Central nervous system efficacy of aumolertinib versus gefitinib in patients with untreated, EGFR-mutated, advanced non-small cell lung cancer: data from a randomized phase III trial (AENEAS)
Prior presentation: Presented in part at the 2022 American Society of Clinical Oncology conference in Chicago, IL, USA as Poster # 9096.
Abstract
Background
The initial randomized, double-blinded, actively controlled, phase III ANEAS study (NCT03849768) demonstrated that aumolertinib showed superior efficacy relative to gefitinib as first-line therapy in epidermal growth factor receptor (EGFR)-mutated advanced non-small cell lung cancer (NSCLC). Metastatic disease in the central nervous system (CNS) remains a challenge in the management of NSCLC. This study aimed to compare the efficacy of aumolertinib versus gefitinib among patients with baseline CNS metastases in the ANEAS study.
Methods
Eligible patients were enrolled and randomly assigned in a 1:1 ratio to orally receive either aumolertinib or gefitinib in a double-blinded fashion. Patients with asymptomatic, stable CNS metastases were included. Follow-up imaging of the same modality as the initial CNS imaging was performed every 6 weeks for 15 months, then every 12 weeks. CNS response was assessed by a neuroradiological blinded, independent central review (neuroradiological-BICR). The primary endpoint for this subgroup analysis was CNS progression-free survival (PFS).
Results
Of the 429 patients enrolled and randomized in the ANEAS study, 106 patients were found to have CNS metastases (CNS Full Analysis Set, cFAS) at baseline by neuroradiological-BICR, and 60 of them had CNS target lesions (CNS Evaluable for Response, cEFR). Treatment with aumolertinib significantly prolonged median CNS PFS compared with gefitinib in both cFAS (29.0 vs. 8.3 months; hazard ratio [HR] = 0.31; 95% confidence interval [CI], 0.17-0.56; P < 0.001) and cEFR (29.0 vs. 8.3 months; HR = 0.26; 95% CI, 0.11-0.57; P < 0.001). The confirmed CNS overall response rate in cEFR was 85.7% and 75.0% in patients treated with aumolertinib and gefitinib, respectively. Competing risk analysis showed that the estimated probability of CNS progression without prior non-CNS progression or death was consistently lower with aumolertinib than with gefitinib in patients with and without CNS metastases at baseline. No new safety findings were observed.
Conclusions
These results indicate a potential advantage of aumolertinib over gefitinib in terms of CNS PFS and the risk of CNS progression in patients with EGFR-mutated advanced NSCLC with baseline CNS metastases.
Trial registration
ClinicalTrials.gov number, NCT03849768
Abbreviations
-
- AE
-
- adverse event
-
- BICR
-
- blinded independent central review
-
- BL
-
- baseline
-
- cEFR
-
- central nervous system evaluable for response
-
- cFAS
-
- central nervous system full analysis set
-
- CI
-
- confidence interval
-
- CNS
-
- central nervous system
-
- CR
-
- complete response
-
- DCO
-
- data cut-off
-
- DCR
-
- disease control rate
-
- DoR
-
- duration of response
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- EGFR
-
- epidermal growth factor receptor
-
- HR
-
- hazard ratio
-
- IQR
-
- interquartile range
-
- NA
-
- not arrived
-
- NE
-
- not evaluable
-
- NSCLC
-
- non-small cell lung cancer
-
- OR
-
- odds ratio
-
- ORR
-
- objective response rate
-
- PD
-
- progressive disease
-
- PFS
-
- progression-free survival
-
- PR
-
- partial response
-
- RECIST 1.1
-
- Response Evaluation Criteria in Solid Tumors version 1.1
-
- SD
-
- stable disease
-
- TEAE
-
- treatment-emergent adverse events
-
- TKI
-
- tyrosine kinase inhibitor.
1 BACKGROUND
As one of the most common actionable genomic alterations in non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR) mutations are well established as an oncogenic driver that confers sensitivity to EGFR-targeted tyrosine kinase inhibitors (EGFR-TKIs) [1-3]. Despite the significant benefit of survival for these patients receiving first- or second-generation EGFR-TKIs, metastatic disease in the central nervous system (CNS) remains a challenge in management. About 50% of the NSCLC patients harboring EGFR mutations may develop CNS metastases, which is likely due to a combination of prolonged survival on the order of years and limited CNS penetration of first-generation EGFR-TKIs [4]. Evidence for the latter arises from a discrepancy in the frequency of the T790M acquired resistance mutation between intracranial and extracranial recurrence on treatment with first-generation EGFR-TKIs [5].
