Cellular senescence and metabolic reprogramming: Unraveling the intricate crosstalk in the immunosuppressive tumor microenvironment
Fusheng Zhang, Junchen Guo, and Shengmiao Yu contributed equally to this work.
Abstract
The intrinsic oncogenic mechanisms and properties of the tumor microenvironment (TME) have been extensively investigated. Primary features of the TME include metabolic reprogramming, hypoxia, chronic inflammation, and tumor immunosuppression. Previous studies suggest that senescence-associated secretory phenotypes that mediate intercellular information exchange play a role in the dynamic evolution of the TME. Specifically, hypoxic adaptation, metabolic dysregulation, and phenotypic shifts in immune cells regulated by cellular senescence synergistically contribute to the development of an immunosuppressive microenvironment and chronic inflammation, thereby promoting the progression of tumor events. This review provides a comprehensive summary of the processes by which cellular senescence regulates the dynamic evolution of the tumor-adapted TME, with focus on the complex mechanisms underlying the relationship between senescence and changes in the biological functions of tumor cells. The available findings suggest that components of the TME collectively contribute to the progression of tumor events. The potential applications and challenges of targeted cellular senescence-based and combination therapies in clinical settings are further discussed within the context of advancing cellular senescence-related research.
Abbreviations
-
- APC
-
- adenomatous polyposis coli
-
- ATM
-
- ataxia telangiectasia mutated
-
- BCL-2
-
- B cell lymphoma protein-2
-
- BCL-W
-
- B cell lymphoma-W
-
- BCL-xL
-
- B cell lymphoma extra-large
-
- BHLHE40
-
- basic helix-loop-helix family member e40
-
- Blimp-1
-
- B lymphocyte-induced maturation protein
-
- C/EBPβ
-
- CCAAT-enhancer-binding protein beta
-
- CAFs
-
- cancer-associated fibroblasts
-
- CAR-T
-
- chimeric antigen receptor-T
-
- CB2R
-
- Cannabinoid type 2 receptor
-
- CCL-
-
- C-C motif chemokine ligand
-
- CCR-
-
- C-C motif chemokine receptor
-
- CDCP1
-
- CUB domain containing protein 1
-
- CSCs
-
- cancer stem cells
-
- CTLA4
-
- cytotoxic T-lymphocyte associated protein 4
-
- CXCL
-
- C-X-C motif chemokine ligand
-
- DCs
-
- dendritic cells
-
- DDR
-
- DNA damage response
-
- ECM
-
- extracellular matrix
-
- EMT
-
- epithelial–mesenchymal transition
-
- EPHA2
-
- EPH receptor A2
-
- ERK1/2
-
- extracellular signal-regulated kinases 1/2
-
- EVs
-
- extracellular vesicles
-
- FGF10
-
- fibroblast growth factor
-
- FTO
-
- fat mass and obesity
-
- GLYT1
-
- glycine transporter 1
-
- GM-CSF
-
- granulocyte-macrophage colony-stimulating factor
-
- GSDMD
-
- gasdermin D
-
- GSTP1
-
- glutathione S-transferase pi 1
-
- HAPLN1
-
- hyaluronic acid and proteoglycan-linked protein 1
-
- HCC
-
- hepatocellular carcinoma
-
- HGF
-
- hepatocyte growth factor
-
- HIC1
-
- hypermethylated in cancer 1
-
- HIF-1α
-
- hypoxia-inducible factor-1α
-
- ICAM-1
-
- intercellular adhesion molecule 1
-
- IGF2
-
- insulin-like growth factor 2
-
- IGF2BP2
-
- insulin-like growth factor 2 mRNA binding protein 2
-
- IL
-
- interleukin-
-
- ILT4
-
- immunoglobulin-like transcript 4
-
- IRF-3
-
- interferon regulatory factor 3
-
- IκBζ
-
- inhibitor of kappa B zeta
-
- JAK2/STAT3
-
- Janus kinase 2/signal transducer and activator of transcription 3
-
- LAG3
-
- lymphocyte activating 3
-
- LKB1
-
- liver kinase B 1
-
- lncRNA
-
- long noncoding RNA
-
- LXR
-
- liver X receptor
-
- MAPK
-
- mitogen-activated protein kinase
-
- M-CSF
-
- macrophage colony-stimulating factor
-
- MDSCs
-
- myeloid-derived suppressor cells
-
- METTL3/METTL14
-
- methyltransferase-like 3/14
-
- MHC-1
-
- major histocompatibility complex class I
-
- MMP-
-
- matrix metallopeptidase
-
- MSCs
-
- mesenchymal stem cells
-
- mtDNA
-
- mitochondrial DNA
-
- mTOR
-
- mechanistic target of rapamycin kinase
-
- mTORC1
-
- mechanistic target of rapamycin complex 1
-
- NA
-
- not available
-
- NAC
-
- neo-adjuvant chemotherapy
-
- NAD+
-
- nicotinamide adenine dinucleotide
-
- NFAT
-
- nuclear factor of activated T
-
- NF-κB
-
- nuclear factor-κB
-
- NK
-
- natural killer
-
- NRF2
-
- nuclear factor-E2-related factor 2
-
- OIS
-
- oncogene-induced senescence
-
- PAI1
-
- plasminogen activator inhibitor type 1
-
- PD-L1
-
- programmed cell death 1 ligand 1
-
- PFKL
-
- phosphofructokinase liver type
-
- PGE2
-
- prostaglandin E2
-
- PI3K/AKT
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B
-
- PIR-B
-
- paired immunoglobulin-like receptor B
-
- PTEN
-
- phosphatase and tensin homolog
-
- RBM4
-
- RNA binding motif protein 4
-
- ROCK
-
- Rho associated coiled-coil containing protein kinase
-
- ROS
-
- oxygen species
-
- SASP
-
- senescence-associated secretory phenotypes
-
- SDF-1
-
- stromal cell-derived factor 1
-
- SIPS
-
- stress-induced premature senescence
-
- STAT1
-
- signal transducer and activator of transcription 1
-
- TAMs
-
- tumor-associated macrophages
-
- TA-MSCs
-
- tumor-associated-MSCs
-
- TANs
-
- Tumor-associated neutrophils
-
- TBK1
-
- TANK binding kinase 1
-
- TGF-β
-
- transforming growth factor-beta
-
- Th1
-
- T helper type 1
-
- TIS
-
- therapy-induced senescence
-
- TME
-
- tumor microenvironment
-
- TNF-α
-
- tumor necrosis factor-alpha
-
- Tregs
-
- T regulatory cells
-
- TSPO
-
- translocator protein
-
- VECs
-
- vascular endothelial cells
-
- VEGF
-
- vascular endothelial growth factor
-
- VSMCs
-
- vascular smooth muscle cells
-
- WTAP
-
- WT1 associated protein
-
- YTHDF3
-
- YTH N6-methyladenosine RNA binding protein F3
1 INTRODUCTION
Over 60 years ago, Hayflick et al. [1] reported that normal human fibroblasts display stable and terminal growth arrest in culture. The group postulated that this phenomenon, termed “cellular senescence”, is the underlying cause of aging. The mechanisms of both cancer and aging underlie time-dependent accumulation of cellular damage [2]. The initial concept was that tumors in the cellular context (unlimited proliferation and increased tumor survival) and cellular senescence (decreased function and health) are antagonistic [3]. However, more recent findings highlight that senescence can mediate tumorigenesis and progression to some extent [4, 5]. These processes include epigenetic changes, DNA damage, mitochondrial dysfunction, senescence-associated secretory phenotypes (SASP), altered intercellular communication, telomere shortening or dysfunction, oncogene activation, and loss of tumor suppressor function (Figure 1) [6]. Clinical data suggest that tumorigenesis is an age-related process, supporting the utility of senescence as a marker of the tumor cell phenotype [2, 7]. Accumulating evidence suggests that senescence has a major impact on the tumor microenvironment (TME), promoting tumor progression and metastasis [8]. Moreover, the interconnection of different cellular senescence traits may collectively contribute to the dynamic evolution of the TME. For example, DNA damage response pathways enhance the secretion of senescence-associated secretory phenotypes to facilitate esophageal squamous cell carcinoma progression and chemoresistance [9].
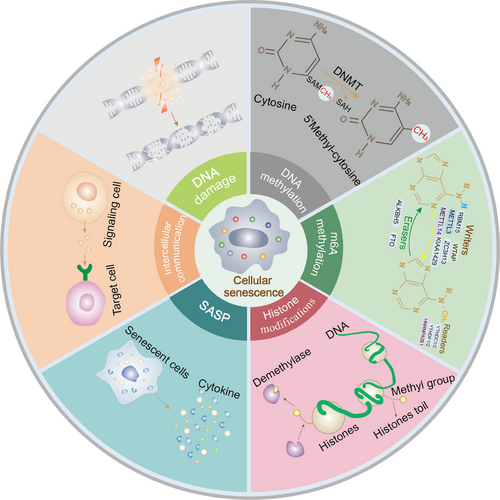
The TME is a highly complex and heterogeneous ecosystem that provides nutrition for tumor growth and proliferation [10]. This milieu encompasses the tumor and the surrounding environment, including immunosuppressive cells [11], immune and inflammatory cells [12], intercellular stroma, microvasculature, and biomolecules (such as inflammatory factors) infiltrating from adjacent areas [13]. Compelling evidence suggests that tumor growth, invasion, metastasis, and drug resistance are inseparable from cellular senescence [14], as cellular senescence-mediated SASP and inflammatory cytokine storms impair anti-tumor immunity [15]. This relationship is based on the contribution of several components of the TME towards generating an immunosuppressive microenvironment that accelerates these events through facilitating immune escape [16]. Senescence-mediated metabolic dysfunction [17], immune cell phenotypic shifts [18], chronic inflammation [19], and TME hypoxia [20] are reported potential causes of such effects. Senescence of different cell types (including tumor and anti-tumor/tumor-promoting immune cells) may be one of factors underlying differential effects on tumors. Tumor cell senescence itself can serve as a tumor-suppressive stimulus, whereas associated phenomena (e.g., SASP) as well as “immunosenescence” induced by immune cell senescence are potential tumor-supportive events (Table 1) [21, 22]. Considering that metabolic reprogramming is involved in the regulation of tumor-associated mechanisms in different immune cells and cellular senescence promotes the development of tumor-associated macrophages (TAMs), T regulatory cells (Tregs), tumor angiogenesis, and cancer-associated fibroblasts (CAFs) through metabolic programming, comprehensive investigation of the impacts of metabolism and cellular senescence on TME evolution and tumor proliferation is an important focus of research [23, 24].
| SASP factor | Senescence trigger | Mechanism | Effect | Ref |
|---|---|---|---|---|
| IL-6 | p27-induced | IL-6 drives bone matrix changes for metastatic ecological niche development | Promotion of breast cancer bone metastasis | [324] |
| HGF, MMPs | SIPS | Mitogenic and extracellular matrix cause TME remodeling | Support of tumor proliferation | [325] |
| CCL2 | OIS | Regulation of NK cells and macrophage function | Inhibition of HCC proliferation | [108] |
| PGE2, COX2 | Oxidative stress | Suppression of anti-tumor immunity | Promotion of HCC | [326] |
| IL-6 | TIS | PI3K/AKT/mTOR activation mediates IL-6 release to support tumor chemoresistance | Promotion of chemoresistance in lymphoma | [327] |
| IL-6 | p27-induced | IL-6 inhibits anti-tumor T cell function and establishes an immunosuppressive TME | Promotion of squamous cell carcinoma | [328] |
| CCL2, CXCL1, IL-15 | OIS | Immune-mediated clearance of senescent cells | Inhibition of HCC | [329] |
| IL-1α | OIS | CD4+ T cells perform clearance of senescent cells to enhance senescence monitoring | Delay of tumor progression | [301] |
|
IL-10, IL-13, GM-CSF, M-CSF |
TIS | JAK2/STAT3 supports tumor growth and chemoresistance by establishing an immunosuppressive TME | Promotion of prostate cancer | [330] |
| CXCL1, CXCL2 | PTEN-loss induced cellular senescence | Gr-1+ myeloid cell-mediated inhibition of senescence reinforcement | Promotion of prostate cancer | [331] |
| CXCL12 | OIS | Senescent cells increase cancer cell survival through CXCL12/CXCR4 signaling | Promotion of thyroid cancer | [332] |
| TGF-β, VEGF, CCL2, CCL20 | OIS | Activation of SASP by IL-1α induces paracrine senescence and affects tumor suppression in vivo | Delay of tumor progression | [333] |
| IL-1α, IL-1β | NA | Uncoupling of SASP from cell cycle exit through IL-1 inactivation | Promotion of pancreatic cancer progression | [334] |
| IL-6, IL-8 | OIS | The transcription factor C/EBPβ cooperates with IL-6 to amplify activation of the inflammatory network, including IL-8 | Inhibition of colorectal cancer | [206] |
| TGF-β | Hypoxia TME | TGF-β signaling induces SASP and generates an immunosuppressive TME | Promotion of lung cancer | [203] |
| IL-1β, IL-6, IL-8 | NAD+ metabolism | The HMGA/NAMPT/NAD signaling axis enhances glycolysis to promote acquisition of pro-inflammatory SASP | Promotion of ovarian cancer | [56] |
| TNF, IL-1β, CCL2 | p53-induced | p53 accumulation induces SASP-associated inflammatory responses to form an immunosuppressive TME | Promotion of HCC | [69] |
- Abbreviations: CCL-, C-C motif chemokine ligand; COX-, cyclooxygenasa; CXCL-, C-X-C motif chemokine ligand; GM-CSF, granulocyte-macrophage colony stimulating factor; HCC, hepatocellular carcinoma; HGF, hepatocyte growth factor; HMGA, high mobility group A; IL-, interleukin-; M-CSF, macrophage-stimulating factor; MMP-, matrix metallopeptidase; NA, not available; NAD, nicotinamide adenine dinucleotide. NAMPT, human niacinamide phosphoribosyltransferase; OIS, oncogene-induced senescence; PGE2, prostaglandin E2; PTEN, phosphatase and tensin homolog deleted on chromosome ten; SASP, senescence-associated secretory phenotypes; SIPS, stress-induced premature senescence; TGF-β, transforming growth factor-β; TIS, therapy-induced senescence; TME, tumor microenvironment; VEGF, vascular endothelial growth factor;.
