR
-
- partial response
-
- RECIST
-
- Response Evaluation Criteria in Solid Tumors
-
- ROS1
-
- proto-oncogene tyrosine-protein kinase ROS
-
- SD
-
- stable disease
-
- SLD
-
- sum of the longest diameter
-
- SS
-
- safety set
-
- TEAE
-
- treatment-emergent adverse event
-
- TRAE
-
- treatment-related adverse event
-
- TPS
-
- tumor proportion score
-
- VEGFR
-
- vascular endothelial growth factor receptor
1 BACKGROUND
Non-small cell lung cancer (NSCLC), contributing to 85% of all lung cancer cases, poses a significant health burden, particularly in regions like China, which accounts nearly a third of all new lung cancer diagnoses globally [1]. Advanced NSCLC without driver alterations has been treated with platinum-based dual-drug chemotherapy [2-4]; however, this conventional approach has limited efficacy, demonstrating an urgent need for alternative therapeutic strategies. Moreover, the burden of chemotherapy extends beyond its limited effectiveness, as its associated adverse effects often deteriorate the quality of life in patients grappling with advanced stages of the disease [5, 6]. Hence, the exploration for chemo-free regimens with better anti-tumor activity is warranted.
The treatment landscape for advanced NSCLC has experienced significant evolution due to the emergence of immune checkpoint inhibitors since 2015 globally and 2018 domestically, particularly those targeting the programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway [7]. The KEYNOTE-042 study marked a pivotal moment in this evolution, demonstrating the efficacy of pembrolizumab monotherapy in patients with untreated NSCLC, especially in tumors with high PD-L1 expression [8]. Following these developments, other anti-PD-1/PD-L1 antibodies, such as sintilimab, a fully human IgG4 monoclonal antibody targeting PD-1, also demonstrated significant improvements in NSCLC when combined with chemotherapy in ORIENT-11 [9] and ORIENT-12 [10] studies.
Anlotinib is a multi-targeted tyrosine kinase inhibitor that primarily targets vascular endothelial growth factor receptors (VEGFRs). The pivotal ALTER 0303 study demonstrated that anlotinib not only prolonged both overall survival (OS) and progression-free survival (PFS) but also had a well-tolerated safety profile, thereby positioning it as a viable third-line or subsequent therapy option for patients with advanced NSCLC [11]. Recent research explored the potential synergistic effects of combining anlotinib with PD-1 blockade therapies, suggesting that anlotinib may enhance the anti-tumor efficacy of PD-1 inhibitors [12]. In line with this hypothesis, our preceding phase I trial explored the combination of sintilimab with anlotinib in patients with treatment-naïve unresectable stage IIIB/C or IV NSCLC. This trial revealed encouraging outcomes: the objective response rate (ORR) was 72.7%, the median PFS reached 15 months, and the toxicity profiles were manageable [13]. These results indicated that the sintilimab-anlotinib combination could be a formidable alternative to conventional platinum-based chemotherapy for this patient group, warranting further evaluation. Therefore, this phase II trial investigated the efficacy and safety of first-line sintilimab and anlotinib in metastatic NSCLC.
2 MATERIALS AND METHODS
2.1 Study design and patients
The SUNRISE study, a phase II, open-label, randomized controlled trial, was conducted across six centers in China. This study enrolled patients with metastatic NSCLC, aged between 18 and 75 years, who had not received prior systemic therapy for metastatic disease and exhibited an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. A critical inclusion criterion was the availability of a tumor sample for PD-L1 expression assessment. For non-squamous cell carcinoma patients, the absence of epithelial growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), or proto-oncogene tyrosine-protein kinase ROS (ROS1) driver gene mutations was mandatory, while those with squamous cell carcinoma either tested negative for these genes or had not undergone prior testing. The study also accommodated patients with asymptomatic brain metastases or those who had received local treatment for stable brain metastases. Individuals with a propensity for bleeding, such as those diagnosed with cavitary squamous cell carcinoma or vascular invasion (as determined by the investigator and confirmed through imaging), were excluded from participation. Detailed inclusion and exclusion criteria are delineated in the study protocol (Supplementary Materials).
The SUNRISE trial is registered at ClinicalTrials.gov (identifier: NCT04124731). The study protocol, which adheres to the principles of the Helsinki Declaration, was approved by the ethics committees of Shanghai Chest Hospital (approval #LS1938), Affiliated Hospital of Qingdao University (approval #QYFYKYLL 908311920), Henan Cancer Hospital (approval #2020052708-004), Cancer Hospital Affiliated to the University of Chinese Academy of Sciences (approval #IRB-2022-5), Anhui Chest Hospital (approval #K2020-010), and The Fourth Hospital of Hebei Medical University (approval #2020172-1). Prior to enrollment, informed consent was obtained from all patients.
2.2 Randomization
Eligible patients with metastatic NSCLC were randomized in a 1:1 ratio to either the sintilimab plus anlotinib group or the chemotherapy group. This randomization employed a stratified block design with a block size of four. The primary stratification factors included histological type (squamous cell carcinoma vs. non-squamous cell carcinoma) and PD-L1 expression level (tumor proportion score [TPS] ≥ 1% vs. TPS < 1%).
