HLX07 alone or combined with serplulimab, cisplatin and 5-fluorouracil for advanced esophageal squamous cell carcinoma: A phase 2 study
Yun Liu, Yanfeng Wang, and Yanrong Zhu contributed equally to this study.
Abstract
Background
The combination of anti-PD-1 antibody serplulimab and chemotherapy is considered standard first-line therapy for advanced esophageal squamous cell carcinoma (ESCC), but few later-line treatments are available. Here we evaluated the therapeutic efficacy of the recombinant, humanized anti-EGFR antibody HLX07 when used alone or together with serplulimab and chemotherapy against advanced ESCC.
Methods
This open-label, non-randomized, two-cohort, phase 2 trial involved patients 18-75 years old with histologically or cytologically confirmed locally advanced, unresectable, or metastatic ESCC, and an Eastern Cooperative Oncology Group performance status of 0-1. Patients who had failed first-line immuno-chemotherapy or at least two lines of other systemic therapy received HLX07 monotherapy intravenously at a dose of 1,000 mg once every 2 weeks (Q2W). Patients with no prior systemic therapy received HLX07 (1,000 mg, day 1) and serplulimab (200 mg, day 1) intravenously Q2W for up to 2 years, concurrently with cisplatin (50 mg/m2, day 1) for up to 8 cycles and 5-fluorouracil (1,200 mg/m2, days 1-2) for up to 12 cycles intravenously Q2W. The primary endpoints were progression-free survival (PFS) and objective response rate (ORR).
Results
Overall, 50 patients were enrolled. In the HLX07 monotherapy group, ORR was 15.0% (3/20), and the median PFS was 1.5 months (95% confidence interval [CI], 1.3 to 3.7). The median duration of response was not reached, and the rate of patients showing an objective response lasting at least 6 months was 66.7% (95% CI, 5.4 to 94.5). Two (10.0%, 2/20) patients experienced grade 3-4 treatment-related adverse events (TRAEs), including hypomagnesemia, hypocalcemia, and fatigue. No patient experienced grade 5 TRAEs. In the HLX07 combination group, the ORR was 60.0% (18/30), and the median PFS was 7.8 months (95% CI, 3.3 to 9.1). Fourteen (46.7%, 14/30) patients experienced grade 3-4 TRAEs, and one (3.3%, 1/30) patient died due to serplulimab-related pneumonitis.
Conclusions
HLX07 monotherapy and its combination with serplulimab and chemotherapy showed manageable toxicity and promising antitumor activity in patients with recurrent or metastatic ESCC. Randomized controlled trials are warranted to further establish the safety and efficacy of HLX07 against ESCC.
Trial registration
This trial was registered at Clinicaltrials.gov (NCT05221658).
List of Abbreviations
-
- CI
-
- confidence interval
-
- COVID-19
-
- coronavirus disease 2019
-
- CT
-
- computed tomography
-
- DCR
-
- disease control rate
-
- DOR
-
- duration of response
-
- EC
-
- esophageal cancer
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- EGFR
-
- epidermal growth factor receptor
-
- ESCC
-
- esophageal squamous cell carcinoma
-
- FP
-
- cisplatin and 5-fluorouracil
-
- HR,
-
- hazard ratio
-
- IgG1
-
- immunoglobulin G1
-
- IHC
-
- immunohistochemistry
-
- irAE
-
- immune-related adverse events
-
- IRRC
-
- independent radiological review committee
-
- mAb
-
- monoclonal antibody
-
- MRI
-
- magnetic resonance imaging
-
- NCI-CTCAE
-
- National Cancer Institute Common Terminology Criteria for Adverse Events
-
- ORR
-
- overall response rate
-
- OS
-
- overall survival
-
- PD-1
-
- programmed cell death protein 1
-
- PFS
-
- progression-free survival
-
- RECIST
-
- Response Evaluation Criteria in Solid Tumours
-
- TKI
-
- tyrosine kinase inhibitor
-
- TRAE
-
- treatment-related adverse event
-
- TTR
-
- time to response
-
- ULN
-
- upper limit of normal
-
- VEGFR
-
- vascular endothelial growth factor receptor.
1 BACKGROUND
Esophageal cancer (EC) is the tenth most common cancer and sixth most common cause of cancer-related death worldwide [1]. The predominant histological subtypes are squamous cell carcinoma and adenocarcinoma, with esophageal squamous cell carcinoma (ESCC) accounting for 85% of EC cases globally [2]. As the symptoms of early-stage ESCC tend to be subtle, most patients are diagnosed at an advanced stage, when the prognosis remains poor. Despite recent advances in systemic therapy, treatment outcomes of ESCC remain dismal, with an overall 5-year survival rate of approximately 20% [3, 4].