The immunosuppressive microenvironment markedly restricts the effectiveness of tumor immunotherapy [25]. To overcome this problem, systematic evaluation of the crosstalk between immune cell phenotypic shifts, as well as metabolic disorders and chronic inflammation caused by cellular senescence is essential. Cellular senescence induces alterations in the surrounding environment, including the composition of the extracellular matrix, intercellular communication, and accumulation of inflammatory factors [2]. Mounting evidence suggests that cellular senescence potentially supports tumor immune evasion by promoting the establishment of an immunosuppressive TME [26]. Notably, senescence contributes to the onset of metabolic reprogramming, while metabolic disorders facilitate phenotypic changes of immune cells [18, 27] and progression of chronic inflammation [28]. Through this process, tumors achieve immune escape and unlimited proliferation, leading to TME hypoxia [29]. Consequently, metabolic reprogramming and chronic inflammation are promoted, providing favorable conditions for a vicious cycle of tumor growth and metastasis [30]. In this review, the mechanisms by which senescence promotes tumor proliferation and metastasis, including modulation of metabolic reprogramming, immune phenotypic shifts, chronic inflammation, TME hypoxia, and tumor biological function, are comprehensively discussed. In addition, potential tumor immunotherapy strategies based on senescence are considered in the context of tumor-targeted combination therapy.
2 CELLULAR SENESCENCE AND METABOLIC REPROGRAMMING REMODEL TME TO THE IMMUNOSUPPRESSIVE PHENOTYPE
Previous research suggests that tumors are inherited diseases caused by multifactorial genetic mutations. Accumulating studies on the tumor phenotype have identified metabolic reprogramming and immune escape as two hallmarks of cancer [31], which promote an acidic TME to support tumor proliferation [32]. Tumor event progression drives the release of additional inflammatory factors by cells through SASP to alter the immune phenotype and form an immunosuppressive TME [33], ultimately inducing a feedback loop of sustained tumor proliferation.
2.1 Cellular senescence and mitochondrial metabolism
Mitochondria play an essential role in the most fundamental intracellular energy conversion processes. Severe impairment of normal cellular energy conversion processes can result from mitochondrial dysfunction, particularly in tissues that significantly depend on mitochondria for chemical energy production [34]. Interestingly, dynamic changes in the TME may complete the regulation of metabolic reprogramming through altering the composition of mitochondrial oxidative phosphorylation complexes and generating excess reactive metabolic by-products. These effects ultimately induce mitochondrial dysfunction and age-related metabolic disorders (including tumor development) [35]. Notably, inefficient adenosine triphosphate (ATP) production is a characteristic of senescent cells. An increase in the adenosine monophosphate/ATP (AMP/ATP) ratio results in a bioenergetic imbalance. This decreased ATP production can be explained by lower oxygen phosphorus efficiency associated with a decrease in mitochondrial membrane potential [36]. Senescent cells mediate an increase in tricarboxylic acid cycle metabolites that play multiple roles in the microenvironment, including lymphangiogenesis, induction of stem cell function, immune regulation, tumorigenesis, DNA methylation, and post-translational protein modifications [37, 38]. Given that senescence is characterized by epigenetic and chromatin modifications, the regulation of these metabolites may have an impact on the senescence phenotype.
Human fibroblast senescence-induced increases in AMP/ATP and adenosine diphosphate/ATP (ADP/ATP) ratios promote activation of AMP-activated protein kinase (AMPK), a key mediator of cellular metabolism, resulting in phosphorylation and activation of p53 [17]. Downregulation of proliferative genes, such as cell cycle protein A, or reduced phosphorylation of retinoblastoma proteins are strongly associated with metabolic programming and senescence. Importantly, AMPK activation can enhance p16 expression by increasing the nuclear presence of the RNA-stabilizing factor human antigen R protein and suppressing the stability of mRNAs encoding cell cycle proteins, leading to a senescent phenotype. This mitochondrial dysfunction-induced senescence phenotype potentially supports tumor progression. For example, earlier studies have reported that aberrant glycolytic flux and T cell depletion caused by mitochondrial defects exacerbate liver cancer [39]. In addition, mitochondrial dysfunction in senescent fibroblasts has been shown to downregulate transient receptor potential cation channel subfamily C member 3 (TRPC3) expression and induce cellular senescence, resulting in the secretion of pro-inflammatory and pro-tumor molecules (such as IL-8, epithelial-derived neutrophil-activating peptide 78 (ENA-78), and growth-related oncogene-alpha (GRO-α)) through SASP acquisition that promote tumor epithelial cell proliferation and prostate cancer growth in vivo [40]. Accordingly, the intrinsic link between mitochondrial dysfunction and senescence contributes to the formation of an immunosuppressive microenvironment and tumor proliferation. Given the significant correlations among senescence, tumors, and inflammation, elucidation of their functions and underlying mechanisms may aid in the identification of novel therapeutic approaches for combating senescence and age-related diseases. DNA damage and epigenetic modifications, both of which are hallmarks of senescence, also have the potential to induce mitochondrial dysfunction. For instance, chronic DNA damage is reported to mediate mitochondrial dysfunction and the development of radiotherapy-resistant glioblastoma [41]. Furthermore, m6A methylation modifications induce mitochondrial dysfunction that affects the metabolic reprogramming of cancer cells, which, in turn, increases cell proliferation, tumor initiation, and metastasis [42]. Similarly, primary tumor senescence can accelerate cancer metastasis via EVs. Specifically, breast cancer senescence-mediated metabolic reprogramming is induced by activating cancer-associated fibroblasts (CAFs) through the release of metabolites (e.g., methylmalonic acid) and promoting the release of IL-6-loaded EVs secreted by bovine milk to accelerate metastatic progression of lung cancer [43, 44]. Based on the collective findings, it is hypothesized that during cell senescence, multiple features (including epigenetic modifications, SASP, and intercellular communication changes) regulate mitochondrial function and mediate the reprogramming of energy metabolism or immune cell phenotypes, consequently favoring tumorigenesis and progression in multiple dimensions. Tumor proliferation, in turn, accelerates the accumulation of DNA damage and epigenetic alterations, and induces mitochondrial dysfunction that leads to complete metabolic reprogramming. Through this process, a tumor-friendly milieu is continuously generated, mediating a vicious microenvironmental cycle that lasts until nutrient depletion.
The existing literature clearly demonstrates that senescent cells exhibit significant alterations in mitochondrial function and metabolism. However, many of the reported observations are limited to relatively small cell populations and may not apply to all subtypes of senescent cells. Indeed, mitochondrial and metabolic parameters are reported to vary depending on the types of senescent cells and stressors. Although evidence of mitochondrial dysfunction in senescent cells cultured in vitro is available, our understanding of the altered mitochondrial function in senescent cells in tissues in vivo is currently limited. Further in-depth characterization of mitochondrial function in different types of senescent cells is warranted.
2.2 Cellular senescence and glucose metabolism
Abnormal sugar metabolism is a feature of tumor metabolic reprogramming. Increased glycolytic activity and lactate fermentation are the primary energy metabolic features associated with high invasiveness of tumor cells [45]. Oxygen consumption is additionally necessary for tumor proliferation and, occasionally, the demands of tumors cannot be met due to low oxygen concentrations. In such cases, tumor cells adjust to these situations by performing adaptive changes to meet their increased bioenergetic and biosynthetic needs through multiple metabolic pathways [46]. Fibroblast senescence is linked to several systems that regulate the microenvironment. These pathways induce mitochondrial dysfunction and increase glycolysis, thereby causing a shift in the energy production from mitochondria to glycolytic sources [47]. The adaptive metabolic alterations contribute to a hypoxic and acidic TME and support tumor proliferation through multiple pathways. In a similar manner to aerobic glycolysis, senescent chondrocytes alter their metabolism, accompanied by a lower energy state and increased AMP/ATP ratio. This process may further accelerate the induction of senescence [48]. Pyruvate, a key metabolite at the intersection of glycolysis and mitochondrial respiration, plays a crucial role in the metabolism of senescent cells [49]. Synthesis of pyruvate during glycolysis requires the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH. Lactate dehydrogenase can replenish the NAD+ pool [50] by converting pyruvate and NAD+ into lactate, which accumulates outside senescent osteosarcoma cells to form an acidic TME in some cases.
The dual nature of cellular senescence (tumor promotion and suppression) creates an apparent contradiction between senescence promotion and tumor evolution. In fact, senescence regulates metabolic reprogramming, creating an environment conducive to tumor growth (including SASP and immunophenotypic suppression). When tumor growth exceeds the rate of senescence-mediated apoptosis, the malignant cycle of continuous tumor proliferation and distant metastasis is accelerated. Interestingly, mitochondrial dysfunction induces glycolysis and mediates senescence/failure of CD4+ or CD8+ T cells, and HCC progression [39]. The involvement of senescence in supporting malignancy through mediation of glycolysis has also been reported in TAMs [51] and CAFs [52]. These events provide the basis for the formation of an immunosuppressive TME and tumor persistence. Characteristics of senescence, such as epigenetic modifications, are additionally involved in the regulation of glycolysis. For instance, YTH N6-methyladenosine RNA binding protein F3 (YTHDF3) promotes aerobic glycolysis in HCC by enhancing phosphofructokinase liver type (PFKL) mRNA and protein expression in an m6A methylation-dependent manner [53]. In addition, Tregs activate a glucose competition-triggered nuclear kinase ATM protein-associated DDR in effector T cells in the TME, causing senescence and altered function that may contribute to tumor progression [54]. Interestingly, metabolic reprogramming-mediated SASP also appear critical for tumor proliferation and metastasis. In the progression of senescence in immune cells, impaired glucose metabolism causes dysregulation of NAD+ metabolism, leading to increased acquisition of SASP (including IL-1β, IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1)) and tumorigenic events [55]. Mechanistically, the high mobility group A/nicotinamide phosphoribosyltransferase/NAD (HMGA/NAMPT/NAD) signaling axis promotes the acquisition of pro-inflammatory SASP (IL-1β, IL-6, and IL-8) through enhanced glycolysis and mitochondrial respiration, ultimately exerting a tumor-promoting effect in ovarian cancer [56]. Notably, EVs are emerging pro-tumor mediators of the SASP, with reports of senescent tumors or normal cells contributing to the release of some SASP (IL-6/IL-12)-loaded EVs and acceleration of tumor progression [57]. For instance, EVs released from senescent fibroblasts contribute to tumor proliferation via the SASP pathway based on interactions with EPH receptor A2 (EPHA2) [58]. In addition, components of EVs derived from senescent fibroblasts can facilitate tumor growth by increasing the incidence of DNA damage and chromosomal instability [59].
Evidence from ongoing research may provide a new paradigm for immune checkpoint therapy of tumors. For example, the restriction of glycolysis in melanoma induces delayed T cell senescence and enhances the therapeutic benefits of immune checkpoint therapy [60]. Accordingly, we hypothesized that age-dependent epigenetic modifications alter immune phenotype and function by causing dysregulation of the glycolytic metabolism and accumulation of metabolite-related products, thereby remodeling an immunosuppressive TME and accelerating tumor proliferation and metastasis. Moreover, tumor immunosuppression induced by crosstalk between other senescence hallmarks, such as SASP, EVs, and DNA damage, and the immune microenvironment is of significant research interest.
2.3 Cellular senescence and lipid metabolism
Multiple enzymes are required for catabolism, digestion and absorption of fat to maintain the balance of the intracellular environment [61]. The high nutritional demands of tumor cells drives them to produce material and energy sources for self-proliferation and metastasis through regulating and utilizing lipid metabolism (fatty acid oxidation) [62]. A typical example is CUB domain-containing protein 1 (CDCP1), which stimulates fatty acid oxidation to supply energy for metastasis of triple-negative breast cancer [63]. Senescence and lipid metabolism are closely associated in multiple aspects of tumor progression. Existing findings suggest that lipid metabolism influences the immune microenvironment by regulating cellular senescence, thereby promoting tumor progression [64]. For example, immunoglobulin-like transcript 4/paired immunoglobulin-like receptor B (ILT4/PIR-B) increases fatty acid synthesis and lipid accumulation in tumor cells by activating MAPK extracellular signal-regulated kinase 1/2 (ERK1/2) signaling and supports lung cancer progression by inducing effector T cell senescence [64]. In addition, specific characteristics of senescence in liver cells may play a role in regulating lipid metabolism to influence tumor progression. Persistence of DDR promotes genomic instability and is involved in the induction of hepatitis B virus-associated HCC events through androgen and lipid metabolism [65]. On the other hand, deficiency of histone methyltransferase SET domain-2 inhibits H3K36me3 enrichment and cholesterol efflux gene expression, resulting in lipid accumulation and abnormal metabolism in HCC [66]. The significance of of lipid metabolism in shaping the immunosuppressive phenotype via regulating immune cells has been established. Lipid accumulation and enhanced metabolism, in turn, stimulate the differentiation and activation of TAMs [67] and confer resistance to anti-angiogenic drugs [68]. Previous studies have shown varying degrees of contribution of multiple cytokines to the development of metabolic dysfunction. In a mouse model of lipid metabolism, accumulation of p53 induced HCC-related SASP inflammatory responses (including upregulation of hepatocyte growth factor (HGF), TNF, IL-1β, and CCL2) and generated an immunosuppressive microenvironment supporting HCC development [69].