2.3 Treatments
In the sintilimab plus anlotinib group, patients were administered sintilimab (200 mg intravenously, every 3 weeks) (Innovent biologics, Inc., Suzhou, Jiangsu, China) alongside anlotinib (12 mg orally, on days 1-14 of each 3-week cycle) (Chia-tai Tianqing Pharmaceutical Co. Ltd., Lianyungang, Jiangsu, China). Sintilimab treatment was continued until the occurrence of progressive disease (PD), intolerable toxicity, withdrawal of consent, initiation of alternative anti-tumor therapies, or death, but not exceeding 24 months (or 24 months). Anlotinib was given under similar conditions but without a predetermined termination point. Dose adjustments were not permissible for sintilimab, while for anlotinib, dose modifications (10 mg/d or 8 mg/d) were allowed according to the protocol-defined dose modification criteria.
Conversely, patients in the chemotherapy group received a standard platinum-based dual-agent chemotherapy regimen. The choice of chemotherapy was based on the NSCLC histological type. Patients with non-squamous cell carcinoma were treated with a regimen of pemetrexed (500 mg/m2 intravenously, on day 1 of each cycle) and carboplatin (area under the curve [AUC] 5, intravenously, on day 1 of each cycle), administered every three weeks for 4 cycles. In cases without disease progression, maintenance therapy with pemetrexed monotherapy was implemented. For squamous cell carcinoma, the regimen comprised gemcitabine (1,000-1,250 mg/m2 intravenously, on days 1 and 8 of each cycle) and carboplatin (AUC 5, intravenously, on day 1 of each cycle), with cycles repeated every three weeks for a total of 6 cycles. Upon confirmed progression via imaging, patients in the chemotherapy group were eligible to switch to single-agent sintilimab treatment.
2.4 Assessments and follow-up
Tumor responses were assessed by investigators in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The safety and tolerability of the treatments were monitored for each patient within 90 days following their final treatment dose. Adverse events (AEs) were classified and graded based on the National Cancer Institute (NCI) Common Terminology Criteria for AEs (CTCAE) version 5.0. The management of AEs adhered to the study protocol.
Archival tumor biopsy samples were collected from patients prior to initiating treatment. The PD-L1 expression was analyzed using immunohistochemistry staining with a 22C3 mouse monoclonal primary antibody on the Dako automated staining platform following the manufacturer's standard protocol (Dako 22C3 PharmDx Assay, Dako Autostainer Link 48, Agilent, Santa Clara, CA, USA). All the areas in each tissue section were evaluated for PD-L1 expression. Tissue sections evaluated to have moderate to strong membrane staining in at least 1% of the tumor cells are considered to be positive for PD-L1 overexpression, while tissue sections with an absence or detection of staining in less than 1% of the cells were considered to be negative [14, 15]. PD-L1 expression was expressed as TPS and grouped by percentage. The slides were scored for PD-L1 membrane staining by two independent pathologists. These evaluations were carried out in a centralized, validated laboratory (Burning Rock Dx, Guangzhou, Guangdong, China).
2.5 Endpoints
The primary endpoint of this study was the ORR, i.e., the proportion of patients with a complete (CR) or partial response (PR). Secondary endpoints included PFS (the time from randomization to disease progression or death, whichever occurred first), duration of response (DoR) (the time from the first documented response to disease progression or death), disease control rate (DCR) (the proportion of patients with CR, PR, and stable disease [SD]), and OS (the time from randomization to death from any cause). Additionally, we evaluated the response in the sintilimab plus anlotinib group utilizing the immune Response Evaluation Criteria in Solid Tumors (iRECIST) criteria as exploratory endpoints. The sum of the longest diameters (SLD) was assessed according to RECIST v1.1 at baseline.
2.6 Statistical analysis
Assuming that the ORR would increase from 25% in the platinum-based dual-drug chemotherapy group to 50% in the sintilimab plus anlotinib group, with a one-sided α of 0.05 and 80% power to demonstrate superiority, we estimated the enrollment of 87 patients. Factoring in a potential 10% dropout rate, 98 patients were needed, with 49 patients in each group.
Efficacy analyses were performed on the full analysis set (FAS), which included all randomized patients as per the intention-to-treat principle. Safety assessments and drug exposure analysis were conducted on the safety set (SS), encompassing all randomized patients who received at least one dose of the study treatment. This population was categorized based on the actual treatment received during the trial.
Descriptive statistical methods were employed to evaluate ORR and DCR, and the 95% confidence interval (CI) was calculated using the Clopper-Pearson method. The Cochran-Mantel-Haenszel test, stratified by histological type and PD-L1 expression level, was used to analyze differences between groups. The ORR and DCR differences and their 95% CI between groups were determined using the unstratified Miettingen-Nurminen method. Median PFS, OS, and DoR, along with their 95% CIs, were estimated using the Kaplan-Meier method, with group comparisons made using the stratified log-rank test. Hazard ratios (HRs) and 95% CIs were calculated using a unstratified Cox proportional hazards model. All statistical analyses were conducted using SAS version 9.4 (or a later version) (SAS Institute, Cary, NC, USA).