The standard first-line systemic therapy for previously untreated recurrent or metastatic ESCC is the blockade of programmed cell death protein 1 (PD-1) in combination with chemotherapy [5-7]. In a randomized phase 3 trial, we demonstrated the superiority of a first-line combination of the anti-PD-1 antibody serplulimab (formerly known as HLX10) with cisplatin and 5-fluorouracil (FP) over placebo plus FP in terms of median progression-free survival (PFS) (5.8 months vs. 5.3 months; hazard ratio [HR] = 0.60; 95% confidence interval [CI], 0.48 to 0.75) and median overall survival (OS) (15.3 months vs 11.8 months; HR 0.68, 95% CI 0.53 to 0.87) [8]. However, patients with recurrent or metastatic ESCC who do not respond to first-line PD-1 inhibitors plus chemotherapy have few treatment options. Investigations for more effective first-line and salvage treatments are needed. Angiogenesis inhibitors such as anlotinib and apatinib, which mainly target the vascular endothelial growth factor receptor (VEGFR), have been explored recently [9, 10]. However, these drugs have not been proven to prolong OS in randomized phase 3 trials involving patients with recurrent or metastatic ESCC. Hence, they have not received clinical approval for this patient population.
About 50-70% of ESCC cases overexpress the epidermal growth factor receptor (EGFR) protein, indicating that targeting EGFR could be a promising strategy [11-14]. In a randomized phase 3 trial, the EGFR tyrosine kinase inhibitor gefitinib significantly improved PFS but did not improve OS as a potential second-line therapy for chemoresistant EC [15]. Regarding anti-EGFR monoclonal antibody treatment, only one phase 1b trial demonstrated a moderate antitumor efficacy of the anti-human EGFR monoclonal antibody SCT200 as monotherapy in patients with recurrent or metastatic ESCC [16]. A randomized phase 2 trial showed that cetuximab (an anti-EGFR antibody) with FP in first-line, compared with FP alone, could improve response rate and median OS of advanced ESCC patients [12]. Therefore, further investigation of anti-EGFR monoclonal antibodies in ESCC patients is warranted.
HLX07 is a novel, recombinant, humanized anti-EGFR monoclonal antibody. In contrast to cetuximab, HLX07 is a mouse-human chimeric IgG1 monoclonal antibody constructed using humanized germline variable regions of heavy and light chains [17]. The complementarity-determining region 2 on the heavy chain is less glycosylated, which increases binding affinity to EGFR and reduces immunogenicity. A phase 1 study showed that HLX07 was safe and tolerable in patients with advanced solid tumors [18]. In this report, we presented the results from our phase 2 study involving patients with locally advanced, unresectable or metastatic ESCC to evaluate the preliminary efficacy and safety of HLX07 alone as a later-line therapy and of HLX07 in combination with serplulimab and chemotherapy as a first-line therapy.
2 MATERIALS AND METHODS
2.1 Study design and participants
This report described a multicenter, two-cohort, phase 2 trial of HLX07 alone or in combination with immunotherapy and chemotherapy to treat advanced ESCC. The work described in this report was conducted across 12 sites in China between August 18, 2022 and January 13, 2023. The trial continues but is no longer enrolling patients. ESCC patients who failed at least one systemic treatment were enrolled into the group receiving HLX07 monotherapy. Treatment-naïve patients were assigned to the group receiving the combination of HLX07 with serplulimab and FP as first-line treatment. Specifically, patients had to exhibit radiological progression or intolerance following a first-line combination of immunotherapy and chemotherapy or, they had to have undergone at least two lines of alternative systemic therapy to be enrolled in the HLX07 monotherapy group. While patients were not allowed to have had any prior treatment for unresectable or metastatic ESCC, except neoadjuvant chemotherapy, concurrent chemoradiotherapy or adjuvant chemotherapy delivered at least six months before enrollment in the study to be enrolled in the HLX07 combination group.
The two groups had similar inclusion criteria: 1) age between 18 and 75 years; 2) histologically or cytologically confirmed locally advanced, unresectable or metastatic ESCC; 3) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; 4) at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors criteria version 1.1 (RECIST v.1.1) [19]; 5) an estimated life expectancy of 12 weeks or longer; and 6) normal organ function as defined in the protocol synopsis (Supplementary Methods). Patients were excluded from the study if they had previous or active autoimmune disease, other malignant disease (except curatively treated skin basal cell carcinoma or cervical carcinoma in situ), medical conditions requiring immunosuppressive medication, active infection with hepatitis B or C virus, active metastasis to the central nervous system, active infection, uncontrolled cardiac disease, or previous treatment with anti-EGFR monoclonal antibody. Further details regarding inclusion and exclusion criteria for this trial are outlined in Supplementary Methods.
The trial was approved by the ethics committees of the leading study site (National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, approval number 21/534-3205) and all other study sites. The trial was conducted in accordance with the Declaration of Helsinki and guidelines of Good Clinical Practice. Written informed consent was obtained from subjects or their legal guardians before enrollment. The trial was registered at Clinicaltrials.gov (NCT05221658).
2.2 Procedures
In the HLX07 monotherapy group, previously treated ESCC patients were administered with HLX07 at 1,000 mg every 2 weeks on day 1 of each cycle. All eligible patients were treated until disease progression, intolerable toxicity, death, investigator's decision to stop, or withdrawal of informed consent. For patients experiencing reversible toxicity of grade 3 or worse related to HLX07, subsequent infusions at a low dose were allowed if the toxicity reverted to grade 1 or baseline levels and the patients met the eligibility criteria for adequate organ function. The minimal HLX07 dose allowed in the study was 600 mg. If grade 3 or worse toxicity persisted even after administration of 600 mg of HLX07, infusion of HLX07 was temporarily suspended and investigators decided whether to discontinue it permanently.