The high metabolic demand of tumors during proliferation is yet to be comprehensively investigated. In cases where the glycolytic metabolism cannot meet the needs of tumors, metabolic reprogramming switches glucose to lipid metabolism to provide the energy required for tumor development. For instance, increased fatty acid uptake promotes ovarian cancer cell survival under cisplatin-induced oxidative stress through enhanced β-oxidation [70]. A feasible hypothesis is that that DNA damage and epigenetic alterations in senescent cells affect the immune cell phenotype through their involvement in the regulation of lipid metabolism, thereby resulting in the formation of an immunosuppressive TME and tumorigenesis. Mechanistically, age-dependent DNA methylation impairs the function of elongation of very long chain fatty acids-like 2 (ELOVL2; a gene highly associated with epigenetic alterations, age prediction, and regulation of lipid metabolism) and disrupts lipid synthesis by inducing an increase in endoplasmic reticulum stress and mitochondrial dysfunction. These effects ultimately contribute to dysfunction of lipid metabolism and accelerated liver senescence [71]. In addition, during this metabolic reprogramming, the immune phenotype and function of B cells, T cells, and macrophages are altered, resulting in remodeling of the immunosuppressive TME and promoting an inflammatory microenvironment that favors the development of malignant disease [72].
2.4 Cellular senescence and amino acid metabolism
Among the several variables closely associated with tumor progression, amino acid degradation is another key aspect of tumor energy metabolism. Activation of glutamine metabolism coordinates non-essential amino acid fluxes within the tumor ecosystem. Mechanistically, RNA binding motif protein 4 (RBM4) modulates the activity of liver kinase B1 (LKB1; a regulator of amino acid metabolism) to favor glutamine-dependent survival of esophageal squamous cell carcinoma cells and counteract tumor cell senescence [73]. Dysregulation of amino acid homeostasis is reported to trigger senescence of epidermal keratinocytes and initiate SASP-dependent skin cancer migration via the glycine transporter protein solute carrier family 6 member 9/glycine transporter 1 (SLC6A9/GLYT1) [74]. Amino acid metabolism is a complex process incorporating multiple pathways that play a role in its contribution to tumor proliferation. The regulation of senescence-related diseases, such as formation of the pre-tumor ecotone, may be influenced by amino acid metabolism. Gasdermin D (GSDMD) and CASP11 are involved in acquisition of SASP (IL-1β and IL-1β-dependent IL-33). IL-33 release, in turn, stimulates Tregs, which contribute to HCC progression [75]. These findings support the concept that amino acid metabolism is involved in remodeling of the immunosuppressive microenvironment through the regulation of Treg cell senescence. The formation of this pre-tumor ecological niche is additionally linked with CAF metabolism and macrophage polarization [76, 77]. Indeed, along with the high energy demand for tumor proliferation, mitochondria may regulate the intrinsic crosstalk of multiple mechanisms (e.g., glycolysis, lipid metabolism, and amino acid metabolism) through metabolic reprogramming to collectively provide energy and raw materials for tumor growth [78]. However, limited information is available on this process at present. The association of different modes of metabolic reprogramming with tumor proliferation and invasion in the microenvironment warrants further investigation.
2.5 Cellular senescence-related metabolites shape the immunosuppressive TME
Accumulating evidence suggests that tumor metabolic reprogramming produces toxic metabolites that reshape the immune microenvironment to promote tumor survival and proliferation. Lactate accumulation and TME acidification exert significant effects on immune cell-mediated anti-tumor responses. Benefiting from the dynamic evolution of the TME, SASP is clearly involved in the shaping of the immunosuppressive TME through the release of multiple inflammatory factors. Interestingly, metabolites support this SASP-mediated release of inflammatory factors to some extent (for instance, glycolysis-derived lactate accelerates HCC growth by promoting the secretion of IL-23 and IL-17 [79]) and exacerbate the suppressive microenvironment (e.g., lactate prevents upregulation of nuclear factors in activated T cells and NK cells, leading to reduced interferon-gamma (IFN-γ) production and weakened melanoma immune surveillance [80]). Cellular senescence stress in CAFs drives glycolysis to produce lactic acid and promote breast cancer growth [52], while lactate released from tumor metabolism helps pre-cancerous cells escape senescence [81]. Tumor progression further promotes an acidic environment and release of associated senescence inflammatory factors as well as persistent tumor immunosuppression. Accordingly, we hypothesized that metabolites and cellular senescence-induced SASP promote each other to create an acidic microenvironment and jointly perpetuate the vicious cycle of tumor development. Interestingly, lactate has been shown to modulate the immunosuppressive phenotype of MDSCs, thereby contributing to radioresistance in pancreatic cancer [82]. Moreover, lactate-induced M2 polarization of TAMs promotes pituitary adenoma invasion through secretion of CCL17 [83]. These findings highlight the failure of tumor immunotherapy to account for the effects of metabolites on the tumor immune microenvironment.
Increased glutamine utilization affects the survival and death of selective types of immune cells and mediates a range of metabolic reprogramming modes to facilitate immunological escape in melanoma and colon cancer (e.g., through suppression of effector T cell survival) [84]. Notably, glutamine-dependent metabolic reprogramming alters the phenotype/function of immune cells by modulating cellular senescence to generate an immunosuppressive TME and drive tumor immune evasion. Tumor growth is associated with ongoing dysregulation of metabolic reprogramming and accelerated accumulation of deleterious metabolites, promoting the cycle of malignancy. The extracellular matrix machinery in CAFs activates glycolysis and glutamine metabolism through metabolic reprogramming to support invasion of head-and-neck cancer cells. This process orchestrates non-essential amino acid fluxes in the TME, leading to disruption of the ecological homeostasis of senescent cells [85]. Similarly, EV-carried circTRPS1 promotes the aggressive phenotype of bladder cancer by regulating the senescence process of CD8+ T cells through the circTRPS8/miR1-141p/GLS3 axis [86]. In addition, mitochondrial glutamine metabolism alters the microenvironment to favor tumor proliferation and treatment resistance in pancreatic and breast cancers [87, 88]. Other specific features of senescence are equally important for tumor growth. Tumorigenesis may be induced by accumulation of DNA damage and epigenetic alterations in cellular senescence, while remodeling by senescent cells could promote the secretion of associated inflammatory mediators to create an environment suitable for tumor survival. Moreover, sustained tumor growth induces a shift in energy metabolism from gluconeogenesis to amino acid metabolism to support tumor immune escape, followed by a vicious cycle of tumor proliferation. In view of the considerable heterogeneity of tumors, detailed analysis of the role of glutamine in each tumor type is essential for understanding the crosstalk in amino acid metabolism and developing novel strategies for targeted tumor therapy.
However, significant variations exist in the diverse tumor metabolic reprogramming products and their modulation of the immune microenvironment, making an exhaustive analysis impossible. The potential involvement of other tumor metabolites is equally interesting. For example, the fibroblast senescence-associated tumor metabolite, methylmalonic acid, promotes CAF activation to advance the progression of lung cancer metastasis [44]. Overall, metabolic reprogramming involved in cellular senescence directly alters the phenotype of immune cells and mediates remodeling of the immunosuppressive TME through the release of metabolites. In turn, proliferating tumors exacerbate metabolic reprogramming and cellular senescence to sustain this neoplastic cycle until systemic nutrient depletion.
3 IMMUNOSUPPRESSIVE TME REMODELING BY CELLULAR SENESCENCE
Clearly, cellular senescence is significantly associated with tumorigenesis and metastatic progression. Considerable research to date has focused on exploring the link between cellular senescence and tumor immune regulation, with the primary aim of clarifying the precise processes underlying a series of tumor immunophenotypic shifts (immunosuppressive TME remodeling) mediated by the senescence program in the TME (Figure 2).
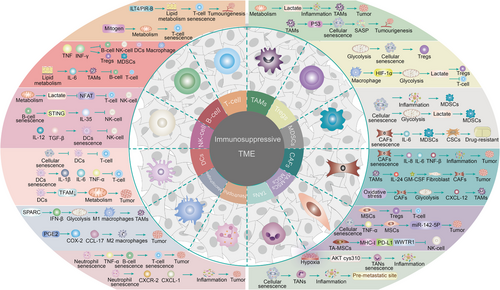
3.1 Cellular senescence and anti‑tumor immune cells in the TME
3.1.1 T cells
T cells, a major component of the human immune system, serve as crucial immunological mediators against infection and tumor development by directly killing target cells, assisting B cells to produce antibodies, stimulating responses to specific antigens and mitogens, and producing cytokines [89, 90]. Compelling evidence suggests that microenvironmental remodeling due to cellular senescence affects T cell function/phenotype and mediates tumor immune evasion [91]. ILT4/PIR-B increases fatty acid synthesis and lipid accumulation in tumor cells by activating MAPK ERK1/2 signaling, which promotes tumor growth and progression by reprogramming lipid metabolism to induce effector T cell senescence [64, 90]. Similarly, melanoma cells display active glucose metabolism and an imbalance in lipid metabolism, inducing mitogen-activated protein kinase signaling and transcriptional signaling activators to coordinate metabolic programming and reduce effector T cell activity during T cell senescence [92]. Metabolic reprogramming is therefore essential for the senescence and exhaustion of immune cells. Moreover, sterol regulatory element binding transcription factor 2/liver X receptor (SREBP2/LXR) signaling and metabolic pathways in the TME are altered by cholesterol deficiency induced by metabolic dysregulation, triggering T cell senescence or dysfunction [93]. Senescence or exhaustion of specific immune cells can induce dysfunction of other immune cell types through a number of pathways, such as antigen presentation and cytokine activation. For example, dendritic cells (DCs) increase CD103+ expression on CD8+ T cells to enhance the immune response [94]. Furthermore, co-stimulation of DCs and CD28 is essential for the maintenance of CD8+ T cell immune potency [95]. However, with the onset of senescence, stimulation of T cells by DCs is significantly reduced [96], which is detrimental to the anti-tumor immune response. Notably, epigenetics and EVs can also induce T cell exhaustion through senescence reprogramming. Increasing evidence suggests that senescent T cells experience metabolic insufficiency as well as altered signaling cascades and epigenetic landscapes. Consequently, effector immunity is suppressed, leading to poor responsiveness to immune checkpoint blockade therapy [97]. Ubiquitin-specific peptidase 8 (USP8) directly deubiquitinates and stabilizes the type II TGF-β receptor TβRII, thereby increasing its expression in the plasma membrane and tumor-derived EVs and promoting TGF-β/SMAD-mediated invasive metastasis of breast cancer cells [98]. Therefore, the influence of epigenetics and EVs or immunostimulatory effects of other immune cells (e.g., DCs) on T cells should not be overlooked in research on T cell senescence/exhaustion. Improved understanding of the complex process of T cell senescence should facilitate the development of effective anti-cancer therapeutic strategies. For instance, metformin enhances the anti-tumor immune response by stimulating mitochondrial reactive oxygen species (ROS) production and activating nuclear factor-E2-related factor 2 (NRF2) in a glycolysis-dependent manner, inducing autophagy, glutaminolysis, mechanistic target of rapamycin complex 1 (mTORC1) and p62/SQSTM1 activation, and promoting CD8+ T cell proliferation and IFN-γ secretion to activate DCs [99].
3.1.2 B cells
B cells are stimulated by antigens to differentiate into plasma cells, which synthesize and secrete antibodies (immunoglobulins). Activated B cells produce abundant cytokines, participate in immune regulation and the inflammatory response, and are primarily involved in humoral immunity [100]. The maturation of B cells is significantly regulated by changes in the microenvironment mediated by the senescence process. The acquisition of SASP drives the secretion of specific cytokines to reshape the tumor ecological niche and the presence of C-X-C motif chemokine ligand 13 (CXCL13) in the TME induces an increase in CD45+/CD19+/IL-10+ levels and impairs the ability of B cells to mount an anti-tumor immune response, ultimately supporting the development of melanoma metastases [101]. Inflammatory factors (IFN-γ, TNF) released by senescent cells activate immunosuppressive networks that increase MDSCs/Tregs. However, these factors additionally inhibit the functions of B/NK cells/DCs/macrophages and facilitate tumor proliferation through chronic inflammation [102]. Obesity-associated lipid accumulation supports the progression of colorectal cancer through IL-6-regulated macrophage polarization and CCL20/C-C motif chemokine receptor 6 (CCL20/CCR6)-mediated suppression of B and γδ T cells [103]. In addition, senescent neutrophils accumulating in lymphoid tissue impair T and B cell infiltration via upregulation of the pro-inflammatory factor TNFα [104]. Therefore, these immune cells are interlinked and may influence the function and expression of one another through the release of inflammatory factors in the senescent environment to regulate the immune response in the TME. Consistent evidence suggests that epigenetic mechanisms can regulate innate immune cells, as well as T and B lymphocytes. Age-dependent alterations in epigenetic markers may lead to reduced immune function. For example, expression of B lymphocyte-induced maturation protein (Blimp-1) leads to deacetylation of histone H3 associated with the c-Myc promoter, which inhibits B cell maturation [105]. However, the complex cascade limited by the cellular senescence and the precise mechanisms by which senescence programming affects T and B cells remain to be elucidated. These pathways may include inhibition of either cell function and migration or proliferation and apoptosis (attributed to differences in immune cell types and cytokines secreted by SASP and the effects of metabolic reprogramming, such as glycolysis).