Third-generation EGFR-TKIs were specifically developed to target the T790M acquired-resistance mutation arising from progression on first-generation inhibitors as well as to improve the limited CNS penetration of these agents, which is nonetheless superior to chemotherapy. Osimertinib, the first globally approved third-generation EGFR-TKI, is approved as second-line treatment for previously treated T790M mutated NSCLC [6] and for first-line advanced EGFR-mutated NSCLC [7]. The latter is based on the results of the FLAURA trial, where a dramatic reduction in risk of progression or death was demonstrated in the osimertinib arm compared to first-generation EGFR-TKIs in treatment-naïve advanced NSCLC patients with EGFR mutation [8]. A subgroup analysis for CNS metastatic patients was reported in the initial FLAURA manuscript, with the notification of patients not being required to undergo CNS imaging at baseline. A subsequent post-hoc analysis with assessment by blinded independent central review (BICR) indicated osimertinib confers a 52% reduction in the risk of CNS progression in the subset of patients with CNS metastases. However, median CNS progression-free survival (PFS) was not yet reached in the subsequent update due to limited follow-up [9]. Other two third-generation EGFR-TKIs have reported their CNS efficacy in advanced NSCLC. In the FURLONG study [10, 11], the median CNS PFS was 20.8 months with furmonertinib. In the LASER301 study [12], the median CNS PFS of lazertinib was 28.2 months.
In this investigation, we report updated results describing the CNS efficacy of aumolertinib in the first-line setting from the AENEAS trial. AENEAS was a randomized phase III trial comparing aumolertinib to gefitinib in untreated patients with EGFR-mutated locally advanced or metastatic NSCLC. Patients were stratified by EGFR mutation status (Ex19del versus L858R) and baseline CNS metastasis status, and the results demonstrated that aumolertinib substantially lowered the risk of progression and death as compared to gefitinib (hazard ratio [HR] = 0.46; 95% confidence interval [CI], 0.36-0.60; P < 0.001). Importantly, PFS was improved in patients with CNS metastases at baseline (15.3 vs 8.2 months; HR = 0.38; P < 0.001, assessed by investigators) [13]. Here, we report CNS efficacy and safety profiles of aumolertinib versus gefitinib in this important subset of patients, as assessed by BICR and longer follow-up.
2 METHODS
2.1 Study design and patients
The detailed design of AENEAS has been published previously [13]. AENEAS was a multicenter, double-blinded, randomized phase III trial conducted at 53 study sites in mainland China. The trial was conducted in accordance with the protocol, applicable local regulations, and the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practices principles. The ethics committee of Shanghai Chest Hospital (ID: LS1840) and each participating institution's research ethics board approved the study protocol. All patients provided written informed consent before the initiation of any study-related procedure. The trial was registered at ClinicalTrials.gov with the identifier NCT03849768.
Patients with previously untreated metastatic or locally advanced NSCLC with EGFR sensitizing mutations were enrolled and randomly assigned in a 1:1 ratio to orally receive either aumolertinib (Jiangsu Hansoh Pharmaceutical Group Co. Ltd., Lianyungang, Jiangsu, China) or gefitinib (AstraZeneca Pharmaceutical Co. Ltd., Cambridge, London, UK) in a double-blinded fashion. Patients were stratified by EGFR mutation status (Ex19del versus L858R, confirmed by a central laboratory [Teddy Clinical Research Laboratory Co. Ltd., Shanghai, China] using the Cobas EGFR Mutation Test [version 2; Roche Molecular Systems Inc, Pleasanton, CA, USA], as detected in tumor tissue samples or blood samples) and baseline CNS metastasis status. At baseline, patients were required to have at least one measurable lesion, defined as ≥ 10 mm, and baseline assessment was performed using Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). Baseline CNS imaging was mandatory, and for patients with identified CNS metastases, follow-up CNS imaging was performed with the same methodology as baseline and then assessed by BICR. Patients with asymptomatic, stable CNS metastases that did not require steroids for at least 2 weeks before starting the study drug were included. The presence of baseline CNS metastases defined the CNS Full Analysis Set (cFAS), and the CNS Evaluable for Response (cEFR) was the subset of patients with at least one measurable CNS lesion of at least 10 mm in the longest diameter.
2.2 Procedures
An interactive web response system (software developed by Shanghai Shanhu Health Technology Co. Ltd., Shanghai, China) randomly assigned eligible patients 1:1 to receive either 110 mg aumolertinib or 250 mg gefitinib, administered orally once daily. Treatment with the study drug was continued until disease progression, withdrawal of consent, the development of unacceptable adverse effects, or the fulfillment of other discontinuation criteria. Treatment with the study drug beyond disease progression was permitted if the patients continued to derive clinical benefits as assessed by the treating investigator. Upon disease progression, patients in the gefitinib arm who acquired an EGFR T790M mutation were eligible to crossover to aumolertinib treatment. For patients with CNS metastases, follow-up imaging of the same modality as the baseline CNS imaging was performed every 6 weeks (±7 days) from the start of treatment until 15 months, after which imaging was performed at 12-week (±7 days) intervals. CNS response was assessed by neuroradiological-BICR provided a baseline CNS target lesion at least 10 mm in the longest diameter could be identified.