In the HLX07 combination group, treatment-naïve ESCC patients received serplulimab at 200 mg and HLX07 at 1000 mg once every 2 weeks on day 1 of each cycle for up to 2 years. Concurrently, patients received cisplatin (50 mg/m2) on day 1 for up to 8 cycles and continuous daily administration of 5-FU (1,200 mg/m2) on days 1 and 2 of each cycle for up to 12 cycles. Both chemotherapeutic drugs were administered intravenously every 2 weeks. Patients were treated until disease progression, death, unacceptable toxicity, withdrawal of consent, or a decision by investigators. Doses of HLX07, cisplatin and 5-FU were allowed to be modified according to the occurrence and severity of treatment-related adverse events (TRAEs). The dose of serplulimab could not be modified.
All patients were assessed regularly for efficacy and safety, including history and physical examinations, evaluations of adverse events, and routine bloodwork at study entry and before each treatment cycle. Computed tomography (CT) or magnetic resonance imaging (MRI) were performed every 6 weeks for the first 48 weeks from enrollment and every 12 weeks thereafter. The response was assessed according to RECIST v.1.1 criteria by an independent radiological review committee (IRRC) consisting of six radiologists from medical centers that were not involved in the study, and by local investigators. When stable disease (SD) was assessed to be the best response, it must also meet the present protocol-specified criteria of a minimum period of 42 days from baseline. Otherwise, that patient's best response was evaluated based on the subsequent assessments. During the follow-up period, patients were contacted every 12 weeks to assess their survival. Adverse events and abnormal laboratory findings were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (NCI-CTCAE v.5.0) and attributed to each study drug as appropriate. Adverse events of special interest (infusion-related reaction, rash, hypomagnesemia, and immune-related adverse events) related to the study drugs were recorded. Serious adverse events were defined as those that led to death, endangered patients’ lives, required hospitalization or an extension of any current hospitalization, led to permanent or severe disability/malfunction, or caused congenital anomalies/birth defects. Serious TRAEs were defined as serious adverse events that were treatment-related.
Besides, patients in the HLX07 monotherapy group had to provide tumor tissue for EGFR expression assessment before enrolment. The expression of EGFR was assessed by immunohistochemistry with the undiluted rabbit monoclonal CONFIRM anti-EGFR (5B7) antibody (catalog no. 790-4347, Ventana, USA) in a central laboratory. Immunohistochemical staining was semi-quantitated as no staining (0), weak staining (1+), moderate staining (2+), or strong staining (3+) according to the percentage of EGFR-positive tumor cells. An EGFR H-score for each assessable patient was calculated by multiplying the staining intensity by the percentage of positive cells, ranging from 0 to 300, according to the described method [20]. We classified patients as showing low EGFR expression if H-score < 200, and as showing high expression if H-score ≥ 200 [20]. Patients enrolled in the HLX07 combination group were assessed for their EGFR expression when their tissue samples were provided later on during the study.
2.3 Outcomes
The two primary endpoints were objective response rate (ORR), defined as the proportion of patients with partial or complete response, and PFS, defined as the time from study enrollment to disease progression or death from any cause. Both endpoints were assessed by the IRRC and by local investigators according to RECIST v.1.1 criteria. Secondary endpoints included the disease control rate (DCR), defined as the proportion of patients with an overall response or stable disease; time to response (TTR), defined as the time from enrollment to first documentation of an overall response; duration of response (DOR), defined as the time from the first documentation of an overall response to radiological disease progression; OS, defined as the time from enrollment to death from any cause; the 6-month PFS rate, defined as the proportion of patients with PFS at 6 months; and safety, defined as the occurrence of TRAEs according to NCI-CTCAE v.5.0. An exploratory endpoint was the investigation of an association between treatment efficacy and H-score of EGFR expression.
2.4 Statistical analyses
The data cutoff date for this present analysis was July 4, 2023. No statistical calculations were performed to estimate the minimal sample sizes before enrollment. Instead, sample sizes were selected because they were expected to provide adequate data for assessing the preliminary efficacy and safety of HLX07 regimens. Data in this trial were analyzed statistically using SAS 9.4 (SAS Institute, Cary, USA). Primary and secondary endpoints of efficacy were reported as the number (percentage) of patients (n [%]) together with two-sided 95% Clopper-Pearson exact CIs. Endpoints were not compared statistically between the HLX07 monotherapy and combination groups because this was not the objective of the trial. Efficacy endpoints were calculated across the intention-to-treat population, while safety endpoints were calculated across all patients who received at least one dose of study medication. Survival curves for each group were estimated using the Kaplan-Meier product limit method, and medians and their two-sided 95% CIs were determined from the curves. The Kaplan-Meier method was also used to estimate the PFS rate at 6 months with corresponding 95% CIs. Safety endpoints were reported in terms of n (%). No interim or sensitivity analyses were planned or performed.
