Anti-tumor drug supervision in China from 2010 to 2024: the evolution and prospect of drug review standards
List Of Abbreviations
-
- CDE
-
- Center for Drug Evaluation
-
- FDA
-
- Food and Drug Administration
-
- HER2
-
- human epidermal growth factor receptor 2
-
- NDA
-
- new drug application
-
- NMPA
-
- National Medical Products Administration
-
- OS
-
- overall survival
-
- PD-1
-
- programmed cell death protein 1
-
- PFS
-
- progression-free survival
-
- PRO
-
- patient-reported outcomes
-
- RCT
-
- randomized controlled trial
-
- R&D
-
- research and development
-
- SAT
-
- single-arm clinical trials
-
- US
-
- United States
1 BACKGROUND
Over the past decade, the research and development (R&D) of anti-tumor drugs in China has undergone a remarkable leap, maintaining a high level of motivation.
With respect to pharmaceutical R&D paradigms in China, the domestic market was once dominated by generic drugs. A progressive transition towards the development of innovative pharmaceuticals is emerging, consequent to the reforms in drug evaluation and approval mechanisms and the promotion of novel drug development in China. The introduction of new drugs from other countries to China used to lag behind. Now a progressive approach is being taken towards synchronization in global R&D. The emergence of new players in the pharmaceutical industry and the enhancement of corporate R&D competencies have further facilitated the internationalization of China's drug R&D endeavors. The rapid advancement in pharmaceutical R&D has significantly enhanced China's drug regulatory capabilities. In August 2015, the State Council of the People's Republic of China promulgated the “Opinions on Reforming the Approval Procedures for Drugs and Medical Devices” marking the official commencement of reforms in the drug evaluation and approval system. The Chinese drug regulatory authorities are integrating resources and optimizing drug review processes through the development of a scientifically complete system of Good Review Practice.
2 DEVELOPMENT OF INNOVATIVE DRUGS IN CHINA
Innovative drugs, including new drugs, improved new drugs (those improved formulations or dose forms with existing active ingredients), biosimilars, and domestic generic drugs with unimported original drugs, constitute essential assurances for patients in China and offer better accessibility of medications. To better understand the changes since the inception of the drug evaluation and approval reforms in 2015, we have collected data on new drug applications (NDAs) for the aforementioned types of drugs submitted to the Center for Drug Evaluation (CDE) under the National Medical Products Administration (NMPA) in China, spanning from January 2010 to March 2024.
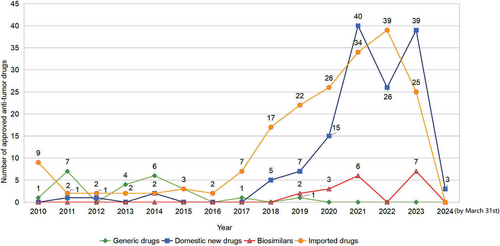
A total of 374 NDAs for anti-tumor drugs had been approved from January 1, 2010 to March 31, 2024, including 186 (49.7%) imported drugs and 188 (50.3%) domestic drugs. The domestic drugs included 139 (37.1%) innovative/improved new drugs, 31 (8.3%) generic drugs, and 18 (4.8%) biosimilars, respectively.
Before 2018, China's pharmaceutical market mainly relied on domestic generic drugs. However, since 2018, the number of drug authorizations granted to innovative drugs has been on the rise, with a notable increase in domestically developed innovative drugs (Figure 1). The emergence of an upward trend in domestically R&D innovative drugs is due to better regulatory frameworks, faster review processes, and the introduction of technical standards. The Chinese government has implemented policies promoting anti-tumor drug R&D, including priority review, conditional approval, and breakthrough treatment designation. From 2010 to 2015, the CDE approved 45 anti-tumor drugs with an average review time of 580 days. From 2016 to 2024, the average review time for 329 applications dropped to 280.2 days. These measures enhanced review efficiency and transparency, offered a more predictable environment for drug development, and boosted the industry's confidence and interest in R&D.
The advent of these domestic drugs has addressed much of the unmet needs of Chinese patients, transformed tumor therapeutics profoundly, and achieved success in the international market, thereby providing additional treatment options for patients worldwide. Bruton's tyrosine kinase inhibitor Zanubrutinib (Brukinsa®), which was granted an accelerated approval by the United States (US) Food and Drug Administration (FDA) in 2019, was tested in a pivotal study—a single-arm trial (SAT) —conducted in Chinese patients after consultation with the CDE to reach consensus.