2.3 Outcomes
The data cut-off date of this study was August 06, 2021. The primary objective for this subgroup analysis was to estimate CNS PFS by neuroradiological-BICR (Fantastic Bioimaging Co. Ltd., Shanghai, China), with CNS PFS defined as the time from random assignment until the date of objective CNS progression or death resulting from any cause. CNS endpoints included CNS objective response rate (ORR), duration of response (DoR) and disease control rate (DCR). Responses of CNS lesions require confirmation by neuroradiological-BICR per RECIST 1.1 guidance.
CNS ORR was defined as the proportion of patients with a best overall CNS response of complete response (CR) or partial response (PR) relative to the total number of patients with baseline CNS disease. CNS DCR was defined as the percentage of patients who had a best overall CNS response of CR, PR, or stable disease (SD) ≥ 5 weeks before any progressive disease (PD) event. CNS DoR was defined as the time from documentation of CNS response (CR or PR) to intracranial disease progression or death.
Adverse events (AEs) were monitored continuously from informed consent to 28 days after the last dose of randomized treatment. The severity of AEs was graded using the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.03.
2.4 Statistical methods
CNS PFS was summarized using the Kaplan-Meier method. The Kaplan-Meier estimates of the 25th, 50th (median), and 75th percentiles, along with their corresponding two-sided 95% CI for PFS, were presented by the randomized treatment arm. A log-rank test that was stratified by EGFR mutation type was used to compare the PFS distributions of the two arms, thus determining whether there was a statistical difference between the arms. The stratified Cox proportional hazards model, as adjusted by EGFR mutation type, was used to estimate the PFS HR, along with the two-sided 95% CI of the two treatment arms. CNS DoR was analyzed using the same methods as the PFS. CNS ORR was analyzed based on the confirmed responses using the Cochran-Mantel-Haenszel test stratified by EGFR mutation type. The exact 95% CI for ORR and DCR was calculated using the Clopper-Pearson method. The incidence of the first event being CNS progression, with no prior non-CNS progression or death, was estimated through competing risk analysis using the Cause-Specific Hazard model. Safety analyses were performed on the safety analysis set, which included all randomized patients with CNS metastases who received the study drug at least once. All the analysis was performed using the Statistical Analysis System (SAS v9.4; SAS Institute, Cary, North Carolina, US).
3 RESULTS
3.1 Patient disposition and baseline characteristics
Of the 429 patients enrolled and randomized in the AENEAS trial, 106 patients (aumolertinib arm, n = 51; gefitinib arm, n = 55) were found to have CNS metastases at baseline by neuroradiological-BICR. These 106 patients were included in cFAS. Among them, 60 patients (aumolertinib arm, n = 28; gefitinib arm, n = 32) had at least one measurable CNS lesion. These 60 patients were included in cEFR (Figure 1). Demographics were generally balanced between treatment arms in cFAS and cEFR. The aumolertinib arm trended towards a higher Eastern Cooperative Oncology Group (ECOG) performance score. In cFAS, 3 patients (5.9%) in the aumolertinib arm and 4 patients (7.3%) in the gefitinib arm received prior brain radiotherapy (Table 1).
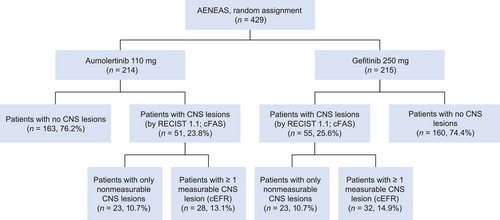
Flow diagram of the study.
Abbreviations: cEFR, CNS evaluable for response; cFAS, CNS full analysis set; CNS, central nervous system.