3.1.3 NK cells
NK cells, an essential component of the immune system, are of significant research interest owing to their ability to directly identify and kill tumor cells. Immunological regulation of tumors by NK cells in the TME has recently been a focus of investigation. Existing studies involving a mouse model of senescence suggest tumor immune escape is promoted by a reduction in NK cell-mediated immunosurveillance [106]. This effect may be attributed to the dynamic evolution of chemokine (CXCL12, IFN-γ, and CCL27) and cytokine profiles (IL-1β and IL-6) in the senescent TME that suppresses NK cell function and recruitment at endometrial tumor sites [107]. Interestingly, oncogene-induced senescence induces hepatocytes to secrete cytokines that promote immune-mediated clearance, thus preventing tumorigenesis via a process termed “senescence surveillance”. Liver cancer cells block the recruitment and maturation of CCR2 myeloid cells and evade senescence surveillance by secreting chemokines that limit the activity of NK cells, accelerating the growth of fully established HCC [108]. Additionally, induction of SASP production in the microenvironment has been shown to promote the release of pro-inflammatory factors (TNF-α, IFN-γ, and IL-2/-4/-6) while decreasing that of anti-inflammatory factors (IL-10), thus reducing the cytotoxicity of NK cells [109] and potentially facilitating tumor evasion of senescence surveillance. Due to the dynamic evolution of the TME, metabolic reprogramming also affects the metabolism and function of NK cells. Moreover, the phenotype and function of NK cells are closely correlated with the status of the other immune cells. For instance, lactate produced by metabolic reprogramming reduces nuclear factor of activated T (NFAT) levels, thereby impairing the function of T cells and activation of NK cells and ultimately facilitating the immune escape of melanoma [80]. In addition, cGAMP/IL-35 (STING/IL-35) axis in B cells, which stimulates the interferon response, reduces NK cell proliferation and attenuates the NK-driven anti-tumor response [110]. DC regulation of NK cell responses requires the presence of specific inflammatory factors, such as lymphotoxin alpha (LT-α), IL-12, and TGF-β [111]. These findings support the intrinsic association of immune cells in the TME. However, cytokines released by senescent cells do not always play a pro-tumorigenic role and several variables may counteract tumor development. In a randomized controlled trial of pancreatic cancer, release of senescence-associated CXCL8/IL-8 following radiotherapy facilitated the survival of NK cells and enhanced tumor immune surveillance. These effects could be associated with the reaction of certain cytokines in the TME to the anti-tumor immune response [112]. Similarly, another study showed that CXCR6 suppresses hepatocarcinogenesis by promoting NK T and CD4+ T cell-dependent senescence control [113]. NK cells can also improve the anti-tumor efficacy of chimeric antigen receptor-T (CAR-T) cells by enhancing immune/tumor cell cluster formation and CAR-T cell adaptation to immune cell senescence [114]. Overall, elucidation of the associations and evolution of various components of the microenvironment is critical for the development of NK cell-based tumor immunotherapy. Further studies are additionally necessary to ascertain the effects of different types of cytokines on immune cell function and the role of immune surveillance during the senescence process.
3.1.4 Dendritic cells (DCs)
Immune cells in the TME, such as dendritic cells (DCs), have been reported to have substantial abnormalities brought about by alterations in the TME. In certain cases, the dynamic evolution of the microenvironment clearly contributes to immune dysfunction and tumor escape, including senescence/exhaustion of immune cells and microenvironment-induced immune phenotype changes and functional impairment [115, 116]. It has been shown that DC senescence impairs the function of DCs and reduces the adaptive immune response of the host, mainly because the efficiency of DCs in cross-presentation of cell-associated Ag and subsequent CD8+ T-cell cross-initiation is severely diminished, and that this loss of immune delivery capacity may be attributed to mitochondrial dysfunction [117]. Correspondingly, impaired tumor antigen presentation and migration of DCs during DC senescence limits the stimulation of CD8+ T cells, resulting in an anti-tumor immunodeficiency to melanoma [118]. In addition, senescence of immune cells not only contributes to their own exhaustion, but also alters their immune phenotype and function by secreting associated inflammatory cytokines [119]. Premature (autocrine) senescence of DCs greatly amplifies paracrine immune senescence and induces CD4+ T cell dysfunction through the secretion of inflammatory EVs (loaded with cytokines such as IL-1β, TNF-α, IL-6), ultimately weakening anti-tumor immunity [120]. Therefore, senescence causes a crosstalk of metabolic reprogramming to induce metabolic dysfunction, which ultimately leads to a reduction in the antitumor effect of DCs [117]. The activation of the antitumor immune response is facilitated by the stimulation of antigen presentation between different types of immune cells, and the weakening and exhaustion of DCs leads to an impaired antitumor effect of the remaining immune cells (T cells, NK cells, etc) [95, 121]. However, conflicting evidence indicates that the senescence process may contribute to the anti-tumor immune response under specific circumstances. This function may be attributable to adaptive changes in the microenvironment early in senescence. For example, deletion of TFAM in DCs leads to mitochondrial dysfunction and mtDNA cytoplasmic leakage, inducing activation of the cyclic GMP-AMP synthase/STING (cGAS-STING) pathway in DCs. This process contributes to enhanced tumor antigen presentation and reverses the immunosuppressive TME, eventually retarding lung cancer growth and metastasis [122]. Furthermore, benefiting from altered immunopeptides and upregulated major histocompatibility complex class I (MHC-I) antigen presentation, senescent cells show a strong adjuvant profile which promotes immune recruitment and activation of DCs, as well as efficient delivery of antigens to DCs and anti-cancer immune monitoring of melanoma cells [123]. Hence, senescence-induced metabolic dysfunction and SASP secretion complicate the research on DCs and are essential for the development of new strategies in oncology to explore the functional alterations of DCs by mitochondrial metabolism, glycolysis, and stimulated activation of the remaining immune cells (e.g., T cells and NK cells).
3.1.5 Macrophages
Macrophages can differentiate into activated macrophages with anti-tumor properties (M1 type) or those that favor tumor proliferation (M2 type) [124]. Cellular senescence affects macrophage regulation through multiple pathways (i.e., metabolic dysregulation and altered immune phenotype) and contributes to tumor development via remodeling of the TME. During senescence, the matricellular protein SPARC is secreted, which mediates TANK binding kinase 1 (TBK1), interferon regulatory factor 3 (IRF3), IFN-β, and signal transducer and activator of transcription 1 (STAT1) signaling. Moreover, these factors induce conversion of anti-inflammatory macrophages to a pro-inflammatory phenotype by inhibiting mitochondrial respiration and glycolysis [125]. Correspondingly, during senescence, macrophages not only undergo morphological changes but also contribute to the secretion of SASP and development of sustained DDR. This state of macrophage senescence may contribute to metabolic dysregulation and lipid accumulation through associated inflammatory phenotypes and supply energy for tumor growth and metastasis [126, 127]. Therefore, macrophage senescence participates in metabolic reprogramming and provides conditions suitable for tumor growth. Along with senescence, release of SASP factors alters the phenotype of macrophages to create an immunosuppressive microenvironment that accelerates tumor development [128]. Other senescent cells can also alter the macrophage phenotype to induce dysregulation of TME homeostasis. For example, senescent thyroid (normal and tumor) cells induce polarization of human monocytes into M2-like macrophages via a prostaglandin E2-dependent (PGE2-dependent) mechanism (release of CCL17, cyclooxygenase 2 (COX2)) to facilitate proliferation of thyroid cancer cells [129]. In fact, the observed senescence-related “dysfunction” of macrophages is the result of their functional adaptation to senescence-related changes in the tissue environment. The resulting loss of coordinated macrophage multifunctional capacity disrupts the efficacy of innate and adaptive immunity. The development of therapeutic strategies for tumors requires an understanding of the impact of macrophage senescence/exhaustion states and other senescent cells on the phenotype/function of immune cells as well as the intrinsic associations between multiple cytokines and immune cells.
3.1.6 Neutrophils
Neutrophils are frontline cells that respond to infection and are implicated in a range of inflammatory diseases. Neutrophils have recently received considerable attention in view of their complex roles in tumor progression. These cells undergo immune senescence and regulate tumor progression throughout the process. Mitochondria-dependent neutrophil extracellular traps are formed during neutrophil senescence that promote lung metastasis from breast cancer [130]. In a state of senescence of neutrophils, specific inflammatory factors or mediators are released, in turn, inducing tumor proliferation. Neutrophil senescence induces alterations in the expression levels of inflammatory factors, such as CXCR1, Fc gamma receptor I (FcγRI), HLA-DR, programmed cell death 1 ligand 1 (PD-L1), intercellular adhesion molecule 1 (ICAM-1), and CXCR4, thus disrupting the balance of the inflammatory ecological niche [131]. PIWI-interacting RNA-17560 (piRNA-17560) from senescent neutrophil-derived EVs promotes chemoresistance and epithelial–mesenchymal transition (EMT) in breast cancer through fat mass and obesity-associated protein (FTO)-mediated m6A demethylation [132]. Interestingly, sustained tumor proliferation releases signals (mediated by the chemokine receptor CXCR2) that accelerate the biological senescence of circulating neutrophils. This effect decouples the biology of these immune cells from chronological senescence and favors the accumulation of highly reactive neutrophils in malignant lesions, endowing a potent pro-tumor function [133]. Moreover, this immunosenescence-mediated tumor proliferation appears associated with mitochondria-dependent metabolic dysregulation [130]. Therefore, the impact of metabolic reprogramming should be taken into account when exploring the mechanisms by which immune senescence promotes tumor growth. The biological associations of neutrophils with other immune cells additionally warrant consideration.
3.2 Cellular senescence and immunosuppressive cells in the TME
3.2.1 Tumor-associated macrophages (TAMs)
TAMs are central to the network of immunosuppressive cells and cytokines that coordinate immunosuppression in the TME [134]. TAMs are usually recruited from the periphery as monocytes through chemokine activity and subsequently deposited in tumor tissue. Tissue-resident macrophages migrate to hypoxic or necrotic areas in the tumor, where they develop into TAMs. High glycolytic activity during cellular senescence creates an extremely acidic microenvironment and induces polarization of TAMs, promoting melanoma growth through tumor acidosis [51]. The degree of TAM infiltration in tumors is associated with poor prognosis in the majority of cancer types, including breast cancer, HCC, and gastric cancer [135, 136]. In a mouse model of senescence, dysregulation of mechanistic target of rapamycin kinase (mTOR) signaling was shown to drive the secretion of multiple pro-inflammatory factors (including colony-stimulating factor 1 (CSF1), TNF, IL-1α, and IL-1β [137]) by microglia, which interfered with normal macrophage function. Moreover, secretion of inflammatory factors (e.g., IL-6) promotes the conversion of macrophages into TAMs, stimulating release of CCL20 in the TME. This pathway can promote colorectal cancer progression through chemotactic recruitment of CCR6 that influences B cell and γδ T cell functions [103]. Tumor proliferation is additionally regulated by certain inflammatory mediators that disrupt the equilibrium of the microenvironment and induce a pro-tumor phenotype in macrophages. For instance, pancreatic cancer cell senescence drives tumor growth through secretion of the pro-inflammatory cytokine CCL20 and transformation of macrophages into TAMs [138]. Thus, tumor proliferation induces metabolic dysregulation and the release of inflammatory factors through specific metabolites (e.g., lactate) to disrupt the balance of the microenvironment, ultimately causing a switch of the anti-tumor immune to pro-tumor phenotype (characterized by increased infiltration of TAMs). An earlier study reported the recruitment of macrophages with the TAM phenotype to epithelium of precancerous and malignant lesions in senescent prostate [139]. Increased p53 expression in TAMs induces senescence and activation of the p53-dependent SASP, while an APR-53-induced increase in p246 expression leads to suppression of M2-polarized myeloid cells and increased T-cell proliferation to enhance the efficacy of the immune checkpoint blockade [140]. Further exploration of alterations in the immune phenotype induced by the ageing process should provide valuable insights for the development of novel tumor immunotherapy approaches.
3.2.2 Regulatory T cells (Tregs)
Senescence-mediated changes in immune function have also been reported for Tregs, a subset of key immunosuppressive effector T cells in the TME, whose phenotype and function are extensively influenced by cellular senescence. HCC senescence-mediated acquisition of SASP promotes the release of IL-33 and IL-1β in the TME, which activate Tregs and enhance obesity-associated HCC progression through immunosuppression [75]. Tregs initiate DNA damage in effector T cells caused by metabolic competition in the microenvironment, resulting in senescence and functional alterations of effector T cells [54]. The immunosuppressive TME mediated by Tregs under conditions of senescence is influenced by several factors. Treg exhaustion is inhibited by senescence-mediated secretion of inflammatory mediators, and surviving Tregs, in turn, cause effector T cell senescence, thereby enhancing the formation of an immunosuppressive TME through this dual effect. Correspondingly, glutathione S-transferase pi 1-regulated (GSTP1-regulated) ROS trigger uncontrolled inflammation and immune senescence by inhibiting Treg senescence [141]. In addition, activation of ATM-associated DNA damage causes tumor cell and Treg-induced T cell senescence. Blockage of ATM-associated DNA damage or the MAPK signaling pathway in T cells could prevent the development of lung cancer cell and Treg-mediated T cell senescence, thereby leading to enhanced anti-tumor immunity [142]. Notably, Treg-dependent immune senescence appears to be associated with metabolic reprogramming, with accelerated Treg-mediated glucose depletion inducing cellular senescence and suppression of effector T cell function via TME crosstalk [143]. Furthermore, Parkinsonism-associated deglycase/DJ-1 (PARK7/DJ-1) promotes pyruvate dehydrogenase activity and maintains Treg homeostasis in the microenvironment [144]. The glucose/glycolytic metabolic profile of Tregs in the TME of ovarian cancer is associated with toll like receptor 4 (TLR4)-mediated metabolic regulation [145]. However, due to immune crosstalk attributed to metabolic reprogramming, lung macrophages increase glycolysis and lactate production through the hypoxia-inducible factor-1α (HIF-1α) axis. These effects contribute to effector T cell exhaustion through antigen presentation and the maintenance of Treg function, ultimately creating an immunosuppressive microenvironment that supports metastasis [146]. Therefore, while exploring senescence-based therapeutic strategies for tumors, attention should be paid to the impact of metabolic reprogramming of anti-tumor cells, such as Tregs, on the phenotype/function of pro-tumor immune cells.