3 RESULTS
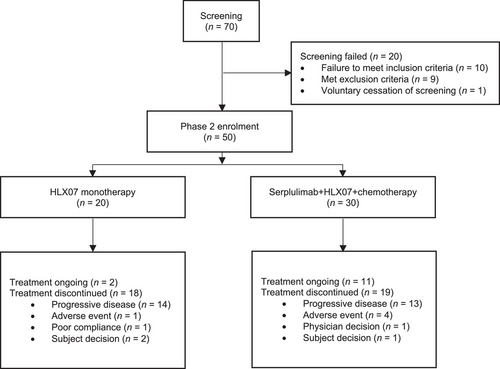
Between August 18, 2022 and January 13, 2023, 70 advanced ESCC patients from 12 hospitals in China were screened for eligibility (Figure 1). Ten failed to meet all inclusion criteria, nine met at least one of the exclusion criteria, and one withdrew consent. The remaining 50 patients with advanced ESCC were enrolled and assigned to receive HLX07 monotherapy as later-line treatment (n = 20) and HLX07 combination therapy as first-line treatment (n = 30), respectively (Table 1). The data cutoff date for the efficacy and safety analyses presented here was July 4, 2023. Unless otherwise noted, the outcomes reported below were those assessed by the IRRC.
| Characteristics |
HLX07 monotherapy (n = 20) |
HLX07 combination therapy (n = 30) |
|---|---|---|
| Age, median (range), years | 59.5 (46-67) | 64.5 (48-74) |
| Sex, n (%) | ||
| Male | 20 (100.0) | 26 (86.7) |
| Female | 0 (0) | 4 (13.3) |
| ECOG performance status, n (%) | ||
| 0 | 8 (40.0) | 12 (40.0) |
| 1 | 12 (60.0) | 18 (60.0) |
| Disease status, n (%) | ||
| Locally advanced, unresectable | 0 (0) | 5 (16.7) |
| Distantly metastatic | 20 (100.0) | 25 (83.3) |
| Metastasisa, n (%) | ||
| Lymph node | 17 (85.0) | 27 (90.0) |
| Liver | 6 (30.0) | 7 (23.3) |
| Lung | 5 (25.0) | 4 (13.3) |
| Bone | 3 (15.0) | 2 (6.7) |
| Other site | 2 (10.0) | 2 (6.7) |
| H-score for EGFR expression based on IHC, n (%) | ||
| ≥ 200 | 7 (35.0) | 5 (16.7) |
| < 200 | 13 (65.0) | 24 (80.0) |
| Not determined | 0 (0) | 1 (3.3) |
| Semi-quantitation of EGFR expression based on IHC, n (%) | ||
| 3+ | 9 (45.0) | 9 (30.0) |
| 2+ | 4 (20.0) | 11 (36.7) |
| 1+ | 7 (35.0) | 8 (26.7) |
| 0 | 0 (0) | 1 (3.3) |
| Not determined | 0 (0) | 1 (3.3) |
| Prior antitumor therapyb, n (%) | ||
| Chemotherapy | 20 (100.0) | 2 (6.7) c |
| Immunotherapy | 19 (95.0) | 0 (0) |
| Anlotinib | 7 (35.0) | 0 (0) |
| Apatinib | 3 (15.0) | 0 (0) |
| KC1036 | 1 (5.0) | 0 (0) |
| Number of prior lines of therapy, n (%) | ||
| 1 | 2 (10.0) | 0 (0) |
| 2 | 10 (50.0) | 0 (0) |
| 3 | 6 (30.0) | 0 (0) |
| 4 | 2 (10.0) | 0 (0) |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry.
- a Some patients were diagnosed with metastasis to more than one site.
- b Some patients in the HLX07 monotherapy group had previously received more than one type of antitumor therapy.
- c Patients received adjuvant chemotherapy.
3.1 Treatment efficacy
The HLX07 monotherapy group was followed up for a median of 7.4 months (95% CI, 5.8 to 9.3). Before the first assessment of efficacy outcomes, four patients were excluded including two patients who withdrew consent and two whose ECOG performance statuses were downgraded. ORR of the 20 patients was 15.0% (95% CI, 3.2 to 37.9), with two patients achieving a complete response and one achieving a partial response (Table 2). Seven (35.0%) patients achieved disease control, including two with complete response, one with partial response, and four with stable disease (Table 2). Of the three patients showing an objective response, the median TTR was 1.4 months (range, 1.3 to 1.4), and the median DOR was not reached. The complete response was observed and lasting for at least 6 months in two patients (Supplementary Figure S1). Both patients were continuing study treatment at the data cutoff date.