3 RESULTS
3.1 Patient characteristics
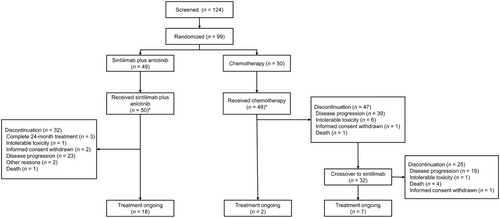
Between November 2019 and March 2023, a total of 124 patients were initially screened at five centers, and 99 were randomized in a 1:1 ratio, with 49 in the sintilimab plus anlotinib group and 50 in the chemotherapy group (Figure 1). Within them, the proportion of patients exhibiting PD-L1 TPS of ≥1% was 67.3% (33 patients) in the sintilimab plus anlotinib group and 68.0% (34 patients) in the chemotherapy group. Additionally, the mean SLD at baseline was 80.0 ± 37.9 mm in the sintilimab plus anlotinib group and 70.2 ± 32.4 mm in the chemotherapy group, while the proportion of patients with brain metastasis was 14.3% and 14.0%, respectively. The baseline characteristics were well balanced between the sintilimab plus anlotinib group and the chemotherapy group (Table 1).
| Variable | Sintilimab plus anlotinib (n = 49) | Chemotherapy (n = 50) | P value |
|---|---|---|---|
| Age, years, median (range) | 63.0 (33, 75) | 65.5 (45, 75) | 0.394 |
| ≤65, n (%) | 29 (59.2) | 25 (50.0) | |
| >65, n (%) | 20 (40.8) | 25 (50.0) | |
| Sex, n (%) | 0.636 | ||
| Male | 41 (83.7) | 40 (80.0) | |
| Female | 8 (16.3) | 10 (20.0) | |
| Smoking history, n (%) | 0.919 | ||
| Yes | 24 (49.0) | 25 (50.0) | |
| No | 25 (51.0) | 25 (50.0) | |
| ECOG PS, n (%) | 0.570 | ||
| 0 | 1 (2.0) | 2 (4.0) | |
| 1 | 48 (98.0) | 48 (96.0) | |
| Tumor histology, n (%) | 0.621 | ||
| Squamous cell | 23 (46.9) | 21 (42.0) | |
| Non-squamous cell | 26 (53.1) | 29 (58.0) | |
| Sum of the longest diameter, mm, mean ± SD | 80.0 ± 37.9 | 70.2 ± 32.4 | 0.169 |
| PD-L1 status, tumor proportion score, n (%) | 0.945a | ||
| <1% | 16 (32.7) | 16 (32.0) | |
| ≥1% | 33 (67.3) | 34 (68.0) | |
| 1-49% | 19 (38.8) | 20 (40.0) | |
| ≥50% | 14 (28.6) | 14 (28.0) | |
| Distant metastases, n (%) | |||
| Brain metastases | 7 (14.3) | 7 (14.0) | 0.967 |
| Liver metastases | 2 (4.1) | 2 (4.0) | 0.984 |
| Bone metastases | 13 (26.5) | 25 (50.0) | 0.016 |
| Others | 34 (69.4) | 36 (72.0) | 0.775 |
| Treatment before recurrence, n (%) | 0.320 | ||
| Surgery | 7 (14.3) | 4 (8.0) | 0.320 |
| Chemotherapy | 4 (8.2) | 3 (6.0) | 0.675 |
| Radiotherapy | 2 (4.1) | 4 (8.0) | 0.414 |
- Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; SD, standard deviation; PD-L1, programmed death-ligand 1.
- a The P value was 0.997 when this variable was categorized into <1%, 1%-49%, and ≥50%.
3.2 Treatment
Within the sintilimab plus anlotinib group, patients underwent a median of 10.6 (range: 1 to 41) cycles of sintilimab treatment. The median duration of anlotinib treatment spanned 7.8 months (range: 1 to 29). As of the cut-off date on March 15, 2023, 18 (36.7%) patients were still undergoing treatment, while 32 (65.3%) had discontinued, predominantly due to disease progression (n = 23, 46.9%). Notably, three patients successfully completed the full 24-month treatment with sintilimab. Among those who experienced PD during sintilimab plus anlotinib treatment, 21 pursued subsequent anti-tumor therapies, including immunotherapy (n = 13, 26.0%), radiotherapy (n = 4, 8.0%), and chemotherapy (n = 16, 32.0%); among them, 7 had PD-1 inhibitors plus chemotherapy, 3 had PD-1 inhibitor plus anlotinib, 2 receive PD-1 inhibitor, bevacizumab plus chemotherapy, 1 received PD-1 inhibitor monotherapy, 2 received chemotherapy plus radiotherapy, 1 radiotherapy only, and 5 chemotherapy only.
In the chemotherapy group, the median number of treatment cycles was 7.9 (range: 1 to 34), 4.6 (range: 1 to 8), and 4.2 (range: 1 to 8) for pemetrexed, gemcitabine, and carboplatin, respectively. Thirty-nine patients (78.0%) had disease progression, among which 32 (65.3%) crossover to sintilimab treatment. By the same cut-off date, 40 patients (80.0%) discontinued chemotherapy, and 9 were still undergoing chemotherapy. Twenty-four transitioned to next-line anti-tumor treatments (including 7 progressed after initial chemotherapy and 17 progressed after the crossover to sintilimab), encompassing chemotherapy (n = 17, 34.7%), immunotherapy (n = 12, 24.5%) and radiotherapy (n = 5, 10.2%). Among the 17 patients who progressed after crossover to sintilimab, 6 received chemotherapy alone, 4 received anlotinib-based therapy (2 cases of combination chemotherapy with anlotinib, 1 case of combination chemotherapy with anlotinib and radiotherapy, and 1 case of anlotinib monotherapy), 4 received a PD-1 inhibitor in combination with anlotinib (including 2 cases of combination radiotherapy), and 3 cases of received PD-1 inhibitor combined with chemotherapy (1 case of combination radiotherapy). Among 7 patients who did not cross to sintilimab, 2 had PD-1 inhibitor plus anlotinib, 2 had bevacizumab and chemotherapy, 1 had PD-1 inhibitor and chemotherapy, 1 had chemotherapy, and 1 had PD-1 inhibitor monotherapy.