3 DRUG REVIEW PRINCIPLE EVOLUTION FOR ANTI-TUMOR DRUGS IN CHINA
Since 2012, the CDE has issued 61 guidance to support anti-tumor drug R&D and address the technical challenges. This has contributed to the improvement of anti-tumor drug review framework in China.
3.1 Scientific and prudent attitude towards single-arm trials
Chidamide (Epidaza®, CS055) represents the first drug to be granted conditional approval based on an SAT [1]. It offers a treatment option for patients suffering from refractory, relapsed peripheral T-cell lymphoma who lack alternative therapies. Subsequently, numerous innovative anti-tumor drugs received conditional approvals. Statistical data show that from May 2014 to May 2021, a total of 19 drugs for 26 indications were granted conditional approvals [2].
SATs inherently carry uncertainties that may pose additional risks to patients. To address this, the CDE has issued three guidance [3-5] which detail the regulatory considerations and technical requirements for the use of SATs in NDAs.
SATs, as pivotal studies, must be executed with scientific precision and prudence with a clear and well-articulated drug action mechanism. SATs should be acceptable only in specific scenarios, such as when control studies are infeasible or when the drug exhibits significantly superior efficacy. Moreover, it is advocated to undertake confirmatory studies as early as possible to mitigate the risk of patients being exposed to drugs with uncertain efficacy and safety profiles. On August 25, 2023, the NMPA issued the “Procedures for Review and Approval of Conditional Approval Applications for Marketed Drugs (interim) (Revised Draft for Public Comments)” [6]. It explicitly stipulates that a confirmatory study should be completed within 4 years after the conditional approval. Evidence from the US FDA demonstrates that it is feasible to complete a confirmatory study within 4 years post-marketing if the confirmatory study is to be commenced once an accelerated approval is granted [7].
3.2 The precision medicine of cancer
Biomarkers serve as crucial instruments for achieving precision therapy. From 2010 to 2023, a total of 109 NDAs for 73 distinct anti-tumor drugs incorporated biomarkers to accurately identify the patient demography for therapeutic intervention [8].
As a novel cognitive paradigm for disease identification, the “pan-tumor” classification aggregates diverse tumors into a singular disease category, considering their genesis. This approach is dedicated to the pursuit of universal therapeutic methodologies [9]. As of February 2024, the NMPA has approved 7 drugs for “pan-tumor”, including Envafolimab, Tislelizumab, Serplulimab, Larotrectinib, Pucotenlimab, Entrectinib and Pembrolizumab. The approvals of these drugs underscore the ongoing evolution of clinical practice in the field of oncotherapy in China [8].
3.3 Diversity of population in clinical trials
Patients suffering from various types of tumors exhibit distinct age distributions and organ function. The CDE has issued several guidances (such as the Guidance for the Application of Physiological Pharmacokinetics Models in Drug R&D for Pediatric Populations) [10] to emphasize research among different populations and to enhance the diversity of subjects, thereby providing more reference information for the post-marketing change management of drugs.
Recruiting patients at different stages of cancer also reflects the diversity of the population. It is a classic practice to sequential progress from the later-line to the front-line population. However, this R&D strategy results in the front-line patients being deprived of timely access to new drugs. Consequently, we advocate for the initiation of development in the front-line as soon as efficacy is observed in later-line patients. Sometimes, developing drugs in early-stage tumors is of great clinical value. For instance, immunotherapy may have more pronounced therapeutic effects in early-stage patients who have not yet undergone immune function deterioration [11].
3.4 Control groups reflect the clinical value of drugs
It is important that the control group in a clinical trial accurately reflects the most efficacious treatment options currently accessible to the intended patients in real-world clinical settings. Only under these conditions can the findings of the trial truly reveal the clinical value of the new drug.
Although some imported drugs received approvals in China, their adoption by the Chinese population did not make much difference to the therapeutics landscape due to economic and other burdens. As such, the urgent issue for patients is to receive drugs that are both efficacious and easily accessible. Accordingly, the CDE does not require a head-to-head comparison with imported drugs.
For example, since Trastuzumab was approved in China in 2002, its widespread clinical adoption was constrained by the high price. Consequently, in the clinical trial of Inetetamab, China's first innovative monoclonal antibody targeting human epidermal growth factor receptor 2 (HER2), Vinorelbine was selected as the control drug in the randomized controlled trial (RCT) rather than Trastuzumab. The outcomes of this RCT provided support for the approval of Inetetamab in China [12].