| cFAS (n = 106) | cEFR (n = 60) | |||
|---|---|---|---|---|
| Characteristics |
Aumolertinib (n = 51) |
Gefitinib (n = 55) |
Aumolertinib (n = 28) |
Gefitinib (n = 32) |
| Age, years, median (IQR) | 58 (50-64) | 61 (54-67) | 56 (51-63) | 63 (54-68) |
| Gender, n (%) | ||||
| Male | 19 (37.3%) | 20 (36.4%) | 8 (28.6%) | 8 (25.0%) |
| Female | 32 (62.7%) | 35 (63.6%) | 20 (71.4%) | 24 (75.0%) |
| EGFR gene mutation type, n (%) | ||||
| Ex19del | 30 (58.8%) | 35 (63.6%) | 17 (60.7%) | 19 (59.4%) |
| L858R | 21 (41.2%) | 20 (36.4%) | 11 (39.3%) | 13 (40.6%) |
| Pathological type, n (%) | ||||
| Adenocarcinoma | 49 (96.1%) | 53 (96.4%) | 27 (96.4%) | 32 (100%) |
| Large cell carcinoma | 0 | 1 (1.8%) | 0 | 0 |
| Other | 2 (3.9%) | 1 (1.8%) | 1 (3.6%) | 0 |
| ECOG performance score, n (%) | ||||
| 0 | 7 (13.7%) | 13 (23.6%) | 4 (14.3%) | 7 (21.9%) |
| 1 | 44 (86.3%) | 42 (76.4%) | 24 (85.7%) | 25 (78.1%) |
| Prior brain radiotherapy, n (%) | 3 (5.9%) | 4 (7.3%) | 3 (10.7%) | 4 (12.5%) |
- Abbreviations: cEFR, CNS evaluable for response; cFAS, CNS full analysis set; CNS, central nervous system; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
- Baseline is defined as the last non-missing observation before the first dose of study treatment.
3.2 CNS PFS
Median follow-up time in the overall AENEAS study population was 26.2 months for the aumolertinib arm and 26.3 months for the gefitinib arm, respectively. The median follow-up time for CNS PFS was 20.9 months in the aumolertinib arm and 13.8 months in the gefitinib arm. A total of 53 events (aumolertinib, n = 20; gefitinib, n = 33) were observed among the 106 patients in the cFAS, while 31 events (aumolertinib, n = 10; gefitinib, n = 21) were observed among the 60 patients in the cEFR (Table 2). In the cFAS, median CNS PFS was 29.0 months (95% CI, 12.3-not arrived [NA]) for aumolertinib versus 8.3 months (95% CI, 6.9-9.7) for gefitinib (HR = 0.31; 95% CI, 0.17-0.56; P < 0.001). Similar results were obtained in the cEFR population (HR = 0.26; 95% CI, 0.11-0.57; P < 0.001) (Table 2, Figure 2A-B). In the cFAS, the 12-month CNS PFS rate was 72.5% for aumolertinib, compared to 30.4% for gefitinib. In the cEFR, the 12-month CNS PFS rate was 73.5% versus 21.6% between treatment arms (Table 2).
| cFAS (n = 106) | cEFR (n = 60) | |||
|---|---|---|---|---|
| CNS response |
Aumolertinib (n = 51) |
Gefitinib (n = 55) |
Aumolertinib (n = 28) |
Gefitinib (n = 32) |
| Total number of events, n (%) | 20 (39.2%) | 33 (60.0%) | 10 (35.7%) | 21 (65.6%) |
| CNS progression | 19 (37.3%) | 32 (58.2%) | 10 (35.7%) | 20 (62.5%) |
| Death | 1 (2.0%) | 1 (1.8%) | 0 | 1 (3.1%) |
| Median CNS PFS, months (95% CI) | 29.0 (12.3-NA) | 8.3 (6.9-9.7) | 29.0 (12.3-NA) | 8.3 (6.9-9.5) |
| HR (95% CI) | 0.31 (0.17-0.56) | 0.26 (0.11-0.57) | ||
| P value | <0.001 | <0.001 | ||
| PFS rate (95% CI) | ||||
| At 6 months | 86.0% (72.9-93.1) | 71.8% (56.3-82.6) | 85.6% (66.0-94.3) | 73.6% (52.3-86.5) |
| At 12 months | 72.5% (57.2-83.1) | 30.4% (16.9-45.0) | 73.5% (52.2-86.5) | 21.6% (7.9-39.7) |
| At 18 months | 59.9% (43.8-72.8) | 8.9% (0.9-28.7) | 64.9% (43.1-80.1) | 0.0% (NA) |
| At 24 months | 56.8% (40.4-70.2) | 8.9% (0.9-28.7) | 64.9% (43.1-80.1) | 0.0% (NA) |
| Best overall responsea, n (%) | ||||
| Complete response | 12 (23.5%) | 3 (5.5%) | 4 (14.3%) | 0 |