| Assessed by IRRC | Assessed by local investigators | |||
|---|---|---|---|---|
| Response |
HLX07 monotherapy (n = 20) |
HLX07 combination therapy (n = 30) |
HLX07 monotherapy (n = 20) |
HLX07 combination therapy (n = 30) |
| Best overall response, n (%) | ||||
| Complete response | 2 (10.0) | 4 (13.3) | 1 (5.0) | 1 (3.3) |
| Partial response | 1 (5.0) | 14 (46.7) | 2 (10.0) | 16 (53.3) |
| Stable disease | 4 (20.0) | 3 (10.0) | 4 (20.0) | 4 (13.3) |
| Progressive disease | 9 (45.0) | 3 (10.0) | 9 (45.0) | 4 (13.3) |
| Not evaluable | 4 (20.0) | 6 (20.0)b | 4 (20.0) | 5 (16.7) |
| Objective response rates, n (%), 95% CI | 3 (15.0), 3.2 to 37.9 | 18 (60.0), 40.6 to 77.3 | 3 (15.0), 3.2 to 37.9 | 17 (56.7), 37.4 to 74.5 |
| Disease control rates, n (%), 95% CI | 7 (35.0), 15.4 to 59.2 | 21 (70.0), 50.6 to 85.3 | 7 (35.0), 15.4 to 59.2 | 21 (70.0), 50.6 to 85.3 |
| Median duration of response, months, 95% CI | NR, 1.4 to NE | 7.2, 4.4 to NE | NR, 2.0 to NE | 7.2, 5.1 to NE |
| Median time to response, months, range | 1.4, 1.3 to 1.4 | 1.4, 1.3 to 3.5 | 1.4, 1.3 to 1.4 | 1.4, 1.3 to 4.4 |
- Abbreviations: CI, confidence interval; IRRC, independent radiological review committee; NE, not evaluable; NR, not reached.
- a Responses were assessed in accordance with the Response Evaluation Criteria in Solid Tumours (version 1.1) [19].
- b Two patients had a first evaluation of stable disease that did not persist beyond the protocol-defined criterion of 42 days and these patients were not assessed subsequently. Therefore their best response was classified as “not evaluable”.
The HLX07 combination group was followed up for a median of 8.3 (95% CI, 8.0 to 8.5) months. ORR was 60.0% (95% CI, 40.6 to 77.3), with four patients achieving complete response and 14 achieving partial response (Table 2). Disease control was achieved in 21 (70.0%) patients, including four with complete response, 14 with partial response, and three with stable disease (Table 2). Among the 18 patients showing an objective response, the median TTR was 1.4 months (range, 1.3 to 3.5) and the median DOR was 7.2 months (95% CI, 4.4 to not evaluable [NE]) (Table 2).
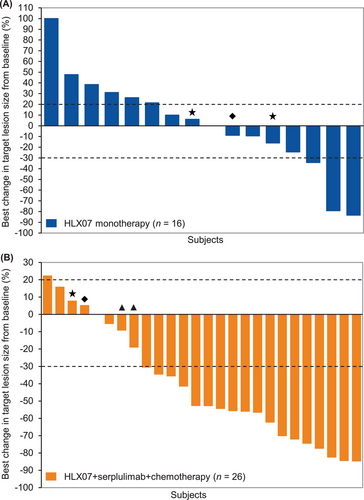
Among the 50 patients treated in the study, records of responses during treatment for the two groups were shown in Figure 2. Seven (35.0%) patients had decreased lesion size from baseline measurement in the HLX07 monotherapy group (Figure 3A). Twenty-one (70.0%) patients had decreased lesion size from baseline measurement in the HLX07 combination group (Figure 3B).
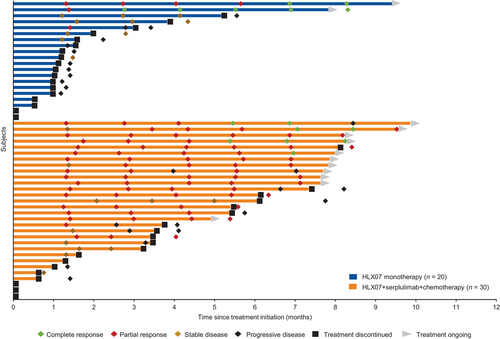
Among the 20 patients in the HLX07 monotherapy group, 16 (80.0%) experienced PFS events, including 11 cases of disease progression and five deaths. Median PFS was 1.5 months (95% CI, 1.3 to 3.7), the PFS rate at 6 months was 11.7% (95% CI, 2.0 to 30.9), and the median OS was 5.8 months (95% CI, 3.5 to NE) (Supplementary Figure S2-S3). Sixteen (53.3%) patients in the HLX07 combination group experienced PFS events including ten cases of disease progression and six deaths. PFS was 7.8 months (95% CI, 3.3 to 9.1) (Supplementary Figure S4), the PFS rate at 6 months was 55.6% (95% CI, 35.0 to 72.0), while the median OS was not reached. As of the data cutoff date, HLX07 combination treatment was ongoing in 11 patients, one of whom had experienced disease progression during the study.
3.2 Safety and tolerability
Among the 20 patients in the HLX07 monotherapy group, 18 (90.0%) experienced at least one TRAE, none of which was serious TRAE (Table 3). Across all grades of severity, the most frequent TRAEs were rash (13 patients, 65.0%), hypomagnesemia (12 patients, 60.0%), hypocalcemia (6 patients, 30.0%), sinus tachycardia (5 patients, 25.0%), hyponatremia (4 patients, 20.0%), dry skin (4 patients, 20.0%), hypokalemia (3 patients, 15.0%), nausea (3 patients, 15.0%), and fatigue (3 patients, 15.0%), respectively. Two (10.0%) patients experienced grade 3 TRAEs of hypocalcemia and fatigue, with one of them experiencing a grade 4 TRAE of hypomagnesemia at the same time. No patients discontinued HLX07 monotherapy because of TRAEs. However, the study treatment for three patients was temporarily interrupted because of COVID-19 infection, urinary tract stones, or acute kidney injury, none of which was related to HLX07. All three patients resumed HLX07 treatment after these events were resolved. No patients experienced infusion-related reactions or died during treatment.