3.3 Anti-tumor response
As assessed by investigators using RECIST 1.1 criteria, the ORR in the sintilimab plus anlotinib group was 44.9% (95% CI = 30.7%-59.8%), compared to an ORR of 18.0% (95% CI = 8.6%-31.4%) in the chemotherapy group (Table 2). This demonstrates a notable increase in ORR of 25.0% in favor of the sintilimab plus anlotinib group (P = 0.003). However, there was no statistically significant difference in the DCR between the groups (77.6% vs. 86.0%, P = 0.316). The median DoR in the sintilimab plus anlotinib group was 21.4 months (95% CI = 15.6-not reached [NR]), in contrast to 13.0 months (95% CI = 5.3-NR) in the chemotherapy group (HR = 0.14, 95% CI = 0.03-0.73, P = 0.009) (Supplementary Figure S1). Efficacy results according to iRECIST criteria are summarized in Supplementary Table S1.
| Variable | Sintilimab plus anlotinib (n = 49) | Chemotherapy (n = 50) | Difference (95% CI) | P value |
|---|---|---|---|---|
| CR, n (%) | 1 (2.0) | 0 (0) | N/A | N/A |
| PR, n (%) | 21 (42.9) | 9 (18.0) | N/A | N/A |
| SD, n (%) | 16 (32.7) | 34 (68.0) | N/A | N/A |
| PD, n (%) | 7 (14.3) | 5 (10.0) | N/A | N/A |
| NE, n (%) | 4 (8.2) | 2 (4.0) | N/A | N/A |
| ORR (95%CI) | 44.9 (30.7 to 59.8) | 18.0 (8.6 to 31.4) | 25.0 (8.2 to 41.8) | 0.003 |
| DCR (95%CI) | 77.6 (63.4 to 88.2) | 86.0 (73.3 to 94.2) | −6.6 (-21.8 to 8.6) | 0.316 |
- Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not estimated; ORR, objective response rate; DCR, disease control rate; CI, confidence interval; HR, hazard ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; N/A, not applicable.
In the subgroup analysis of patients with a PD-L1 TPS of ≥1%, a significantly higher ORR of 54.5% was observed in the sintilimab plus anlotinib group compared to 17.6% in the chemotherapy group (P = 0.002). In patients with squamous NSCLC, sintilimab plus anlotinib resulted in a significantly higher ORR than chemotherapy (43.5% vs. 9.5%, P = 0.013). In patients with brain metastasis, the ORR was numerically higher with sintilimab plus anlotinib than chemotherapy (57.1% vs. 28.6%) (Table 3). The detailed treatment response across subgroups is presented in Supplementary Table S2.
| Variable | Sintilimab plus anlotinib (n = 49) |
Chemotherapy (n = 50) |
Difference (95% CI) | P value |
|---|---|---|---|---|
| Age, responder/total (%) | ||||
| ≤65 | 13/29 (44.8) | 4/25 (16.0) | 28.8 (3.9 to 50.3) | 0.024 |
| >65 | 9/20 (45.0) | 5/25 (20.0) | 25.0 (-2.5 to 50.2) | 0.075 |
| Sex, responder/total (%) | ||||
| Male | 19/41 (46.3) | 5/40 (12.5) | 33.8 (14.5 to 51.2) | <0.001 |
| Female | 3/8 (37.5) | 4/10 (40.0) | −2.5 (-44.1 to 41.0) | 0.916 |
| Smoking history, responder/total (%) | ||||
| Yes | 10/24 (41.7) | 5/25 (20.0) | 21.7 (-4.5 to 45.6) | 0.103 |
| No | 12/25 (48.0) | 4/25 (16.0) | 32.0 (6.1 to 54.3) | 0.016 |
| ECOG PS, responder/total (%) | ||||
| 0 | 1/1 (100.0) | 0/2 (0) | 100.0 (-31.5 to 100.0) | 0.157 |
| 1 | 21/48 (43.8) | 9/48 (18.8) | 25.0 (6.5 to 42.1) | 0.009 |
| Histological subtype, responder/total (%) | ||||
| Non-squamous cell | 12/26 (42.6) | 7/29 (24.1) | 22.0 (-3.4 to 45.3) | 0.089 |
| Squamous cell | 10/23 (43.5) | 2/21 (9.5) | 34.0 (8.0 to 56.3) | 0.013 |
| PD-L1 status, tumor proportion score, responder/total (%) | ||||
| <1% | 4/16 (25.0) | 3/16 (18.8) | 6.3 (-23.7 to 35.4) | 0.674 |
| ≥1% | 18/33 (54.5) | 6/34 (17.6) | 36.9 (14.2 to 56.3) | 0.002 |
| 1%-49% | 11/19 (57.9) | 4/20 (20.0) | 37.9 (7.1 to 62.4) | 0.016 |
| ≥50% | 7/14 (50.0) | 2/14 (14.3) | 35.7 (0.5 to 63.6) | 0.047 |
| Brain metastasis, responder/total (%) | ||||
| Yes | 4/7 (57.1) | 2/7 (28.6) | 28.6 (-23.9 to 68.2) | 0.298 |
| No | 18/42 (42.9) | 7/43 (16.3) | 26.6 (7.3 to 44.4) | 0.008 |
| Liver metastasis, responder/total (%) | ||||
| Yes | 0/2 (0) | 1/2 (50.0) | −50.0 (-92.4 to 46.8) | 0.317 |
| No | 22/47 (46.8) | 8/48 (16.7) | 30.1 (11.6 to 47.0) | 0.002 |
- Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not estimated; ORR, objective response rate; DCR, disease control rate; CI, confidence interval; HR, hazard ratio; ECOG PS, Eastern Cooperative Oncology Group performance status.