| Partial response | 20 (39.2%) | 24 (43.6%) | 20 (71.4%) | 24 (75.0%) |
| Stable disease | 16 (31.4%) | 26 (47.3%) | 2 (7.1%) | 7 (21.9%) |
| Progressive disease | 3 (5.9%) | 1 (1.8%) | 2 (7.1%) | 0 |
| Not evaluable | 0 | 1 (1.8%) | 0 | 1 (3.1%) |
| CNS ORR (95% CI) | 62.7% (48.1-75.9) | 49.1% (35.4-62.9) | 85.7% (67.3-96.0) | 75.0% (56.6-88.5) |
| OR (95% CI) | 1.76 (0.81-3.81) | 1.95 (0.53-7.25) | ||
| P value | 0.149 | 0.312 | ||
| CNS DCR (95% CI) | 94.1% (83.8-98.8) | 96.4% (87.5-99.6) | 92.9% (76.5-99.1) | 96.9% (83.8-99.9) |
| OR (95% CI) | 0.61 (0.10-3.76) | 0.43 (0.04-4.87) | ||
| P value | 0.591 | 0.485 | ||
| Median CNS DoR, months (95% CI) | 27.7 (NA) | 6.9 (5.5-9.4) | 27.7 (NA) | 6.9 (5.5-8.3) |
| HR (95% CI) | 0.18 (0.07-0.44) | 0.15 (0.05-0.41) | ||
| P value | <0.001 | <0.001 | ||
| Estimated percentages remaining in response (95% CI) | ||||
| At 6 months | 83.1% (64.0-92.6) | 62.4% (40.0-78.5) | 82.4% (59.6-93.0) | 58.9% (35.7-76.2) |
| At 12 months | 75.7% (55.6-87.6) | 27.4% (10.7-47.3) | 77.2% (53.5-89.9) | 21.4% (6.8-41.4) |
| At 18 months | 75.7% (55.6-87.6) | 11.0% (1.0-34.6) | 77.2% (53.5-89.9) | 0.0% (NA) |
| At 24 months | 75.7% (55.6-87.6) | NA | 77.2% (53.5-89.9) | 0.0% (NA) |
- Abbreviations: cEFR, CNS evaluable for response; cFAS, CNS full analysis set; CI, confidence interval; CNS, central nervous system; DCR, disease control rate; DoR, duration of response; HR, hazard ratio; NA, not arrived; ORR, objective response rate; OR, odds ratio; PFS, progression-free survival.
- a Responses are confirmed, per RECIST 1.1.
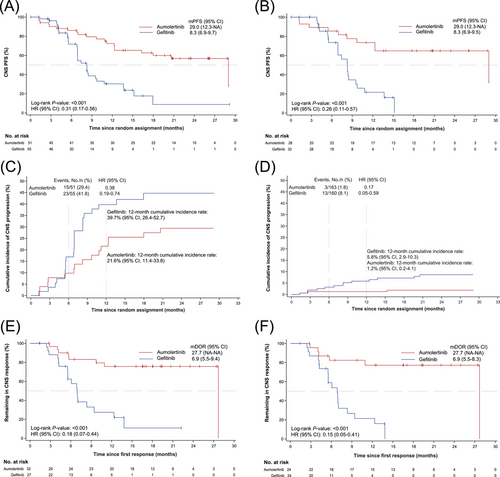
Kaplan-Meier plot of (A) CNS PFS in cFAS and (B) CNS PFS in cEFR. Cumulative incidence of CNS progression in patients (C) with CNS metastases at baseline and (D) without CNS metastases at baseline. Kaplan-Meier plot of (E) CNS DoR in cFAS and (F) CNS DoR in cEFR.
Abbreviations: cEFR, CNS evaluable for response; cFAS, CNS full analysis set; CI confidence interval; CNS, central nervous system; DoR, duration of response; HR, hazard ratio; NA, not arrived; PFS, progression-free survival.
3.3 Risk of CNS progression
Competing risk analysis showed that the estimated probability of CNS progression without prior non-CNS progression or death was consistently lower with aumolertinib than with gefitinib in patients with and without CNS metastases at baseline (Figure 2C-D). Twelve-month estimated probability of CNS progression was 21.6% (95% CI, 11.4-33.8) with aumolertinib and 39.7% (95% CI, 26.4-52.7) with gefitinib in patients with baseline CNS metastases. Twelve-month estimated probability of CNS progression was 1.2% (95% CI, 0.2-4.1) with aumolertinib and 5.8% (95% CI, 2.9-10.3) with gefitinib in patients without baseline CNS metastases.
3.4 CNS response
Aumolertinib demonstrated a higher CNS CR rate compared with gefitinib in both the cEFR (14.3% vs. 0%) and cFAS (23.5% vs. 5.5%). In the cEFR, CNS ORR was 85.7% (95% CI, 67.3-96.0) with aumolertinib and 75.0% (95% CI, 56.6-88.5) with gefitinib (OR = 1.95; 95% CI, 0.53-7.25; P = 0.312). In the cFAS, CNS ORR was 62.7% (95% CI, 48.1-75.9) with aumolertinib and 49.1% (95% CI, 35.4-62.9) with gefitinib (OR = 1.76; 95% CI, 0.81-3.81; P = 0.149) (Table 2). In the cEFR, the median time to response was similar in patients treated with aumolertinib and gefitinib (6.1 vs. 6.0 weeks) (Supplementary Table S1).