| HLX07 monotherapy (n = 20) | HLX07 combination therapy (n = 30) | |||||||
|---|---|---|---|---|---|---|---|---|
| TRAEs | Grades 1-2 | Grade 3 | Grade 4 | Totalb | Grades 1-2 | Grade 3 | Grade 4 | Totalb |
| Any TRAEs, n (%) | 16 (80.0) | 1 (5.0) | 1 (5.0) | 18 (90.0) | 15 (50.0) | 11 (36.7) | 2 (6.7) | 29 (96.7)c |
| Serious TRAEs | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (10.0)d | 0 (0) | 4 (13.3)c |
| Most common TRAEs (≥ 10%) in either group, n (%) | ||||||||
| Rash | 13 (65.0) | 0 (0) | 0 (0) | 13 (65.0) | 15 (50.0) | 3 (10.0) | 0 (0) | 18 (60.0) |
| Hypomagnesemia | 11 (55.0) | 0 (0) | 1 (5.0) | 12 (60.0) | 13 (43.3) | 4 (13.3) | 1 (3.3) | 18 (60.0) |
| Hypocalcemia | 5 (25.0) | 1 (5.0) | 0 (0) | 6 (30.0) | 10 (33.3) | 0 (0) | 1 (3.3) | 11 (36.7) |
| Sinus tachycardia | 5 (25.0) | 0 (0) | 0 (0) | 5 (25.0) | 3 (10.0) | 0 (0) | 0 (0) | 3 (10.0) |
| Hyponatremia | 4 (20.0) | 0 (0) | 0 (0) | 4 (20.0) | 11 (36.7) | 0 (0) | 0 (0) | 11 (36.7) |
| Dry skin | 4 (20.0) | 0 (0) | 0 (0) | 4 (20.0) | 6 (20.0) | 0 (0) | 0 (0) | 6 (20.0) |
| Hypokalemia | 3 (15.0) | 0 (0) | 0 (0) | 3 (15.0) | 3 (10.0) | 1 (3.3) | 0 (0) | 4 (13.3) |
| Nausea | 3 (15.0) | 0 (0) | 0 (0) | 3 (15.0) | 16 (53.3) | 0 (0) | 0 (0) | 16 (53.3) |
| Fatigue | 2 (10.0) | 1 (5.0) | 0 (0) | 3 (15.0) | 5 (16.7) | 1 (3.3) | 0 (0) | 6 (20.0) |
| Hypoalbuminemia | 2 (10.0) | 0 (0) | 0 (0) | 2 (10.0) | 6 (20.0) | 0 (0) | 0 (0) | 6 (20.0) |
| Hyperkalemia | 2 (10.0) | 0 (0) | 0 (0) | 2 (10.0) | 1 (3.3) | 0 (0) | 0 (0) | 1 (3.3) |
| Itching | 2 (10.0) | 0 (0) | 0 (0) | 2 (10.0) | 7 (23.3) | 1 (3.3) | 0 (0) | 8 (26.7) |
| Decreased appetite | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 7 (23.3) | 0 (0) | 0 (0) | 7 (23.3) |
| Dermatitis acneiform | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 2 (6.7) | 3 (10.0) | 0 (0) | 5 (16.7) |
| Neutrophil count decreased | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 9 (30.0) | 2 (6.7) | 0 (0) | 11 (36.7) |
| Platelet count decreased | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 9 (30.0) | 0 (0) | 0 (0) | 9 (30.0) |
| White blood cell count decreased | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 7 (23.3) | 1 (3.3) | 0 (0) | 8 (26.7) |
| Alanine aminotransferase increased | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 7 (23.3) | 0 (0) | 0 (0) | 7 (23.3) |
| Aspartate aminotransferase increased | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 7 (23.3) | 0 (0) | 0 (0) | 7 (23.3) |
| Vomiting | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 6 (20.0) | 0 (0) | 0 (0) | 6 (20.0) |
| Oral ulcer | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 4 (13.3) | 0 (0) | 0 (0) | 4 (13.3) |
| Hyperthyroidism | 1 (5.0) | 0 (0) | 0 (0) | 1 (5.0) | 4 (13.3) | 0 (0) | 0 (0) | 4 (13.3) |
| Hypoproteinaemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (10.0) | 0 (0) | 0 (0) | 3 (10.0) |
| Lymphocyte count decreased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (6.7) | 2 (6.7) | 0 (0) | 4 (13.3) |
| Anemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 20 (66.7) | 1 (3.3) | 0 (0) | 21 (70.0) |
| Fever | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (10.0) | 0 (0) | 0 (0) | 3 (10.0) |
| Proteinuria | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (10.0) | 0 (0) | 0 (0) | 3 (10.0) |
- Abbreviation: TRAE, treatment-related adverse event.
- a A grade 1-2 event is recorded if it occurred in at least 10% of patients in either group. Any grades 3-4 event is recorded.