3.2.3 Myeloid-derived suppressor cells (MDSCs)
MDSCs are heterogeneous precursor cells of bone marrow-derived dendritic cells (DCs), macrophages, and granulocytes that exert immunosuppressive effects through multiple pathways [147, 148]. The senescent secretome associated with SASP contains colony-stimulating factors and chemokines that stimulate the production of MDSCs by enhancing myelopoiesis in the bone marrow and spleen [149]. Immunosuppressive MDSCs suppress acute inflammatory responses through chemotactic recruitment into inflamed tissues and expansion in localized areas, thereby suppressing the activities of several components of innate and adaptive immunity [150]. For instance, MDSCs have been shown to stimulate the activity of immunosuppressive Tregs, along with upregulation of amino acid catabolic enzymes and secretion of senescence-associated inflammatory cytokines (IL-10 and TGF-β) [151]. Elevated concentrations of inflammatory factors (e.g., IL-22, IL-6, and TNF-α) appear to be associated with maintenance of the immunosuppressive function of MDSCs and acceleration of gastric cancer progression in aging patients [152]. In parallel, MDSC-induced immunosuppression disrupts the balance between senescent and tumor cells, in addition to interfering with the maintenance of energy metabolism and histone homeostasis to promote an immunosuppressive TME [151]. Increased fatty acid oxidation and lactate production from glycolysis in the TME are key immunometabolic pathways underlying the functional activation of MDSCs and maintenance of an immunosuppressive TME [153]. In addition, owing to crosstalk between metabolic reprogramming and immune senescence, MDSCs regulate the functions of anti- /pro-tumor immune cells through specific pathways (involving selective growth factors and receptors) to enhance immunosuppressive effects on the TME. Mechanistically, senescence of MDSCs promotes lung cancer growth via enhancement of chemotaxis [154]. Senescent CAFs affect the metabolic program of MDSCs through regulating IL-6- and IL-33-mediated chronic inflammation and promote the resistance of cancer stem cells (CSCs) in the TME to chemotherapy [155].
3.2.4 Tumor-associated neutrophils (TANs)
Cytokine and epigenetic signaling in the local TME induce the polarization of neutrophils into pro-tumor TANs [156]. Increased release of CXCR4 and CD62, accompanied by the formation of a senescent ecotone, promote neutrophil infiltration in the TME and conversion to TANs to drive pre-metastatic ecotone formation during metastasis in breast cancer [130]. Earlier reports suggest that tumor progression is accelerated by the conversion of neutrophils into TANs and alterations in the tumor microecological balance due to hypoxia and metabolic reprogramming. Acrolein produced under hypoxic conditions contributes to the tumor microenvironment of pancreatic cancer by directly interacting with Cys310 of AKT to induce infiltration of TANs and increase the degree of chronic inflammation [157]. Moreover, glycolytic properties of the basic helix-loop-helix family member e40 (BHLHE40) promote overactivation of TANs to favor tumor proliferation [158]. Interestingly, senescent TANs can pass through mitochondria-dependent neutrophil extracellular traps to participate in the establishment of pre-metastatic ecological niches in breast cancer [130]. In addition, TANs may be recruited by senescent colorectal cancer cells to mediate inflammatory cell infiltration into tumors [159]. Thus, hypoxia/metabolism and cellular senescence may be involved in the recruitment of TANs in the TME. The potential correlation between cellular senescence (including tumor/non-tumor senescence) and hypoxia/metabolic changes on induction and infiltration of TANs is worth further exploration. The biological functions and types of neutrophil subpopulations remain poorly understood at present. Tumor immunotherapy may therefore benefit from research focusing on immune senescence and the impact of different neutrophil subsets on tumor progression.
3.2.5 Mesenchymal stem cells (MSCs) and tumor-associated-MSCs (TA‑MSCs)
Complex interactions exist between MSCs and the TME. As antigen-presenting cells, MSCs influence tumor progression by modulating the tumor-adaptive immune response [160]. Data from several studies suggest that senescence-regulated MSCs promote tumor formation. For instance, previous reports have demonstrated that senescence in MSCs induces the secretion of multiple cytokines in the secretome to exacerbate the inflammatory response at a systemic level. As a result, the immunomodulatory activity of MSCs is diminished, promoting the proliferation or migration of tumor cells [161]. Two subsequent investigations confirmed that MSCs stimulate the development of gastric cancer cells via miR-142-5p/DEAD-box helicase 5 (miR-142-5p/DDX5), concomitant with SASP-mediated secretion of inflammatory mediators, including IL-1β, IL-6, and TNF-α [162, 163]. However, normal MSC function cannot be sustained without energy supply from mitochondrial metabolism. Following metabolic reprogramming, oxidative stress-induced disruption of mitochondrial metabolism promotes human MSCs and lipid metabolism via senescence-associated genes in MSCs, supporting the progression of multiple myeloma [164, 165]. Furthermore, due to the intrinsic interconnectivity of the immune network, MSC senescence mediates the activation of Tregs and suppresses effector T cell proliferation to generate an immunosuppressive environment [166]. Therefore, metabolic dysregulation-mediated immune cascades may influence tumor progression through regulation of MSCs and the senescence process. However, conflicting studies have shown anti-inflammatory and anti-ageing effects of MSCs, which could be attributed to their self-renewal and multidirectional differentiation capacity as well as senescent state [167, 168]. Notably, tumor-secreting cytokines can facilitate the conversion of MSCs into TA-MSCs, which strongly promote proliferation. Indeed, tumor-cultured MSCs can remodel the TME and allow tumor cells to escape senescence, thereby accelerating metastasis [169]. Interestingly, this senescence escape appears to be associated with TA-MSC-mediated immunosuppression of the TME, in view of the finding that high levels of immunomodulatory molecules (e.g., MHC-I, PD-L1, PDL-2, and the transcriptional co-activator WW domain containing transcription regulator 1 (WWTR1)) in senescent neuroblastoma TA-MSCs impede NK cell activity and induce immunosuppression [170]. Moreover, MSCs from normal tissue sources or EVs from TA-MSCs, and those derived from abnormal TME exert differential effects on tumor development, which may depend on variations in tissue source and EV contents [171]. However, to date, few studies have investigated the relationship between TA-MSCs and the cellular senescence. Further research on the dual effects of MSCs on the TME and the mechanisms through which cellular senescence promotes conversion of MSCs into TA-MSCs is necessary.
3.2.6 Cancer-associated fibroblasts (CAFs)
CAFs actively promote tumor proliferation and metastasis, mainly through paracrine cytokine activity. Recent studies have demonstrated that breast cancer-associated CAFs can drive the progression of breast cancer by inducing p16-dependent senescence and stimulating paracrine secretion (involving stromal cell-derived factor 1 (SDF-1), IL-6, MMP-2, MMP-9, and TNF-β) [172]. Similarly, CAFs secrete high levels of IL-3 through activated STAT6 to remodel the TME of cervical tissue and amplify autocrine and paracrine secretion in TME through IL-6 and STAT3 to promote cervical tumorigenesis [173]. However, limited studies to date have focused on the intrinsic link between tumor proliferation and functional maintenance of cellular senescence during the phenotypic transformation of CAFs. Considering the multiple ways in which CAFs mediate tumor invasion, this relationship requires extensive investigation. IL-6 and CXCL1 released from senescent cells induce senescence phenotypes in CAFs [174], which, in turn, promote proliferation/metastasis of oral and pancreatic cancers through the release of inflammatory factors, such as MMP-2 and IL-8 [175, 176]. Therefore, through secretion of specific cytokines, tumor proliferation conditions can sustain the function of senescent cells and induce senescence of CAFs. Senescent CAFs continue to release inflammatory mediators to maintain/enhance the pro-tumor effects of the TME. Given the complex exchange of information in the TME, exploration of the mechanisms underlying the transition from the senescent fibroblast to the pro-tumor phenotype of CAFs is necessary. Earlier, Li et al. [177] suggested that fibroblasts in the senescent environment mediate CAF function via the Stat3 pathway (including high expression of the CAF markers alpha-smooth muscle actin (α-SMA) and vimentin (VIM)) as well as release of CAF-specific factors (CXCL12, fibroblast growth factor 10 (FGF10), and IL-6), which ultimately contribute to migration and invasion of lung cancer cells.
While the enhancement of tumor proliferation through metabolic reprogramming enhances is well established, knowledge on the influence of CAF metabolism on proliferation during the process of CAF senescence is limited. In a model of senescence, CAF mitochondrial oxidative stress triggered lactate production through the regulation of aerobic glycolysis to promote breast cancer growth [52]. Maintenance of tumor cellular senescence and the onset of metabolic dysregulation facilitate the release of methylmalonic acid, a tumor metabolite, which contributes to activation of CAFs and promotes lung cancer progression [44]. In addition, CAFs regulate the process of senescence and produce a nutrient-rich microenvironment through induction of specific metabolic phenotypes, such as mitochondria-mediated glycolysis, thereby providing nutrition for tumor growth [76]. Oxidative stress stimulates CAFs to regulate glycolytic flux and modifies the senescence phenotype to alter CCL2 expression levels and macrophage migration patterns. Moreover, the extracellular matrix in CAFs activates glycolysis and glutamine metabolism to coordinate non-essential amino acid fluxes within the tumor ecotone to reshape the CAF senescence ecotone [85, 178]. Consequently, toxic chemicals are produced by metabolic reprogramming in the TME that activate CAFs and remodel the microenvironment. These effects further increase inflammatory cell infiltration in the TME through various CAF-dependent metabolic modalities (such as glycolysis or amino acid metabolism) and promote immunosuppression by altering the immune cell phenotype. The formation of an immunosuppressive TME further activates the pro-tumor phenotype of CAFs, ultimately resulting in a vicious cycle whereby CAF-dependent metabolic reprogramming continuously maintains an immunosuppressive TME. For example, polarization of macrophages in the immunosuppressive TME facilitates the release of inflammatory factors, such as IL-6, IL-24, and granulocyte-macrophage colony-stimulating factor (GM-CSF). This release maintains homeostasis of the senescent environment to activate the pro-tumor phenotype of CAFs, leading to the formation of a persistent immunosuppressive TME [179]. Additionally, the senescence of CAFs contributes to enhanced MDSC-dependent immunosuppression, which supports intrahepatic cholangiocarcinoma proliferation [155]. Given the complexity of the TME, comprehensive exploration of the intricate crosstalk between immune cells in the TME regulated by CAFs in the ageing microenvironment should yield valuable insights that may aid in the development of effective targeted anti-tumor therapies.
4 CELLULAR SENESCENCE, METABOLISM AND HYPOXIA IN THE IMMUNOSUPPRESSIVE TME
Excessive hypoxia in tumor tissues disrupts the balance in the microenvironment, resulting in the formation of a hypoxic, hypoglycemic, and acidic TME that promotes tumor development [180]. Interestingly, a feedback loop exists between hypoxia and tumor proliferation, with accelerated tumor growth leading to excessive hypoxia. In turn, this effect provides favorable conditions for pancreatic cancer growth and metastasis through a variety of mechanisms, including metabolic reprogramming and immunosuppression by the hypoxic TME [181]. The associations among senescence-mediated tumor progression, formation of the hypoxic TME, and the onset of metabolic reprogramming warrant further investigation.
TME is a complex, dynamically changing ecosystem in which components and metabolic processes are interconnected. Previous studies have shown that senescence can induce multiple aspects of dysregulated metabolic programming (mitochondrial metabolism, glycolysis, and lipid metabolism), which collectively contribute to tumor growth. Along with its involvement in the onset of tumor proliferation, hypoxia is implicated in senescence-dependent metabolic reprogramming and HIF-1α/HIF-2α-mediated regulation of tumor progression. For example, translocator protein (TSPO) deficiency induces mitochondrial dysfunction, leading to hypoxia and angiogenesis in patients with glioblastoma, and promotes glioblastoma growth through glycolysis metabolism [182]. Mitochondrial dysfunction, in turn, facilitates tumor cell escape from senescence and continued proliferation. A hydride transfer complex in hypoxic tumor cells that reprograms NAD metabolism to overcome tumor cell senescence by transferring reducing equivalents from NADH to NADP+ may additionally be involved in this process [183]. Correspondingly, energy conversion, which serves as a key mechanism of hypoxia development, is required for utilization in tumor metabolism. Cellular senescence exacerbates the disrupted energy metabolism, chronic inflammation, and hypoxia in the TME via the AMPK signaling pathway to further drive HCC progression [184]. Hypoxia-mediated HIF-2α activates lipid synthesis and lipid metabolic distribution through upregulation of the phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT/mTOR (PI3K/AKT/mTOR) pathway to support HCC distancing from senescence [185]. This lipid metabolic remodeling may affect the developmental trajectories of immune cells. For example, hypoxia-mediated HIF-1α induces the mediator complex subunit 23/peroxisome proliferator activated receptor-gamma (MED23/PPAR-γ) signaling pathway, in turn, promoting the production of adipose Tregs [186]. Ultimately, these events cause an imbalance in immune regulation that affects the process of tumor cell senescence. Tumor cell development is additionally dependent on various factors other than immunosuppression, such as stromal cell support (including angiogenesis, differentiation of CAFs, and stem cell activity). The hypoxic environment can induce fibroblast senescence-dependent SASP through the cGAS/STING/nuclear factor-κB (cGAS/STING/NF-κB) pathway to generate a pro-inflammatory and pro-tumor environment [187]. Furthermore, hypoxia-mediated acquisition of paracrine SASP contributes to pathological angiogenesis [188]. Hence, in the hypoxic TME, dysregulation of metabolic reprogramming leads to diverse senescence outcomes, including senescence and acquisition of SASP by non-tumor cells (immune and stromal cells), generating an immunosuppressive TME and immunosenescence. This process eventually promotes the sustained survival of tumor cells by facilitating senescence escape. Further oncological studies are required to establish the differences between physiological and pathological hypoxia, as well the diverse impacts on the TME. Pathological hypoxia clearly promotes tumor growth through senescence-dependent metabolic reprogramming, while physiological hypoxia inhibits SASP through AMPK-mediated mTOR to delay tumor progression. This finding may be utilized to develop potential novel strategies for minimizing the deleterious paracrine effects of senescent cells in the TME [189].
5 CELLULAR SENESCENCE AND CHRONIC INFLAMMATION IN THE IMMUNOSUPPRESSIVE TME
Initially, inflammation was interpreted as a response of the immune system to tumor cells. Following substantial advancements in cancer research, Virchow proposed that “tumors originate from chronic inflammation” and referred to tumors as “wounds that never heal” [190]. Subsequent studies have confirmed that chronic inflammation serves as a high-risk factor for the development of numerous malignant tumor types and is involved in multiple tumorigenic processes (including malignancy, invasion, and metastasis). Accordingly, chronic inflammation has been highlighted as a critical biological feature of tumors [31].