In the cEFR, the median confirmed CNS DoR was 27.7 months in the aumolertinib arm versus 6.9 months in the gefitinib arm (HR = 0.15; 95% CI, 0.05-0.41; P < 0.001). Similar results could be found in the cFAS (Figure 2E-F). In the cEFR, the estimated percentage remaining in CNS response at 12 months was 77.2% for aumolertinib, compared to 21.4% for gefitinib (Table 2).
The median best percentage change from baseline in CNS target lesion size in the aumolertinib arm was −56.5% (range, −100.0% to 32.0%) versus −50.5% (range, −74.1% to −4.5%) in gefitinib arm (Figure 3, Supplementary Table S2). The proportion of patients with ≥ 30% reduction, ≥ 50% reduction, and ≥ 75% reduction in target lesion was 85.7%, 71.4%, and 28.6% in the aumolertinib arm respectively, compared to 87.5%, 56.3%, and 0% in the gefitinib arm, respectively (Supplementary Table S2). More patients in the aumolertinib arm achieved a consistent decreasing trend in CNS target lesions compared with the gefitinib arm (Figure 4).
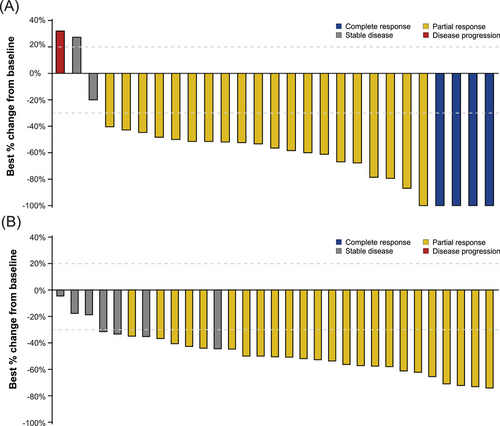
Best percentage change from baseline in CNS target lesion size (cEFR) by (A) aumolertinib and (B) gefitinib.
Abbreviations: cEFR, CNS evaluable for response; CNS, central nervous system.
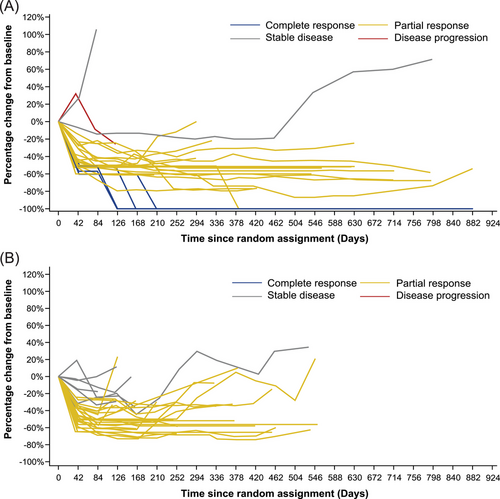
Percentage change from baseline in CNS target lesion size (cEFR) by (A) aumolertinib and (B) gefitinib.
Abbreviations: cEFR, CNS evaluable for response; CNS, central nervous system.
3.5 Concordance between CNS response and systemic response
By grouping the patients according to the best overall systemic response, we found that most patients with a best overall systemic response of CR and PR showed a decreasing trend in CNS target lesions. A higher proportion of patients with a best overall systemic response of SD and PD in the aumolertinib arm showed an increasing trend in CNS target lesions (Supplementary Figure S1). Changes in CNS lesions were well correlated with systemic responses. In the cEFR, the concordance rate between CNS ORR and systemic ORR in the aumolertinib arm was 89.3%. Among the 28 patients in the aumolertinib arm, 22 (78.6%) had both CNS and systemic response, and 3 (10.7%) had neither CNS nor systemic response. The concordance rate in the gefitinib arm was 78.1%. Among the 32 patients in the gefitinib arm, 20 (62.5%) had both CNS and systemic response, and 5 (15.6%) had neither CNS nor systemic response (Table 3). Regarding systemic efficacy, in the cFAS, median systemic PFS was 15.3 months (95% CI, 10.8-20.9) for aumolertinib and 8.1 months (95% CI, 5.5-8.3) for gefitinib (HR = 0.35; 95% CI, 0.22-0.56; P < 0.001). Median overall survival was NA (95% CI, 23.1-NA) for aumolertinib and 22.8 months (95% CI, 20.1-NA) for gefitinib (HR = 0.73; 95% CI, 0.41-1.31; P = 0.288) in cFAS (Supplementary Figure S2).