3.4 Survival
As of the cut-off date on March 15, 2023, the median follow-up was 21.2 months (95% CI = 13.5-26.7) in the sintilimab plus anlotinib group and 22.0 months (95% CI = 17.9-29.7) in the chemotherapy group.
Within the sintilimab plus anlotinib group, 28 PFS events (57.1%) were documented. Comparatively, in the chemotherapy group, there were 43 PFS events (86.0%). The combination therapy of sintilimab and anlotinib notably prolonged PFS, achieving a median of 14.4 months (95% CI = 8.0-22.9), which was significantly longer than that with standard platinum-based chemotherapy (median: 5.6 months, 95% CI = 3.5-6.9) (HR = 0.39, 95% CI = 0.23-0.67, P < 0.001) (Figure 2A). In the subgroup of patients with a PD-L1 TPS of ≥ 1%, the median PFS in the sintilimab plus anlotinib group extended to 19.3 months (95% CI = 9.8-NR), compared to 4.4 months (95% CI = 3.1-6.2) in the chemotherapy group (HR = 0.23, 95% CI = 0.11-0.46, P < 0.001). In addition, in the subgroup analysis of patients with squamous NSCLC, the median PFS was 8.5 months (95% CI = 1.6-17.1) in the sintilimab plus anlotinib group compared to 4.9 months (95% CI = 3.0-6.6) in the chemotherapy group (HR = 0.46, 95% CI = 0.21-0.98, P = 0.039) (Figure 2B). In patients with brain metastasis, the median PFS was 18.4 months with sintilimab plus anlotinib versus 5.6 months with chemotherapy (Figure 2B).
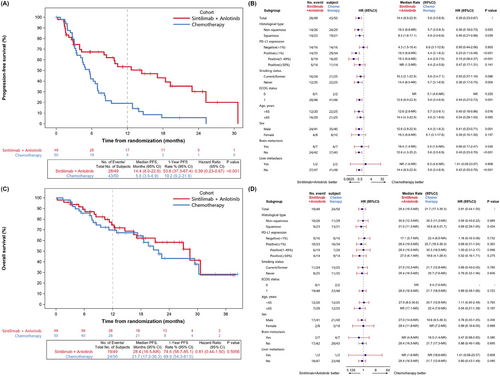
The OS data were immature, with 19 deaths (38.8%) reported in the sintilimab plus anlotinib group, in comparison to 24 deaths (48.0%) in the chemotherapy group. The 12-month OS rate was 74.6% (95% CI = 58.7%-85.1%) with sintilimab plus anlotinib and 69.9% (95% CI = 54.4%-81.0%) with chemotherapy, respectively. In the sintilimab plus anlotinib group, the 24-month OS rate was 58.4% (95% CI = 40.4%-72.6%), compared with 43.2% (95% CI = 26.0%-59.2%) in the chemotherapy group (Figure 2C). The subgroup analysis of OS is presented in Figure 2D.
3.5 AEs
In the sintilimab plus anlotinib group, 96.0% (48 patients) experienced treatment-emergent AEs (TEAEs), slightly lower than the 98.0% (48 patients) in the chemotherapy group (Table 4). The rate of grade 3 or higher TEAEs was 30.0% in the sintilimab plus anlotinib group, compared to 49.0% in the chemotherapy group. The rates of TEAEs leading to treatment discontinuation were similar in both groups (8.0% vs. 8.2%), as were the occurrences of serious TEAEs (20.0% in the sintilimab plus anlotinib group vs. 26.5% in the chemotherapy group). No TEAE leading to death in both groups. The most common TEAEs are listed in Supplementary Table S3.