Our group demonstrated specific associations between metabolism and senescence, with tumor proliferation inducing metabolic disruption to regulate the senescence process and the induction of SASP in cellular senescence accelerating metabolic dysregulation to support the development of chronic inflammation. Dysregulation of tumor metabolism promotes the release of several metabolites or inflammatory mediators through cellular senescence pathways, thereby triggering tumor immunosuppression and chronic inflammation in the surrounding microenvironment. For instance, metabolic programming and cellular senescence-regulated IL-17A shapes the inflammatory TME of lung cancer via the Janus kinase 2/STAT3 (JAK2/STAT3) pathway [191]. Glycolysis and lipid metabolism in prostate cancer cells promote the activation of mTOR and NF-κB signaling and release of inflammatory mediators, including IL-15, TNF-α, IL-1β, CXCR2, and CXCL5, to produce an immunosuppressive and inflammatory TME [192-195]. To date, a limited number of specific inflammatory factors have been identified that induce chronic inflammation and an immunosuppressive TME via the metabolic programming-cellular senescence route, which may be effectively utilized to inform studies on the metabolic-senescence-inflammation axis in tumors. Furthermore, the TME is significantly altered with tumor growth. The numbers of dysfunctional CD8+ T cells, immunosuppressive CD4+ T cells, Tregs, and regulatory B cells are increased, whereby CD4+ T cells tend to have a pro-inflammatory Th2 phenotype. In addition, DCs exhibit maturation and functional defects, adapting phenotypes in response to local inflammatory signals [196]. Similarly, senescent CAFs affect metabolic programs in MDSCs through regulation of IL-6- and IL-33-mediated chronic inflammation and promote cancer stemness in the TME, increasing resistance to chemotherapy and creating an immunosuppressive environment [155]. Metabolic reprogramming in tumor cells accelerates immune cell senescence/depletion to facilitate senescence escape by tumors. Senescence of immune cells in the TME triggers SASP release (including IL-α, IL-β, IL-6, IL-8, IFN-γ, TNF, and CXCL11) and further supports immunosuppression and chronic inflammation [197-201]. Immune suppression mediated by cellular senescence-dependent SASP contributes to the onset and maintenance of chronic inflammation. This includes SASP-mediated activation of TGF-β signaling and IL-6/IL-8 secretion, both of which accelerate chronic inflammation and breast cancer progression [197, 202]. Considering that hypoxia and senescence are interlinked (TGF-β release in hypoxic TME drives the senescence phenotype and SASP secretion [203]) and activation of the AMPK signaling pathway in cellular senescence exacerbates the dysregulation of metabolic reprogramming, hypoxic activation and chronic inflammation in the TME [184], we recommend viewing the tumor hypoxic ecotone as a complete ecosystem. Tumor proliferation consumes oxygen to create a hypoxic environment and mediates metabolic reprogramming to release inflammatory factors that create an immunosuppressive and inflammatory TME, in turn, activating the senescence phenotype. Senescence-dependent activation of SASP creates favorable conditions for tumor proliferation and promotes a vicious cycle of senescence-hypoxia-metabolism-chronic inflammation.
6 CELLULAR SENESCENCE REGULATES TUMOR BIOLOGICAL FUNCTIONS
Senescence, metabolism, and hypoxia have been identified as regulatory factors in multiple tumor-related biological processes, including resistance to therapy, autophagy, angiogenesis and biorhythms (Figure 3).
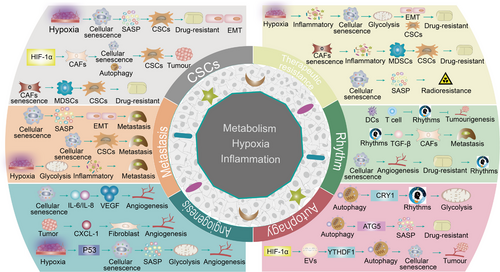
6.1 Cellular senescence and cancer stem cells (CSCs)
CSCs are a primary cause of tumor resistance and disease recurrence. Hypoxia and metabolic reprogramming are critical components of the TME that are essential for rapid tumor growth and maintenance of CSC function [204]. Notably, cellular senescence and CSC function are commonly regulated by overlapping signaling pathways, including p16, p21, and p53, suggesting that cellular senescence drives genetic reprogramming and stem activation to contribute to CSC-mediated metastasis and treatment resistance [205]. In addition, increased cancer stemness may be associated with the CAF-mediated promotion of MDSC function and chemoresistance [155]. However, accumulation of senescent cells over time may mediate the expression and secretion of SASP-associated pro-inflammatory chemokines and cytokines, such as IL-6, IL-8, and IL-1α, which have been shown to support the pro-tumorigenic phenotype of CSCs via paracrine signaling in prostate cancer [201, 206]. The hypoxic TME is hypothesized to activate CSCs from a dormant state, thereby increasing their survival following exposure to stress conditions. Earlier studies have confirmed that hypoxia-mediated HIF1-α and HIF2-α contribute to the survival and activation of CSCs in the TME and underlie glioma resistance and EMT [207, 208]. This pro-tumor effect may be attributed to release of a SASP-like secretome from the hypoxic microenvironmentincluding inflammatory factors, such as IL-8, IL-1β, IL-2, IL-212, CXCL213, and CXCL212 [209]. Interestingly, a link between hypoxia and senescence in CSCs has been confirmed. In this study, hypoxia affected senescence and maintained the pro-oncogenic stem cell phenotype by regulating HIF-1α-mediated expression of p21 in CSCs and other progenitor cells [210]. Additionally, tumor cell-mediated metabolic reprogramming is reported to contribute to the maintenance of cancer stemness. For example, in breast cancer cells, dysregulation of mitochondrial metabolism induces glycolysis to support the proliferation of CSCs [211]. HIF-1 activation drives autophagy, glycolysis, and fibroblast senescence in CAFs, ultimately sustaining cancer stemness through metabolic reprogramming to promote breast cancer growth [212]. This finding suggests that intrinsic crosstalk between hypoxia and metabolic reprogramming in the senescent TME creates an inflammatory milieu conducive to activation and stemness maintenance of CSCs.
6.2 Cellular senescence and angiogenesis
Angiogenesis, a process that provides the nutrients and oxygen required for tumor growth, is crucial factor in tumor invasion and metastasis [213]. Numerous studies have investigated the effects of angiogenesis in the TME on tumor invasion and metastasis. Ischemic retinal cellular senescence-mediated SASP is reported to promote pathological angiogenesis by reshaping the inflammatory environment through secretion of IL-6, IL-8, and vascular endothelial growth factor (VEGF) [188]. Moreover, CXCL1 secreted by colorectal cancer cells promotes tumor angiogenesis by inducing a senescent phenotype in stromal fibroblasts [214]. Recent studies have focused on inducing pathological vascular normalization of tumors and limiting their malignant progression triggered by increased hypoxia and senescence [215]. Hypoxia-dependent regulation of the m6A reader, YTH N6-methyladenosine RNA binding protein F2 (YTHDF2) (associated with senescence characteristics), is implicated in enhanced inflammation and angiogenesis critical for HCC invasion [216]. Furthermore, hypoxia-induced SASP releases the pro-inflammatory factor, TNF-α, which activates NF-κB, leading to VEGF production. This sequence of events promotes angiogenesis and metastasis [217]. Interestingly, angiogenesis is facilitated by metabolic reprogramming in hypoxic TME. HIF-1α regulates P53 expression in hypoxic tumor cells, which influences cellular senescence and promotes tumor proliferation through enhanced glycolysis and angiogenesis [218]. High BCAT1 expression supports angiogenesis and malignant progression of gliomas by regulating hypoxia, glycolysis, and cellular senescence to induce an immunosuppressive state [219]. In malignant TME, both senescent and non-senescent vascular endothelial cells (VECs) and vascular smooth muscle cells (VSMCs) appear to accelerate tumor growth by supporting angiogenesis to an extent. The involvement of vascular endothelial cells (VECs) and VSMCs in angiogenesis in a senescent TME has been further explored. Th17/IL-17 induces VEC senescence via activation of the NF-κB/p53/retinoblastoma (NF-κB/p53/Rb) signaling pathway [220]. Notably, exendin-4 alleviates angiotensin II-induced senescence in VSMCs by inhibiting Rac1 activation through a cyclic adenosine monophosphate/protein kinase A-dependent (cAMP/PKA-dependent) pathway [221]. Subsequently, inflammatory factors released by senescent VECs and VSMCs may stimulate immunosuppression and angiogenesis [222, 223]. However, in a malignant TME, reduced senescence accompanied by VECs and VSMCs has also been shown to promote angiogenesis and metastasis. Vascular endothelial growth factor (VEGF) is considered the most potent and abundant vascular growth factor in angiogenesis, with an established role in promoting tumor progression. Cellular senescence (with the exception of VECs and VSMCs) is reported to induce the release of SASP (VEGF, IL-6, IL-8, CXCL1, and TNF-α) to reshape the inflammatory environment, thus supporting pathological angiogenesis and bladder cancer growth. This process is accompanied by an adaptive response to hypoxia and metabolic reprogramming [224]. Targeting pro-angiogenic cellular senescence may provide an innovative avenue for treatment of solid tumors, given the therapeutic benefits of anti-angiogenic therapy. Moreover, the specific mechanisms by which senescence affects other pathways of VEGF and TME angiogenesis (e.g., hypoxia and metabolic reprogramming) require further investigation.
6.3 Cellular senescence and tumor metastasis
Senescence-mediated enhancement of tumor invasion and metastasis has been demonstrated in cervical cancer [225], breast cancer [226], melanoma [227], and prostate cancer [228]. Tumor metastasis is a multi-step process designated the “invasion-metastasis cascade” [213, 229]. Therefore, the precise involvement of senescence at each stage of this process, from primary tumor cell development to the formation of metastatic ecological niches, should be explored. For example, senescence-related reprogramming promotes cancer stemness in lymphoma and is a key factor in metastasis [205]. Cellular senescence-mediated acquisition of SASP induces tumor EMT and distant metastasis [230]. Moreover, vascular permeability factor/VEGF (VPF/VEGF) delays and induces escape from senescence in human dermal micro-VECs [231], which lends credence to the possibility of transendothelial migration of tumor cells. Given the effects of hypoxia and metabolic reprogramming on immunosuppression and chronic inflammation, it is hypothesized that these factors, in conjunction with cellular senescence, promote tumor proliferation and metastasis. For example, overexpression of TNF receptor-associated factor 6 (TRAF6) and H2A histone family member X (H2AX) and γH2AX-mediated HIF-1α enrichment in the senescent environment lead to HIF-1α-driven glycolysis and an inflammatory response, promoting metastasis of lung cancer [232, 233]. Virtually all established mechanisms of tumor metastasis involving CSCs [234], angiogenesis [218], EMT [235], chemoresistance [236], and autophagy [237] are related to hypoxia and metabolism to varying degrees. However, the potential roles of cellular senescence and metabolic reprogramming in other metastatic processes, such as signaling networks for the entry and exit of tumor cells from dormancy as well as tumor-associated metastatic colonization and evolution, are yet to be determined.
6.4 Cellular senescence and therapeutic resistance
Tumors frequently develop resistance to multiple treatment modalities, ranging from conventional radiotherapy and chemotherapy to modern targeted treatments and immunotherapy, resulting in distant metastasis. In-depth understanding of senescence-mediated mechanisms of resistance is necessary to improve therapeutic efficacy. D-galactose is reported to induce cell senescence in astrocytes, leading to chemoresistance and astrocytoma metastasis [238]. RagC GTPase activates mTOR signaling and tumor chemoresistance by promoting senescence in HCC [239]. Interestingly, the mechanisms underlying TME tandem-induced therapeutic resistance are multifaceted. For instance, hypoxia-mediated HIF-1α regulates cellular senescence in the TME to achieve colorectal cancer chemoresistance [240]. Notably, hypoxia induced by tumor proliferation can trigger mitochondrial dysfunction to regulate glucose uptake and utilization, in addition to promoting angiogenesis and chemoresistance in renal cell carcinoma [241]. This mitochondrial dysfunction may be attributed to an immunosuppressive inflammatory environment produced by hypoxia and metabolic reprogramming triggered by the activation of senescence signaling during tumor proliferation and in the TME. The cascade includes multiple cell biological processes (such as angiogenesis, EMT, and CSCs) that co-support tumor chemoresistance [242]. Autophagy-associated ATG5 controls mitotic slippage, thus inducing cell senescence-dependent SASP and promoting pancreatic cancer chemoresistance, migration, invasion, and angiogenesis via paracrine secretion [243]. Angiogenesis in the senescent TME may drive endocrine secretion in breast cancer cells to achieve resistance to tamoxifen [244]. Furthermore, activation of the NF-κB/HOX transcript antisense RNA (NF-κB/HOTAIR) axis links cellular senescence, DDR, and chemoresistance in ovarian cancer [245]. Interestingly, this chemoresistance continues to be enhanced through several ways to achieve sustained tumor malignancy. Senescent CAFs affect metabolic programs in MDSCs through regulation of IL-6- and IL-33-mediated chronic inflammation and promote cancer stemness in the TME to confer resistance to chemotherapy [155]. Moreover, chemotherapy-induced cellular senescence facilitates CSC growth to enhance tumor chemoresistance [246].
Radioresistance presents another considerable therapeutic challenge. Ionizing radiation effectively induces senescence in glioma cells. Senescent stromal cells can support the proliferation of neighboring glioma cells by secreting pro-inflammatory SASP factors, ultimately inducing radioresistance and disease recurrence [247]. Telomere dysfunction, a feature of cellular senescence, is additionally reported to promote radioresistance of oral cancer cells [248]. Current research on cellular senescence and tumor radioresistance has focused on the inflammatory environment shaped by the acquisition of SASP [249]. Further investigation of the crosstalk between specific features of senescence (DNA damage or epigenetics) and tumor biological behavior (autophagy, EMT, angiogenesis) would be of significant interest [250, 251].