| CNS responsea | CNS non-response | |||||||
|---|---|---|---|---|---|---|---|---|
| Systemic response | Total | CR | PR | Total | SD | PD | NE | |
| Aumolertinib (n = 28) | ||||||||
| Systemic responsea (n, %) | Total | 22 (78.6%) | 1 (3.6%) | |||||
| CR | 0 | 0 | 0 | 0 | 0 | |||
| PR | 3 | 19 | 1 | 0 | 0 | |||
| Systemic non-response (n, %) | Total | 2 (7.1%) | 3 (10.7%) | |||||
| SD | 1 | 1 | 1 | 1 | 0 | |||
| PD | 0 | 0 | 0 | 1 | 0 | |||
| Gefitinib (n = 32) | ||||||||
| Systemic responsea (n, %) | Total | 20 (62.5%) | 3 (9.4%) | |||||
| CR | 0 | 0 | 0 | 0 | 0 | |||
| PR | 0 | 20 | 2 | 0 | 1 | |||
| Systemic non-response (n, %) | Total | 4 (12.5%) | 5 (15.6%) | |||||
| SD | 0 | 4 | 4 | 0 | 0 | |||
| PD | 0 | 0 | 1 | 0 | 0 | |||
- Abbreviations: cEFR, CNS evaluable for response; CNS, central nervous system; CR, complete response; NE, not evaluable; PR, partial response; PD, progressive disease; SD, stable disease.
- a Responses are confirmed, per RECIST 1.1.
3.6 Longitudinal summarization of CNS lesion status
Summarization of CNS lesion status at baseline and data cut-off in the overall study population showed that there were fewer patients with CNS lesions at data cut-off in the aumolertinib arm compared with baseline (Supplementary Figure S3). In patients with baseline CNS metastases, 15.7% in the aumolertinib arm developed new CNS lesions, while 32.7% in the gefitinib arm developed new CNS lesions. The median time to develop new CNS lesions in the aumolertinib and gefitinib arms was 6.2 and 8.2 months, respectively. In patients without baseline CNS metastases, 2.5% in the aumolertinib arm developed new CNS lesions, while 14.4% in the gefitinib arm developed new CNS lesions. The median time to develop new CNS lesions in the aumolertinib and gefitinib arms was 9.0 and 9.7 months, respectively (Supplementary Table S3).
3.7 Safety
The safety profile of aumolertinib in this analysis was consistent with the previous results of the overall trial population as no additional or novel safety signals were observed (Supplementary Tables S4-S5).
4 DISCUSSION
In this post-hoc analysis of the AENEAS study, we found that aumolertinib demonstrated a clinically meaningful 69% reduction in the risk of CNS progression or death compared to gefitinib in patients with EGFR-mutated, advanced NSCLC who had not received prior treatment and had CNS metastases. In addition, aumolertinib was safe and tolerable. This finding is consistent with the subgroup analysis of PFS by baseline CNS metastases in the AENEAS study (15.3 vs 8.2 months; HR = 0.38; P < 0.001, assessed by investigators) [13].
The other three third-generation EGFR-TKIs have reported their CNS efficacy in advanced NSCLC. In the FLAURA study [9], median CNS PFS was not reached with osimertinib (95% CI, 16.5 to not calculable), and median CNS DoR was 15.2 months (95% CI, 4.2-18.7). A recent systematic review investigated the efficacy of osimertinib for EGFR mutant NSCLC with CNS metastases, which pooled 11 trials without neuroradiological-BICR review. The combined median overall PFS of untreated CNS metastasis patients achieved by osimertinib was 12.21 months [14]. In the FURLONG study [10, 11], the median CNS PFS was 20.8 months with furmonertinib (95% CI, 15.2-25.3), and the median CNS DoR was not reached (95% CI, 10.3 to not calculable). In the LASER301 study [15], without neuroradiological-BICR review, the median overall PFS achieved by lazertinib was 16.4 months in patients with CNS metastasis at baseline. Subsequent subset analysis of the LASER301 study revealed that the median CNS PFS of lazertinib was 28.2 months (95% CI, 14.8-28.2) [12]. Aumolertinib provides promising and durable management of CNS metastasis with an encouraging median CNS PFS of 29 months and median CNS DoR of 27.7 months in this subset of the AENEAS study.
CNS penetration has been proven to play a key role in intracranial anti-tumor activity. In preclinical in vivo rat studies, aumolertinib has been shown to achieve drug concentrations in the brain at least 7 times those of plasma (data not shown), which is consistent with our findings in its CNS efficacy. In this manuscript, we present an analysis of the subgroup of AENEAS patients identified to have baseline CNS metastases by neuroradiological-BICR, which demonstrates a clinically meaningful 69% reduction in the risk of CNS progression or death in the cFAS for aumolertinib treatment relative to gefitinib. Additionally, trends favoring aumolertinib over gefitinib were also overserved with respect to CR rate, ORR and best percentage change from baseline in CNS target lesion size. The concordance rate between CNS ORR and systemic ORR was also higher in the aumolertinib arm. Furthermore, fewer patients in the aumolertinib arm developed new CNS lesions than those in the gefitinib arm, regardless of baseline CNS metastases, indicating a protective effect of aumolertinib on the development of new CNS metastases. These results, together with the preclinical findings, support aumolertinib's promising efficacy in the CNS. Among the few patients who developed new CNS lesions, the median time to develop new CNS lesions seemed to be shorter with aumolertinib versus gefitinib. Meanwhile, prolonged CNS PFS and fewer new CNS lesions development were observed in the aumolertinib arm. Given the limited reports of intracranial efficacy of third-generation EGFR-TKIs [9, 10] and similar phenomena observed in immunotherapy [16], such findings are especially encouraging and may help in investigating the management of this difficult-to-treat subgroup of patients in the clinical practice [17, 18].