| Event | Sintilimab plus anlotinib (n = 50) | Chemotherapy (n = 49) |
|---|---|---|
| Any AE, n (%) | 49 (98.0) | 48 (98.0) |
| Any TEAE, n (%) | 48 (96.0) | 48 (98.0) |
| Grade 3 or higher TEAEs | 15 (30.0) | 24 (49.0) |
| Serious TEAEs | 10 (20.0) | 13 (26.5) |
| TEAEs leading to dosage reduction | 7 (14.0) | 9 (18.4) |
| TEAEs leading to treatment interruption | 18 (36.0) | 17 (34.7) |
| TEAEs leading to treatment discontinuation | 4 (8.0) | 4 (8.2) |
| TEAEs leading to trial withdrawal | 1 (2.0) | 5 (10.2) |
| TEAEs leading to death | 0 (0) | 0 (0) |
| Any TRAE, n (%) | 48 (96.0) | 48 (98.0) |
| Grade 3 or higher TRAEs | 14 (28.0) | 24 (49.0) |
| Serious TRAEs | 8 (16.0) | 9 (18.4) |
| TRAEs leading to dosage reduction | 7 (14.0) | 9 (18.4) |
| TRAEs leading to treatment interruption | 17 (34.0) | 15 (30.6) |
| TRAEs leading to treatment discontinuation | 4 (8.0) | 3 (6.1) |
| TRAEs leading to trial withdrawal | 1 (2.0) | 5 (10.2) |
| TRAEs leading to death | 0 (0) | 0 (0) |
- Abbreviations: AE, adverse event; TEAE, treatment-emergent adverse event; TRAE, treatment-related adverse event.
Treatment-related adverse events (TRAEs) were reported in 96.0% of patients in the sintilimab plus anlotinib group and 98.0% in the chemotherapy group. Notably, grade 3 or higher TRAEs were less frequent in the sintilimab plus anlotinib group (28.0%) than in the chemotherapy group (49.0%). The sintilimab plus anlotinib group also had a lower rate of TRAEs, leading to trial withdrawal (2.0% vs. 10.2%). Serious TRAEs (16.0% vs. 18.4%), TRAEs resulting in dosage reduction (14.0% vs. 18.4%, anlotinb dose reduction in 7 cases who received sintilimab plus anlotinib, and dose reduction of pemetrexed in 4, gemcitabine in 8, and carboplatin in 10 cases in the chemotherapy group), TRAEs leading to treatment interruption (34.0% vs. 30.6%), and TRAEs leading to treatment discontinuation (8.0% vs. 6.1%) were comparable between the two groups (Table 4). Grade 3 or higher hematological TRAEs (e.g., anemia) were hardly reported in patients who received sintilimab plus anlotinib, while the rates of grade 3 or higher anemia, thrombocytopenia, leukopenia, and neutropenia were all over 10% in the chemotherapy group. TRAEs occurring at a rate greater than 10% in either group are detailed in Table 5.
| TRAE | Sintilimab plus anlotinib (n = 50) | Chemotherapy (n = 49) | ||
|---|---|---|---|---|
| All Grade | Grade 3 or higher | All Grade | Grade 3 or higher | |
| Hematological toxicities, n (%) | ||||
| Anemia | 17 (34.0) | 0 (0) | 44 (89.8) | 10 (20.4) |
| Thrombocytopenia | 6 (12.0) | 0 (0) | 22 (44.9) | 7 (14.3) |
| Leukopenia | 5 (10.0) | 1 (2.0) | 24 (49.0) | 6 (12.2) |
| Neutropenia | 4 (8.0) | 1 (2.0) | 20 (40.8) | 6 (12.2) |
| Lymphopenia | 1 (2.0) | 0 (0) | 5 (10.2) | 1 (2.0) |
| Non-hematological toxicities, n (%) | ||||
| Hyponatremia | 17 (34.0) | 1 (2.0) | 13 (26.5) | 2 (4.1) |
| Hypothyroidism | 15 (30.0) | 0 (0) | 0 (0) | 0 (0) |
| Hypoalbuminemia | 15 (30.0) | 0 (0) | 7 (14.3) | 0 (0) |
| TSH increased | 15 (30.0) | 0 (0) | 2 (4.1) | 0 (0) |
| Hand-foot syndrome | 14 (28.0) | 0 (0) | 2 (4.1) | 0 (0) |
| Fatigue | 13 (26.0) | 0 (0) | 13 (26.5) | 0 (0) |
| Joint pain | 13 (26.0) | 0 (0) | 4 (8.2) | 0 (0) |
| Bilirubin increased | 13 (26.0) | 1 (2.0) | 3 (6.1) | 0 (0) |
| γ-glutamyltransferase increased | 11 (22.0) | 0 (0) | 6 (12.2) | 0 (0) |
| AST increased | 11 (22.0) | 0 (0) | 11 (22.4) | 0 (0) |
| Cough | 10 (20.0) | 0 (0) | 5 (10.2) | 0 (0) |
| Hair loss | 9 (18.0) | 0 (0) | 5 (10.2) | 0 (0) |
| ALT increased | 9 (18.0) | 0 (0) | 15 (30.6) | 1 (2.0) |
| Hyperuricemia | 9 (18.0) | 0 (0) | 4 (8.2) | 0 (0) |
| Hemoptysis | 7 (14.0) | 0 (0) | 2 (4.1) | 0 (0) |
| Rash | 7 (14.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Amylase increased | 6 (12.0) | 0 (0) | 0 (0) | 0 (0) |
| Fever | 6 (12.0) | 0 (0) | 0 (0) | 0 (0) |
| Back pain | 5 (10.0) | 0 (0) | 2 (4.1) | 0 (0) |
| Hyperthyroidism | 5 (10.0) | 0 (0) | 3 (6.1) | 0 (0) |
| Toothache | 5 (10.0) | 0 (0) | 0 (0) | 0 (0) |
| Proteinuria | 5 (10.0) | 0 (0) | 2 (4.1) | 0 (0) |
| Dysphagia | 5 (10.0) | 0 (0) | 1 (2.0) | 0 (0) |
| Dysphonia | 5 (10.0) | 0 (0) | 0 (0) | 0 (0) |
| Epistaxis | 5 (10.0) | 0 (0) | 0 (0) | 0 (0) |
| Free triiodothyronine decreased | 5 (10.0) | 0 (0) | 3 (6.1) | 0 (0) |
| Blood pressure increased | 5 (10.0) | 2 (4.0) | 0 (0) | 0 (0) |
| Decreased appetite | 3 (6.0) | 0 (0) | 10 (20.4) | 0 (0) |
| Hyperglycemia | 2 (4.0) | 0 (0) | 5 (10.2) | 0 (0) |
| Pain | 1 (2.0) | 0 (0) | 5 (10.2) | 1 (2.0) |
| Nausea | 0 (0) | 0 (0) | 9 (18.4) | 0 (0) |
- Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; TSH, thyroid-stimulating hormone.