Notably, immunotherapy enhances the anti-tumor activity of chemotherapy via suppression of NK cell-induced senescent cells [252]. Therefore, elucidation of the mechanisms underlying cellular senescence-mediated chemoresistance/radioresistance may yield promising insights for application in tumor immunotherapy. Future research should focus on the effects of signaling exchanges of metabolic programming, hypoxia, and other TME components on cellular senescence features, such as DNA damage and epigenetics.
6.5 Tumor therapy-induced cellular senescence
Cancer cells can enter a state of senescence upon exposure to chemotherapy, radiation, or targeted treatments, a process termed “therapy-induced senescence” (TIS). This state entails arrest of the cell cycle, which is primarily mediated through activation of p53/p21WAF1 and/or p16INK4A/Rb pathways [253]. In tumors such as malignant pleural mesothelioma, an increase in markers of senescence, such as PAI1 and p21WAF1, has been reported following neo-adjuvant chemotherapy (NAC) [254]. While the primary goal of cancer therapy is to reduce tumor growth by triggering cell death, the treatment can also damage the TME, including stromal cells, leading to significant accumulation of senescent cells that can affect various components of the TME and potentially alter the overall response to the tumor. Secreted factors of senescent cells, known as the senescence-associated secretory phenotype (SASP), are capable of promoting growth, new blood vessel formation, migration, and invasion of surrounding cells, impacting the overall behavior of cancer and stromal cells in the TME [255].
Furthermore, the effects of SASP vary depending on the targeted cell and its surrounding environment, adding to the complexity of the function of senescent cells within the TME. Accumulating SASP factors, including IL-6, IL-8, and TNF-α, can promote the senescent state through self-stimulating and neighboring stimulating mechanisms, inhibiting key processes of tumor growth and metastasis [256]. Conversely, SASP components, such as VEGF, EGF, and FGF, may erroneously promote differentiation and the formation of new blood vessels in adjacent tissues. Furthermore, several SASP factors and pathways, including TGF-β1, NF-kB, CXCL1, IL-8, and cGAS-STING signaling, are capable of fostering senescence in surrounding cells via paracrine effects [257]. Therefore, the efficiency of TIS in the context of damaged TME is influenced by various elements, including the volume of secreted molecules, the signaling pathways activated, and the temporal dynamics of SASP evolution. Such factors reflect the complexity and variability of the senescence response and its changes depending on particular parameters and pathophysiological settings [258, 259]. TIS can induce cellular senescence via SASP in a manner that indirectly influences tumor growth by promoting activities, such as tumor cell proliferation, formation of new blood vessels, transition to a more invasive cell type, epithelial-mesenchymal transition (EMT), and ability of the tumor to evade the immune system.
6.6 Cellular senescence and autophagy
In the malignant TME created by tumor progression, autophagy acts as a dynamic degradation and recycling system that regulates tumor survival and metastasis while influencing tumor aggressiveness [260]. Activation and inhibition of autophagy represents an adaptive change in tumor response to hypoxia and metabolism. Compelling evidence supports the theory that autophagy-related mechanisms of tumor progression are linked to factors such as metabolic and senescence [261].
Theoretically, autophagy should exert an anti-tumor effect based on its powerful stabilizing function and timely removal of “dangerous molecules” from the body. Nevertheless, autophagy has both beneficial and detrimental effects on tumors. Given the pro-tumorigenic nature of autophagy, autophagy of non-tumor cells provides nutrients to tumor cells and suppresses CD8+ T cell-mediated anti-tumor immunity, thereby promoting the survival and metastasis of cancer cells [262, 263]. Indeed, it is currently thought that this difference is a conflict between the anti-tumor and pro-tumor effects of autophagy, which may depend on the stage and environment in which autophagy occurs. For example, before tumorigenesis, activation of autophagy inhibits tumorigenesis; in advanced tumor stages, activation of autophagy may in turn promote tumor growth and metastasis [264]. Artemisinin inhibits cell growth in colorectal cancer by promoting ROS-dependent cellular senescence and autophagy [265]. Autophagy-associated ATG5 in breast cancer supports tumor chemoresistance by inducing SASP-associated paracrine tumorigenicity [243]. This may be attributed to differences in the type of cellular processes and inflammatory factor release mediated by autophagy/senescence at different stages of the tumor. In the TME, autophagy can be induced by stress signals both inside and outside the advanced tumor cell, including metabolic stress, hypoxia, senescence stress and immune signals. HIF-1α overexpressing in MSCs derived EVs ameliorate hypoxia-induced pancreatic β-cell apoptosis and pancreatic β cell senescence by activating YTHDF1-mediated autophagy [266]. Cannabinoid type 2 receptor (CB2R) activation regulates transcription factor EB-mediated (TFEB-mediated) autophagy and accelerates mitochondrial damage and inflammatory factor release through lipid metabolism [267]. However, in the face of hypoxic, metabolic, and cellular senescence stress, autophagy may work synergistically in multiple ways to maintain tumor proliferation. In the hypoxic TME, CSCs senescence leads to the release of inflammatory products through metabolic reprogramming to shape the immunosuppressive TME and chronic inflammatory environment conducive to testicular cancer proliferation and metastasis [268]. Subsequently, activation and homeostatic maintenance of autophagy drive the tumorigenicity and cellular differentiation of CSCs, while autophagy-mediated activation of CSCs drives tumor angiogenesis [269], EMT [270], therapeutic resistance [271] in diverse levels. Notably, autophagy also further accelerates tumor invasion and metastasis by promoting an intrinsic angiogenesis-EMT/EMT-chemoresistance/angiogenesis-chemoresistance association through the lung cancer metastasis cascade and feedback loops [272-274]. These findings highlight the critical interaction between autophagy and senescence and further confirm the biological significance of cellular senescence in TME hypoxia and metabolic programming.
6.7 Cellular senescence and rhythms
Circadian rhythms exert a significant impact on physiological and metabolic functions (including sleep, immune, endocrine and vascular biology) and their disruption is associated with increased risk of tumor metastasis [275]. Disturbances in biological rhythms lead to increased ROS production and melatonin secretion, which affect sleep, ultimately causing cellular senescence. These findings indicate that hormone secretion and metabolic regulation serve as a bridge between circadian rhythms and cellular senescence [276]. Considering the strong association of senescence with age-related metabolic diseases, senescence-regulated early growth response 1 (EGR-1) induces rhythm disruption and accelerates age-related metabolic dysfunction in the liver [277]. Disruption of circadian rhythms causes an imbalance in immune cells, which may affect the tumor immune response and consequently influence cancer progression. Loss of rhythmic KLF transcription factor 4 (KLF4) expression in aged macrophages is associated with disruption of circadian innate immune homeostasis, which may underlie the loss of protective immune responses in age-related diseases, including tumors [278]. Conversely, maintenance of circadian rhythm homeostasis in DCs and CD8+ T cells is reported to enhance the anti-tumor immune response and suppress melanoma growth [279]. However, due to interactions of tumor cells with stromal cells in the TME, expression of the circadian regulatory gene Bmal1 is dysregulated, inducing fibrinolytic enzymes to promote conversion between CAFs and myofibrillar fibroblasts via activation of TGF-β, thereby promoting proliferation and metastasis of colorectal cancer [280]. Disruption of circadian rhythms in the microenvironment is significantly associated with angiogenesis and chemoresistance [281, 282]. In addition, autophagy regulates biological rhythms by degrading the core clock protein CRY1, which controls glucose metabolism [283]. Therefore, circadian dysregulation-mediated metabolic reprogramming of senescence initiates tumorigenesis and supports metastasis through a cascade of biological behaviors.
However, limited information is available on the unique expression and functional patterns of the cell-autonomous biological clock in different tumor types. Furthermore, little is known regarding the potentially common core clock signals existing between cancer and pluripotent stem cells that may diverge at some stage of cellular differentiation. These insights are critical for the development of effective therapeutic strategies, which may involve restoring functional clocks and reversing tumor epigenome plasticity.
6.8 Extracellular matrix
The extracellular matrix (ECM), a crucial component of the TME, is composed of a number of proteins, including collagen, fibronectin, hyaluronic acid, laminin, and proteoglycans [284]. Earlier research has conclusively shown that ECM integrity markedly declines with age. Reduced collagen density and variations in size and thickness of ECM fibers that weaken structural integrity are two physical ageing-associated alterations in ECM [285]. Compared to their more mature, senescent counterparts, young dermal fibroblasts exhibit a higher output of ECM constituents, including proteoglycans, glycoproteins, and proteins that maintain cartilage integrity [286]. Young fibroblasts are responsible for producing key elements of the extracellular matrix (ECM), in particular, hyaluronic acid and proteoglycan-linked protein 1 (HAPLN1) [287], which plays a vital role as a crosslinker to stabilize aggregates of proteoglycan monomers. Notably, the production of HAPLN1 decreases in aged fibroblasts, highlighting the intricate relationship between tumor cells and fibroblasts. Earlier studies have additionally demonstrated the significance of LOXL1 in facilitating cancer cell migration via the ECM, with impaired secretion reported in aging fibroblasts [288]. The changes in ECM components secreted by aging fibroblasts lead to a reduction in collagen density and degree of fiber crosslinking, alongside significant enhancement of fiber alignment. These modifications collectively facilitate the invasion of melanoma cells.
Accumulation of senescent matrix components linked to the SASP with age can promote the release of soluble factors that drive remodeling of the ECM and contribute to matrix sclerosis within the local environment. This process is critical in linking the ECM to the TME, as it enhances ECM crosslinking and sclerosis, facilitating tumor dissemination and advancement [289]. While senescence alters the ECM structure, the local build-up of senescent stromal cells associated with SASP may create tumor-specific habitats necessary for disease progression. For instance, lung malignancies are directly correlated with age and increased ECM rigidity, crosslinking, and collagen density [290], highlighting the importance of ascertaining the role of SASP cell accumulation in tumorigenesis within these soft tissue settings during senescence. Similarly, changes in ECM density due to senescence are linked with the development of breast cancer, whereby reduced integrity of the basement membrane or myoepithelial layer can enhance tumor cell interactions with ECM components, potentially establishing a pre-metastatic niche [291].
Remodeling of the ECM during tumor development has a significant impact on immune cell function. Components of the ECM, such as collagen and fibronectin, activate the PI3K/AKT pathway, which aids in the immune evasion of cancer stem cells (CSCs) [292]. Furthermore, ECM proteins, such as monomeric collagen type I, attract immunosuppressive cells, including T regulatory cells (Tregs) and Tumor-Associated Macrophages (TAMs), which support CSC survival by inhibiting the recruitment of anti-tumor immune cells [8]. Conversely, stabilizing collagen to reduce ECM density and tumor stiffness can facilitate T cell migration and improve the efficacy of anti-PD-1 therapy [293]. These findings highlight the critical role of physical alterations in the ECM in regulating immune and tumor cell migration. Ageing directly influences the specificity and functionality of immune cells in both the systemic and localized environments. Elucidation of the effects of ECM structure and functionality on tumor growth should provide opportunities to identify novel therapeutic targets that can inhibit both tumor proliferation and metastasis.
7 POTENTIAL CLINICAL APPLICATIONS OF CELLULAR SENESCENCE
The mechanisms by which senescence promotes tumorigenesis have been extensively investigated, providing valuable insights into the potential clinical applications of senescence in tumor therapy (Table 2).
| Drug | Target | Mechanism | Effect | Ref |
|---|---|---|---|---|
| ABT-263 | BCL-2, BCL-W, BCL-xL | Removal of senescent cells through induction of apoptosis by ABT263 | Rejuvenation of stem cells | [297] |
| GX15-070 | BCL-2 | Targeting of BCL-xL to induce senescent cell death improves the efficacy of bromodomain and extra-terminal protein inhibitors | Inhibition of breast cancer | [335] |
| Dasatinib | SRC family protein tyrosine kinase inhibitor | Reprogramming of liver metabolism by modulating the senescent secretome | Inhibition of HCC | [5] |
| Irinotecan | TOP1 inhibitor | Enhancement of SASP improves ovarian tumor sensitivity to immune checkpoint blockade | Inhibition of ovarian cancer | [336] |
| Metformin | Gluconeogenesis inhibition | Enhancement of the anticancer efficacy of CDK4/6 inhibitors by reprogramming the profiles of the SASP | Inhibition of head-and-neck squamous cell carcinoma | [337] |
| NVP-BSK805 | JAK2 inhibitor | Enhancement of the efficacy of chemotherapy by activating senescence-associated anti-tumor immunity | Inhibition of prostate cancer | [330] |
| Rapamycin | mTOR inhibitor | Reduction of the mRNA levels of IL-6 and other cytokines to inhibit the secretion of inflammatory cytokines by senescent cells | Inhibition of prostate cancer | [201] |
| Sertraline | Serotonin reuptake inhibitor | Inhibition of mTOR signaling to induce tumor cell death | Inhibition of HCC | [338] |
| Y-27632 | ROCK inhibitor | Reduction of IL-1α, IL-1β, IL-6, and IL-8 secretion | Delay of tumor growth | [339] |
| BIRB 796 | p38 MAPK | Inhibition of SASP in human fibroblasts | Reduction of the inflammatory response | [340] |
| Kaempferol | NF-κB | Blockage of NF-κB, p65 and IκBζ signaling pathways | Reduction of the inflammatory response | [341] |
| Corticosterone | IL-1α/NF-κB | Inhibition of IL-1α signaling and NF-κB transactivation activity | Suppression of inflammation | [342] |
| Ruxolitinib | JAK1/2, ROCK | Reduction of impairment in DCs function by ROCK | Enhancement of anti-tumor immunity | [343] |
| Quercetin | PI3K/AKT, BCL-2 | Targeting of PI3K/AKT and BCL-2 to selectively kill and clear senescent cells | Removal of senescent cells | [298] |
| Fisetin | PI3K/AKT, NF-κB | Inhibition of the survival of pro-inflammatory factors and induction of apoptosis in senescent cells | Inhibition of SASP | [344] |
| Piperlongumine | PI3K/AKT/mTOR | Reduction of senescent cell survival through induction of apoptosis | Removal of senescent cells | [345] |
- Abbreviations: AKT, Protein Kinase B; BCL-2,B cell lymphoma protein-2; BCL-W,B cell lymphoma-W; BCL-Xl, B cell lymphoma extra-large; DCs, dendritic cells; HCC, hepatocellular carcinoma; IL-, interleukin-; JAK2/STAT3, Janus kinase 2/signal transducer and activator of transcription 3; SASP, senescence-associated secretory phenotypes; PI3K, Phosphatidylinositol-3-kinase; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NF-κB, Nuclear Factor-kappa B; ROCK, Rho associated coiled-coil containing protein kinase.