Although the present results characterized the promising CNS efficacy of aumolertinib, we are aware that our study may have some limitations. First, this is a post-hoc subgroup analysis of a phase III trial in which patients were stratified by baseline CNS metastasis status. Although baseline characteristics in this study seem to be generally balanced, other potential confounding factors among patients suggest that we should be cautious when interpreting these findings. The small sample size may also limit getting statistically meaningful data from the cEFR. The incidence of prior brain radiotherapy at baseline is very low in both treatment arms. Although such a small factor can hardly alter the results, lacking in patients with prior brain radiotherapy hindered us in exploring its potential impact on CNS outcomes, thus limiting the interpretation of these results. Additional studies carried out in a larger number of patients with CNS metastases are needed to guide the management of these patients [19].
5 CONCLUSIONS
In summary, aumolertinib improved CNS PFS outcomes and reduced the risk of CNS progression relative to gefitinib, as first-line therapy in EGFR-mutated advanced NSCLC. No new safety signals were observed. Aumolertinib thus provides a promising and durable treatment option in patients with EGFR-mutated advanced NSCLC, which have a high incidence of CNS metastases at diagnosis.
AUTHOR CONTRIBUTIONS
Shun Lu, Hongying Wei and Qiong Wu contributed to the conceptualization. Hongying Wei provided administrative support. All authors contributed to the provision of study materials or patients, as well as the collection and assembly of data. Shun Lu, Hongying Wei and Qiong Wu contributed to the data analysis and interpretation. Shun Lu, Hongying Wei, Jiawei Wei, and Zheyu Zhang contributed to the manuscript drafting and revision. All authors reviewed and approved the final manuscript. The corresponding author takes full responsibility for the accuracy of all data and descriptions in this work.
ACKNOWLEDGMENTS
This study was funded by Hansoh Pharmaceutical Group Co. Ltd, Pujiang Program [Grant No. 22PJ1420700 to J.W.], National Natural Science Foundation of China (82030045 and 82241227 to S.L.), National Multi-disciplinary Treatment Project for Major Diseases (2020NMDTP to S.L.), Collaborative Innovation Center for Clinical and Translational Science by Ministry of Education & Shanghai (CCTS-202204 and CCTS-202304 to S.L.), Shanghai Chest Hospital Basic Research Project (2023YNKT-1 to S.L.). We, the authors, thank all patients in the study and their families, along with the investigators and staff at the participating centers. We also thank the study teams from Hansoh Pharmaceutical Group Co. Ltd., including Sui-Sui Dong, Jiawei Wei, Xiaoling Qian, Xue Sun, Zhenzhong Su, Qiu Sun, and Ziqiang Chen for their support, as well as Fantastic Bioimaging (Shanghai, China) for BICR assessment. We thank Rakesh Ojha for medical writing assistance and Ming Li for statistical analysis assistance. The funders were involved in the study design, data collection, data analysis, interpretation of data, writing of the report, and the decision to submit the paper for publication.
CONFLICTS OF INTEREST STATEMENT
Disclosures provided by the authors are available with this article at DOI https://doi.org/10.1200/JCO.21.02641
CLINICAL TRIAL INFORMATION
ClinicalTrials.gov number, NCT03849768. The protocol is available through this link: https://classic.clinicaltrials.gov/ProvidedDocs/68/NCT03849768/Prot_001.pdf
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
AENEAS was a multicenter, double-blinded, randomized phase III trial conducted at 53 study sites in mainland China. The ethics committee of Shanghai Chest Hospital (ID: LS1840) and each participating institution's research ethics board approved the study protocol. The trial was conducted in accordance with the protocol, applicable local regulations, the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practices principles. All patients provided written informed consent before initiating any study-related procedure.
Open Research
DATA AVAILABILITY STATEMENT
Upon request and subject to certain criteria, conditions, and exceptions, Hansoh will provide access to individual de-identified participant data from Hansoh-sponsored interventional clinical studies conducted for medicines for indications that have been approved. Data requests may be submitted to [email protected].