The rate of immune-related adverse events (irAEs) was significantly higher in the sintilimab plus anlotinib group (74.0%) compared to the chemotherapy group (12.2%) (Supplementary Table S4). The most common irAEs in the sintilimab plus anlotinib group were hyponatremia (32.0%, grade 3 or higher 2.0%), followed by anemia (28.0%), increased thyroid-stimulating hormone (24.0%), and hypoalbuminemia (24.0%), all grade 1-2.
4 DISCUSSION
In our SUNRISE study, the efficacy and safety profiles of sintilimab and anlotinib as a chemo-free regimen were compared with standard platinum-based dual-drug chemotherapy in the first-line treatment of metastatic NSCLC. The investigation data yielded a superior ORR and PFS against the standard platinum-based chemotherapy, especially in patients with a PD-L1 TPS of ≥1% and squamous NSCLC. The combination of sintilimab and anlotinib is well-tolerated and resulted in a numerically lower rate of grade 3 or higher TRAE, especially less hematological toxicities than chemotherapy. To our knowledge, this randomized controlled trial substantiated the advantages of combined immunotherapy and anti-angiogenic therapy over the standard platinum-based chemotherapy in treating metastatic NSCLC.
In a previous KEYNOTE-042 study, the improvements of ORR and PFS with pembrolizumab monotherapy over chemotherapy were unsatisfactory [8]. Additionally, in similar PD-L1 positive populations, nivolumab plus ipilimumab reported a similar ORR of 35.9% versus 30.0% with chemotherapy. The median PFS was 5.1 months with nivolumab plus ipilimumab, as opposed to 5.6 months with chemotherapy (HR = 0.82) [16]. In the present study, the sintilimab plus anlotinib group showed significantly improved ORR and prolonged PFS compared with chemotherapy. Particularly in the PD-L1 TPS ≥ 1% subgroup, we observed an increased ORR of 54.5% and an extended median PFS of 19.3 months, suggesting a numerical improvement over these previous studies. These outcomes, while not directly comparable to earlier studies, indicate a potential advancement in efficacy with combination modality. In the realm of NSCLC treatment, the synergy between anti-angiogenic therapies and immunotherapies is gaining increasing recognition [17, 18]. Herein, the improved ORR and prolonged PFS with sintilimab plus anlotinib echoed the findings of the LEAP-007 trial (pembrolizumab plus lenvatinib) [19] and other smaller-scale studies exploring combinations like camrelizumab plus apatinib [20] or famitinib [21]. On the other hand, negative or marginal findings were reported with first-line ramucirumab plus pembrolizumab [22], but better outcomes were observed in immunotherapy-treated patients [23]. The bi-specific antibody (PD-1/VEGF) ivonescimab, in combination with chemotherapy as first- or second-line therapy, showed promising anti-tumor activity in patients with advanced or metastatic immunotherapy-naïve NSCLC [24]. Hence, future studies are warranted to verify the combination of targeting immune checkpoint and VEGF for efficacy in NSCLC.
Significantly, this study exclusively enrolled patients with metastatic NSCLC with a relatively high baseline SLD compared with previous studies (e.g., IMpower150 study [25]), underscoring the promising efficacy of sintilimab plus anlotinib in patients with more advanced disease, and echoing with the report from a previous study that patients with high baseline SLD might benefit from immunotherapy [26]. In the meantime, the subgroup analysis revealed benefit from sintilimab plus anlotinib among squamous NSCLC, and encouraging anti-tumor activity in patients with brain metastasis, despite no significantly difference due to a relatively small sample size. These findings underscore the potential of sintilimab plus anlotinib as a first-line treatment option for patients with metastatic NSCLC, especially those with positive PD-L1 expression and squamous NSCLC. The encouraging efficacy in patients with high tumor burden and brain metastasis demonstrated potential advantage of sintilimab plus anlotinib, which need further validation due to the limited sample size of subgroups.