The persistence of senescent cells leads to chronic inflammation that is detrimental to homeostasis of the organism. The screening and development of therapeutic agents to inhibit SASP or remove senescent cells from the tissue microenvironment is a current research priority [8]. Senolytics, the primary agents of senotherapy, interfere with the cell survival pathway to achieve selective elimination of senescent cells [294]. Senescent cells can be selectively induced undergo apoptosis by RNA interference or drugs that block the anti-apoptotic pathways of senescent cells (including B cell lymphoma protein-2/B cell lymphoma extra-large (BCL-2/BCL-xL), PI3K/AKT, p53/p21, ephrins, tyrosine kinases, and HIF-1α pathways [295]). The small molecule inhibitors, ABT-737 and ABT-263, target BCL-2, BCL-W, and BCL-xL. In a mouse model of senescence, these agents specifically induced apoptosis and eliminated senescent hematopoietic stem cells in vivo [296, 297]. Other studies have shown that senescent human umbilical vein cells and adipocytes can be effectively eliminated by dasatinib and quercetin [298] and aurora kinase inhibitors stimulate the clearance of senescent lung cancer cells [299].
Interestingly, senescent cells can recruit immune cells by non-autonomous cell secretion to elicit an immune response for self-elimination. IL-15-activated NK cells selectively target and remove senescent decidual cells through granule cytosolic action [300]. Precancerous senescent hepatocytes rely on the adaptive immune response mediated by T cells, monocytes, and macrophages to regulate the immunological clearance of senescent cells through release of inflammatory factors [301]. In pancreatic cancer, senescence therapy produces SASP and promotes the accumulation of stimulated CD8+ T cells, thereby accelerating the clearance of senescent cells induced by chemotherapy with gemcitabine to retard pancreatic cancer progression [302]. These investigations support the utility of promising preclinical therapeutic strategies entailing the recruitment and activation of anti-tumor immune cells for removal of senescent cells from the TME. However, senescent cells have the ability to evade immune surveillance and avoid clearance by immune cells. For example, senescent fibroblasts recognize each other through the non-classical MHC molecule HLA-E and the inhibitory receptor NKG8A on NK and CD8+ T cells, thereby inhibiting their clearance by the immune system [303]. One potential novel approach for senescent cell elimination is targeted blockade of the mutual recognition between HLA-E and NKG2A, which may activate clearance of senescent cells by the immune response [303]. Accordingly, precise activation of the immune response in a specific manner to eliminate harmful senescent cells that persistently reside in the TME after chemotherapy, while simultaneously preventing SASP-induced inflammatory responses that support tumor growth is critical to achieve effective therapy.
Given the SASP-mediated induction of immunosuppressive TME and chronic inflammation, reducing or inhibiting SASP has emerged as a viable strategy for suppressing the negative function of senescent cells. Newly emerging classes of SASP inhibitors are collectively termed “senomorphics”. Blockage of specific signaling pathways involved in acquisition of SASP, such as p38/MAPK, mTOR, and NF-κB, may effectively improve anti-tumor effects. Mechanistically, rapamycin blocks SASP release by selectively interfering with IL-1α translation via mTOR, ultimately impeding the tumor growth-supporting actions of senescent fibroblasts [201]. Similarly, metformin inhibits prostate cancer progression by inhibiting activation of the IKK/NF-κB pathway and SASP [304], while anakinra inhibits IL-6/IL-8 secretion and blocks NF-κB signaling to delay tumor metastasis [305]. Signaling blockade upstream of SASP, such as p38/MAPK or PI3K/AKT/mTOR, may additionally induce tumorigenesis and recurrence due to the fact that SASP is coordinated by multiple signaling pathways that are not unique to cellular senescence [306]. Future studies should focus on the development of SASP inhibitors that prevent the activation of this signaling and ultimately, tumor recurrence.
Significant advancements have been made in the field of tumor immunotherapy in recent years. Immunotherapeutic agents, such as PD-1/PD-L1, cytotoxic T-lymphocyte associated protein 4 (CTLA4) and lymphocyte activating 3 (LAG3), have been widely investigated as antibodies against immunosuppressive receptors [307]. Elucidation of the diverse mechanisms of the immunosuppressive TME is essential for the development of personalized and precise immunotherapeutic approaches for cellular senescence. Senescent cells and other stromal components alter their original cellular signaling patterns via the immune checkpoint pathway to achieve tumor immune escape. Current strategies for tumor immunotherapy involve blocking of immunosuppressive signals in senescent cells. For example, in senescent mice, innate immune activation triggered by STING agonists increases the sensitivity of breast cancer cells to the immune checkpoint blockade [308]. Furthermore, inhibition of the PD-1/PD-L1 pathway in vitro restores T cell function in aged mice [309].
Metabolic reprogramming and hypoxia are extensively involved in the complex crosstalk of the TME in cellular senescence, supporting tumor progression through inducing an immunosuppressive microenvironment and chronic inflammation [310]. Therefore, the consideration of multifaceted combination therapies is necessary. A number of studies have focused on avoiding chemotherapeutic drug-induced senescence of tumor cells. The use of chemotherapeutic agents to kill tumor cells, followed by treatment with a small-molecule inhibitor of cellular senescence (ABT-263), significantly prolonged suppression of breast cancer cells and improved the efficiency of lung and breast cancer therapy [311]. APR-246, in combination with pembrolizumab, inhibits macrophage M2 polarization and promotes T cell proliferation through effects on p53-signaling-dependent SASP, suggesting that immune checkpoint blockade responsiveness can be improved by modulation of TAM reprogramming by the TME [140]. Importantly, combined inhibition of cellular senescence, autophagy, and glycolysis is reported to markedly reduce the stemness of glioma subpopulations to favor tumor cell death [312]. Given the holistic nature of the TME, integration of these strategies with targeted therapies for senescence may present a promising research direction for the development of potent anti-tumor agents.
8 CONCLUSIONS AND FUTURE PERSPECTIVES
To survive in hostile environments (e.g., hypoxia and chronic inflammation), tumor cells employ various strategies (such as metabolic reprogramming involved in immune cell phenotypic changes) to produce an immunosuppressive TME. At present, researchers are unable to fully explain the complex associations among various components of cellular senescence and the TME (including metabolism, hypoxia, chronic inflammation, and multiple immune cells, among other factors) (Figure 4). As a result, it is difficult to provide a proper framework for elucidating the intricate crosstalk between various factors. Based on the currently available data, the following conclusions can be drawn: 1) the dynamic evolution of cellular senescence is an adaptive shift of the tumor to confer a malignant TME; 2) to meet the conditions required for self-proliferation, senescence drives the metabolic reprogramming of tumor cells and supports tumor growth by modulating immune cell function and phenotype to create an immunosuppressive TME; and 3) tumor proliferation triggers the formation of a hypoxic TME, which, together with cellular senescence, supports immune suppression and chronic inflammation that favor tumor proliferation. Additionally, these immunosuppressive signals are transferred to distant sites via EVs and SASP, which generate suitable conditions for tumor cell metastasis and invasion by suppressing normal immune function to produce an immunosuppressive TME. Metabolic reprogramming and hypoxia regulate a wide range of biological behaviors through cellular senescence. The senescence process further supports tumor proliferation and metastasis through the interplay of multiple mechanisms (including CSCs, angiogenesis, and chemoresistance), generating a positive feedback loop associated with poor prognosis. In addition, the mechanisms by which cellular senescence regulate tumor progression may provide useful clinical information for the development of therapeutic regimens to improve patient outcomes. Integration of targeted cellular senescence-based tumor therapies with immunotherapy or metabolic and hypoxia-based targeted therapies may provide novel guidelines for clinical therapy.
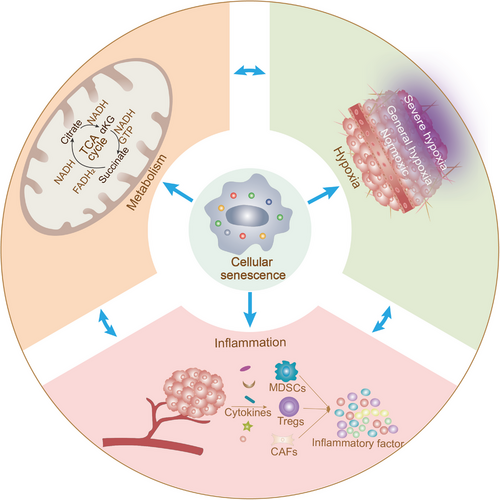
Stromal and immune cell senescence may collectively drive the progression of tumor cells from their original state to a highly invasive and metastatic state, involving changes in cell-secreted factors, biophysical structure of the TME, and even macroscopic characteristics of tumors. However, numerous concerns regarding the study of cellular senescence and tumor progression need to be addressed.
Currently, gold standard features and consistent biomarkers for identifying the state of cellular senescence are lacking. Detection of senescent tumor cell markers may benefit from the use of use of galactose-conjugated fluorescent nanoparticles [313], radioactive beta-galactosidase (β-gal) positron emission computed tomography [314], and a noninvasive imaging system [315]. However, senescence associated β-gal is expressed in specific cells and its overexpression in macrophages poses a challenge for developing β-gal-based screening strategies [316, 317]. Moreover, the impacts of cells with distinct genetic backgrounds, tissue types of different origins, or cellular senescence induced by different drugs on detection of senescence markers require consideration.
Senescent cells can enhance their senescence phenotype in an autocrine manner while also transmitting senescence signals to neighboring cells via paracrine means. Thus, the senescence burden in the TME increases over time, causing a time-dependent effect of SASP, and therefore, cellular senescence should be considered a dynamic evolution process with varying anti-tumor and pro-tumor characteristics [318]. However, the mechanisms underlying this senescence signaling are yet to be fully elucidated. Analysis of the effects of varying types of SASP/EVs released by hypoxia and metabolism at different states on the communication of intercellular information is of significant interest [266]. Furthermore, the difference between tumor cell and non-tumor cell senescence should be noted. Non-tumor cell (including immune cell) senescence releases inflammatory factors that can play an anti- or pro-tumor (more likely pro-tumor) role, whereas tumor cell senescence results in a delay in tumor growth to some extent. Nevertheless, “immune senescence” induced by associated phenomena accompanying tumor cell senescence (e.g., SASP) promotes tumor progression [319]. Moreover, individual differences in the senescence of each immune cell type in the TME are of considerable interest. Future research should therefore focus on mechanisms that can promote tumor cell senescence/apoptosis without inducing the onset of cellular inflammation.
Tumor heterogeneity poses a serious challenge to targeted tumor therapy. At present, effective tumor-targeted therapeutic agents based on cellular senescence are lacking. Agents for drug development should exhibit preferential affinity for tumor cells to avoid detrimental side-effects caused by the induction of senescence in normal cells [320]. Importantly, tissues of elderly patients commonly contain a high proportion of senescent cells, and agents targeting senescence may compromise the structural integrity of tissues or disrupt tissue blood circulation by interfering with VECs [321]. Pharmacological therapy against cancer should take into account the temporal and spatial nature of cellular senescence and the effectiveness of combination therapy requires further evaluation. The application of chemotherapeutic agents, followed by those that remove senescent cells, may prevent the development of chronic inflammation in the TME. Moreover, combinations of targeted agents against hypoxia, metabolism, and immunotherapeutic strategies may improve therapeutic efficacy.
Single-cell RNA sequencing and spatial transcriptome analyses have facilitated the study of tumor heterogeneity and spatial specificity. Notably, single-cell sequencing technologies have been successfully employed to clarify the mechanisms linked to cellular senescence and tumor stem cells, tumor immune escape, DDR, and tumor metabolic aberrations. These developments provide the opportunity to establish the spatial and temporal structures of tumors and the TME [322, 323]. Further elucidation of the mechanisms by which different subpopulations of senescent cells accomplish complex interoperability and coordination in time and space may enhance our understanding of the dynamics of senescent TME.
Cellular senescence and metabolic disorders induce dysfunction of specific immune cells and alter the phenotype/function of the remaining immune cells through intercellular communication (e.g. SASP), thereby shaping the immunosuppressive TME and inflammatory environment. Further single-cell sequencing and spatial transcriptomics studies are warranted to explore the phenotypic/functional effects of cellular senescence on different immune cells and their subpopulations in the TME.
In summary, in-depth exploration of the complex correlations and mechanisms of cellular senescence in the TME (including the effects of metabolic reprogramming and hypoxia-mediated crosstalk between immune cell functions and tumor biological behaviors) should facilitate clarification of the impact of cellular senescence on tumor progression and aid in the development of effective therapeutic strategies. In view of this complex crosstalk, further attention should be paid to multifaceted combination therapies targeting cellular senescence, hypoxia, metabolism, and the immune system.
AUTHOR CONTRIBUTIONS
Fusheng Zhang drafted this review and designed the figures; Junchen Guo and Shengmiao Yu completed the data collection and provided editorial assistance; Youwei Zheng, Meiqi Duan, Liang Zhao and Yihan Wang gave some valuable suggestions; Xiaofeng Jiang and Zhi Yang provided the design and revision of the manuscript; Xiaofeng Jiang and Zhi Yang obtain funding supports. All authors made substantial, direct and intellectual contribution to the review. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This study was supported by grants from the Liaoning Nature Science Foundation of China (No. 2022JH2/101300042), The National Natural Science Foundation of China (No. 82173194, 81672877), the Key Research Project of Liaoning Provincial Department of Education of China (No. ZD2020004).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