With the emergence of innovative drugs and the increase of imported drugs in China, Chinese clinical practices are gradually improving, and the gap between clinical practices in China and in other countries is gradually narrowing. As clinical practice evolves, regulatory requirements are also changing accordingly. In 2020, the CDE issued the “Guidance for Clinical R&D of Anti-tumor Drugs Oriented by Clinical Value [13]”. This document elucidates that innovative drug development should aim highly to provide patients with better (more effective, safer, or more convenient) treatment options.
For example, with the approvals of multiple domestic monoclonal antibodies targeting programmed cell death protein 1 (PD-1), the CDE now requests that the combination of anti-PD-1 with chemotherapy, rather than chemotherapy alone, should be employed as a control group in the RCT study for first-line therapy of late-stage non-small cell lung cancer.
3.5 Select endpoints that best demonstrate clinical benefit
In China, the new “Provisions for Drug Registration” [14] and the accompanying “Guidance for Conditional Approval of Drugs for Marketing (interim) [15]” stipulate that the selection of surrogate endpoints should be based on their predictive capacity for clinical benefit.
For malignant tumors, overall survival (OS) is the gold standard for evaluating the efficacy of drugs. Using appropriate surrogate endpoints can expedite the introduction of drugs into the market, thereby permitting patients to access novel therapies earlier. It is essential that these surrogate endpoints can predict clinical benefit, and their association with OS in the domain of anti-tumor drugs is contingent upon the intrinsic properties of the tumor. The CDE has promulgated guidance for the selection of surrogate endpoints in clinical trials [16, 17].
In instances where the progression of certain tumors is notably rapid, a significant extension of progression-free survival (PFS) is deemed a clinical benefit. Therefore, it is considered appropriate to use PFS as the primary endpoint. A phase III RCT on patients with hormone receptor-positive metastatic breast cancer who had not responded to previous endocrine therapy revealed that the combination of Dalpiciclib, the first domestic cyclin-dependent kinase 4/6 inhibitor, with the elective oestrogen receptor degrader Fulvestrant significantly prolonged the PFS compared to Fulvestrant administered as a monotherapy (15.7 months vs. 7.2 months, hazard ratio of 0.42), with a prolonged OS [18]. Based on the results of this study, the NMPA approved Dalpiciclib for patients with recurrent or metastatic breast cancer in 2021.
Both tumor and anti-tumor therapies cause significant pain in patients and severely impact their quality of life. Therefore, it is recommended that patient-reported outcomes (PRO) be adopted as an evaluation metric of clinical benefit. Although PRO has not yet been used as a primary endpoint for anti-tumor drugs, it helps explain clinical data that cannot be revealed by other traditional assessment metrics.
4 CONCLUSIONS AND PROSPECTS
Since China started the reforms in the drug review and approval system in 2015, the number of approvals of new drugs has been on the rise, with a steady increase in the number of applications that are globally synchronized. These achievements have reduced the disparity between domestic and international clinical practices, allowing Chinese patients to receive advanced therapeutic drugs and making it easier for pharmaceutical companies to synchronize global R&D strategies.
The accelerated R&D and launch of new drugs will ultimately reshape the landscape of tumor therapies, bringing more and diverse treatment options to patients in China. Moving forward, China's drug regulatory authorities are committed to advancing regulatory innovation for anti-tumor drugs to align with the evolving clinical treatment needs and drug R&D demands. We will introduce and integrate advanced international evaluation methods to refine the drug approval process. Regulatory innovation will prioritize the drugs targeting rare and pediatric tumors. We aim to enhance R&D efficiency and facilitate effective treatments through policy support. Additionally, we will promote the convergence of R&D and clinical application of anti-tumor drugs to advance China's anti-tumor drug industry. China's drug regulatory authorities will remain committed to offering a greater number of high-quality, safe, and effective therapeutic drugs for cancer patients.
AUTHOR CONTRIBUTIONS
Research concept and design: Jun Ma and Zhimin Yang. Collection and/or assembly of data and policy: Ling Tang, Yuanyuan Song. Analysis and interpretation of data and policy: Ling Tang, Yuanyuan Song, Hong Zhang, Ruimin Hao, Xin Tong, Xing Ai. Article written: Ling Tang. All authors approved the final manuscript.
ACKNOWLEDGMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
FUNDING INFORMATION
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used in the current study are available from the corresponding author upon reasonable request.