Despite no OS benefit being observed in the LEAP-007 study [27], our current study demonstrated a numerically higher 24-month OS rate with sintilimab plus anlotinib over chemotherapy, which indicated the potential of treatment modality with anti-angiogenic therapies plus immunotherapies. Notably, a high rate of crossover to sintilimab treatment was observed in our study, which was as high as 65.3%. Such a high crossover rate and proportion of patients who received next-line anti-tumor treatment might have contributed to a relatively high 24-month OS rate in the control group, surpassing typical historical data in this context [2-4], and the cross of survival curve between the two groups. This was common in trials that allowed cross-over and analysis with immature OS data [28, 29]. Further analyses will be required to comprehensively interpret these findings. In addition, this study was not powered to detect the OS difference between patients who received sintilimab plus anlotinib and those with chemotherapy. Future larger-scale studies with OS being the primary endpoint will be required to comprehensively interpret the survival benefit from sintilimab plus anlotinib in metastatic NSCLC.
A key drawback of chemotherapy, aside from its limited efficacy, has been the associated adverse effects, often significant in nature [5, 6]. In our SUNRISE study, we observed that a substantial 49.0% of patients in the chemotherapy group experienced grade 3 or higher TRAEs. Notably, hematological toxicities were significantly lower in the sintilimab plus anlotinib group compared to the chemotherapy group, as evidenced by the lower rates of anemia, thrombocytopenia, leukopenia, neutropenia, and lymphopenia. In the context of chemo-free regimens, such as nivolumab plus ipilimumab in the CheckMate227 study, the rate of grade 3 or higher TRAEs was reported at 32.8% [16]. Similarly, in the LEAP-007 trial that investigated the combination of immunotherapy and small molecule anti-angiogenic drugs, a considerable increase in grade 3 or higher TRAEs was noted in the combination group (57.9%), in contrast to pembrolizumab monotherapy (24.4%) [27]. Additionally, combinations like camrelizumab with apatinib [20] or famitinib [30] also demonstrated a high rate of severe AEs. However, in comparison, our study offers an encouraging perspective with the sintilimab plus anlotinib combination. The rate of grade 3 or higher TRAEs in this group was notably lower, standing at 28.0%, a marked reduction from the 49.0% observed in the chemotherapy group. The rate of irAEs was higher in the sintilimab plus anlotinib group (74.0%) compared to the chemotherapy group (12.2%), as could be expected based on the differences in the nature of the drugs [31, 32], however, no additional concern was raised. Although the present study does not provide long-term safety data, previous data on immune checkpoint inhibitors suggest a low rate of late-onset AEs [32]. Furthermore, anlotinib has been on the market for 6 years now with real-world data confirming its long-term safety [33-37].
It is imperative to consider the inherent limitations. The primary endpoint of the study is the ORR, which may not fully capture the broader clinical benefits of the treatment regimen. Nevertheless, it can be acceptable to use the ORR as the primary endpoint for a phase II trial [38, 39] since it objectively reflected of the drug's antitumor activity within a short evaluation timeframe. In addition, the PFS benefits of sintilimab and anlotinib were also demonstrated in the analysis. Besides, another limitation was the choice of chemotherapy as the comparator, reflective of the standard treatment at the time of study initiation. In addition, even though immunotherapy in combination with chemotherapy is now the standard of care, not all patients are suitable, and the study design allowed for crossover. Lastly, because of the relatively small sample size, the subgroup analysis was based on even smaller numbers of patients, and the subgroup analyses should be interpreted with caution. Despite these limitations, in the context of high costs associated with internationally available immunotherapy products (e.g., pembrolizumab), sintilimab and anlotinib, both being covered by medical insurance, may offer a cost-effective and safe alternative for Chinese patients with metastatic NSCLC.
5 CONCLUSIONS
The SUNRISE study has shown significant ORR and PFS benefits of first-line sintilimab plus anlotinib over conventional chemotherapy, with a manageable and tolerable safety profile. This combination offers a new treatment option. However, the present study indicated no differences in OS between these treatment modalities, and further analyses with mature survival data and research with a larger sample size and more rigorous study is needed.
AUTHOR CONTRIBUTIONS
Baohui Han, Tianqing Chu, and Hua Zhong had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Baohui Han, Tianqing Chu, and Hua Zhong were involved in the study of concepts and design. All authors (Baohui Han, Tianqing Chu, Hua Zhong, Zhuang Yu, Jing Wang, Yanqiu Zhao, Xiaoqian Mu, Xinmin Yu, Xun Shi, Qingming Shi, Maojing Guan, Cuimin Ding, Nan Geng, and Jialin Qian) involved in investigation, analysis and interpretation of data. Tianqing Chu and Hua Zhong were involved in the draft of the manuscript. All authors read, critically revised, and approved the manuscript.
ACKNOWLEDGEMENTS
The study was supported by the “Science and Technology Innovation Action Plan” Medical Innovation Research Special Project of Shanghai (Grant No. 21Y11913500); the key project of the Medical and Health Technology Development Research Center of the National Health Commission (Grant No. WKZX2023CX030003). Funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
All authors have no conflicts of interest to declare.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol, which adheres to the principles of the Helsinki Declaration, was approved by the ethics committees of Shanghai Chest Hospital (approval #LS1938), Affiliated Hospital of Qingdao University (approval #QYFYKYLL 908311920), Henan Cancer Hospital (approval #2020052708-004), Cancer Hospital Affiliated to the University of Chinese Academy of Sciences (approval #IRB-2022-5), Anhui Chest Hospital (approval #K2020-010), and The Fourth Hospital of Hebei Medical University (approval #2020172-1). Prior to enrollment, informed consent was obtained from all patients.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov (identifier: NCT04124731).
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available within the article and its Supplementary Materials.