- b Included TRAEs of grades 1 to 5.
- c Included one patient in the HLX07 combination group who experienced an event of grade 5 (serious), which was immune-related pneumonitis.
- d One was serplulimab-related, another was HLX07-related, and the other one was chemotherapy-related.
Among the 30 patients in the HLX07 combination group, all but one (96.7%) patient experienced at least one TRAE. The most frequent TRAEs were anemia (21 patients, 70.0%), rash (18 patients, 60.0%), hypomagnesemia (18 patients, 60.0%), nausea (16 patients, 53.3%), hypocalcemia (11 patients, 36.7%), hyponatremia (11 patients, 36.7%), neutrophil count decreased (11 patients, 36.7%), and platelet count decreased (9 patients, 30.0%) (Table 3). Only under half of the patients (n = 14, 46.7%) experienced TRAEs that were grade 3, grade 4, and grade 5. Nevertheless, no patients discontinued HLX07 combination therapy because of TRAEs. However, the study treatment was temporarily interrupted in six patients because of rash, dermatitis acneiform, or conjunctivitis, and they resumed HLX07 treatment after these events resolved. No patients discontinued dual chemotherapy because of TRAEs. Doses of FP were reduced in six patients who developed seven TRAEs including two (6.7%) with decreased platelet count, one (3.3%) with decreased neutrophil count, one (3.3%) with anemia, one (3.3%) with anorexia and nausea, and one (3.3%) with fatigue. Four (13.3%) patients developed serious TRAEs: two events were related to serplulimab, one to HLX07, and one to chemotherapy. Two (6.7%) patients experienced a total of three immune-related adverse events: decreased platelet count (grade 1), hyperthyroidism (grade 1), and pneumonitis (grade 5). Two (6.7%) patients experienced infusion-related reactions.
3.3 Exploratory endpoint
Among the 20 patients in the HLX07 monotherapy group, seven (35.0%) had an EGFR H-score of ≥ 200, while 13 (65.0%) had an EGFR H-score of < 200. ORR did not differ substantially between those with high H-scores (14.3%) or those with low H-scores (15.4%) (Supplementary Table S1). Median PFS was numerically slightly longer for those with high H-scores (1.8 months [95% CI, 0.8 to NE] vs. 1.5 months [95% CI, 1.3 to 3.7]) (Supplementary Table S1). Among the 30 patients in the HLX07 combination group, EGFR expression in tumors was analyzed in 29 patients because we lacked a suitable tumor tissue sample from one patient. Five (17.2%) patients showed an H-score ≥ 200, while 24 (82.8%) showed an H-score < 200. Those with high H-scores showed a numerically higher ORR (80.0 vs. 58.3%) and longer median PFS (9.1 months [95% CI, 0.3 to NE] vs. 7.8 months [95% CI, 2.9 to NE]) (Supplementary Table S1).
4 DISCUSSION
In this study, we explored the preliminary efficacy of HLX07 monotherapy as a late-line treatment, or of HLX07 in combination with serplulimab, cisplatin, and 5-fluorouracil as a first-line treatment. Our findings show that HLX07 monotherapy conferred encouraging antitumor efficacy with a manageable toxicity profile in previously treated advanced ESCC patients. More importantly, the addition of HLX07 to serplulimab and chemotherapy for these patients also exhibited notable antitumor activity in the first-line setting.
ESCC is known to be an immunocompetent tumor, therefore a high proportion of patients with advanced ESCC can achieve a response with immunotherapy plus chemotherapy as a first-line treatment [21]. However, those who are refractory or progress after the standard-of-care therapy have limited treatment options; salvage chemotherapy only produces moderate antitumor activity as a second-line or later option in this setting [22]. Thus, the development of targeted therapies may help to provide alternative options for these subsets of patients. To date, no approval has been granted for the use of targeted drugs in advanced ESCC. In terms of anti-EGFR monotherapy in ESCC, findings from the studies of anti-EGFR TKIs and anti-EGFR monoclonal antibody SCT200 have been published. Previously, anti-EGFR TKIs showed modest activities with ORRs of 2.8% to 16.7%, including gefitinib [23], erlotinib [24], and icotinib [25]. However, a randomized phase 3 trial comparing second-line gefitinib with placebo in esophageal cancer failed to meet the primary endpoint of a significant OS benefit with gefitinib compared to placebo (3.7 months vs. 3.6 months; HR = 0.90; P = 0.293) [15]. SCT200 showed an encouraging ORR of 16.7% with a median DOR of 3.9 months as a second-line treatment for ESCC [16]. Comparing the findings of the HLX07 monotherapy group with that of anti-EGFR TKIs and SCT200, the ORR was similar (15.0% versus 16.7%). Moreover, the median DOR in the HLX07 monotherapy group was longer than in the SCT200 trial (> 6.0 months vs. 3.9 months). The results from anti-EGFR antibodies, especially HLX07 monotherapy treatment, provided further evidence to support the targeting of the EGFR pathway as a treatment strategy for advanced ESCC.
The results from our HLX07 combination group supported the combination of an anti-EGFR monoclonal antibody and immunotherapy for treating advanced ESCC. Such a combination has been explored against other cancers as a way to overcome immunosuppression within the tumor microenvironment, such as squamous cell carcinoma of the head and neck as well as colorectal cancer [26]. For example, the combination of cetuximab and an anti-PD-1 inhibitor nivolumab or pembrolizumab has shown promising efficacy against recurrent or metastatic head and neck squamous cell carcinoma [27, 28]. Here we found that the combination of HLX07, serplulimab, and chemotherapy was effective as a first-line treatment against advanced ESCC. Patients showed an ORR of 60.0% and median PFS of 7.8 months (95% CI, 3.3 to 9.1), which are comparable to, or potentially better than, the ORR of 58% and median PFS of 5.8 months (95% CI, 5.7 to 6.9) for serplulimab in combination with chemotherapy. The currently reported median PFS of 7.8 months is also better than that reported for first-line combination therapies of chemotherapy with pembrolizumab (6.3 months) [5], nivolumab (5.8 months) [6], or camrelizumab (5.7 months) [7]. The potentially longer median PFS in our study may mean that HLX07 potentiates anti-tumor immune responses within the tumor microenvironment, leading to more durable clinical benefits in those patients who respond to the treatment.
The TRAEs observed in our patients receiving late-line HLX07 monotherapy, including rash and hypomagnesemia, were consistent with those frequently experienced by patients treated with EGFR inhibitors such as cetuximab and SCT200. However, the incidence of grade 3 or worse TRAEs was lower with HLX07 in our study compared to SCT200 in a previous trial (10.0% vs. 33.3%) [16]. Similarly, the safety profile of patients receiving first-line HLX07 in combination with serplulimab and chemotherapy in our study was consistent with that previously reported for the individual therapeutic regimes. The incidence of grade 3 or worse TRAEs was lower in our study (46.7%) compared to trials involving first-line combinations of chemotherapy with either pembrolizumab (71.9%) or camrelizumab (63.4%) [5, 7].
In this study, we observed a numerically higher ORR and longer median PFS among those patients with high EGFR H-scores who were treated with the HLX07 combination therapy, but not for those with high EGFR H-scores who were treated with HLX07 monotherapy. Owing to the small sample size of patients with high EGFR H-scores in both treatment arms (n = 5 for the HLX07 combination group; n = 7 for the HLX07 monotherapy group), our study was not powered for a rigorous statistical comparison or correlation analysis. The dependence of a PFS or clinical benefit on tumor EGFR level therefore remains to be clarified.
Our findings should be interpreted with caution due to the small, ethnically homogeneous sample size. Future work should further explore the efficacy and safety of HLX07, both as monotherapy and in combination with serplulimab and chemotherapy, in larger and more diverse patient populations.
5 CONCLUSIONS
Our findings suggest encouraging efficacy and manageable safety of HLX07 as monotherapy or in combination with serplulimab and chemotherapy. This trial supports the need for larger investigations of these treatment regimens as potential first- and later-line therapies for advanced ESCC.
AUTHOR CONTRIBUTIONS
Jing Huang and Qingyu Wang contributed to the study design as well as the analysis and interpretation of data. Yun Liu, Yanfeng Wang, Yanrong Zhu, Tao Wu, Zhenyang Liu, Jin Zhou, Yuan Yuan, Mudan Yang, Bo Liu, Zhenbo Tan, Wu Zhuang, Jiayan Chen, Ning Li, Ying Wang, Xuhui Hu, Lin Wang, Haoyu Yu, Qingyu Wang, Jun Zhu, and Jing Huang contributed to data acquisition. Yun Liu, Jing Huang and Qingyu Wang contributed to the writing of the manuscript, which Yanfeng Wang, Yanrong Zhu, Tao Wu, Zhenyang Liu, Jin Zhou, Yuan Yuan, Mudan Yang, Bo Liu, Zhenbo Tan, Wu Zhuang, Jiayan Chen, Ning Li, Ying Wang, Xuhui Hu, Lin Wang, Haoyu Yu, and Jun Zhu reviewed and approved. Jing Huang and Qingyu Wang had full access to all data in the study and share final responsibility for the decision to submit the manuscript for publication.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the patients participating in this trial, their families, the study personnel at each study site, as well as the clinical study team at Shanghai Henlius Biotech Inc, comprising Lu Luo (clinical procedures), Yali Liu (statistical analysis), Wenting Qiu, Jiancheng Cheng and Wenjie Zhang. Medical writing assistance was provided by Zhi Hao Kwok, Shiqi Zhong and Chen Hu of Shanghai Henlius Biotech Inc.
CONFLICT OF INTEREST STATEMENT
Xuhui Hu, Lin Wang, Haoyu Yu, Qingyu Wang, and Jun Zhu are employees of Shanghai Henlius Biotech Inc. None of the other authors has any competing interests to declare.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committees at the leading center (National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, approval No. 21/534-3205), as well as each participating site, and performed in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines. Written informed consent was obtained from each subject or each subject's guardian before enrolment. This trial is registered on clinicaltrial.gov (NCT05221658). This study was funded by Shanghai Henlius Biotech Inc. (Shanghai, China).
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed in this study are available from the principal investigator (Jing Huang) and the sponsor (Shanghai Henlius Biotech Inc) on reasonable request.




