Immune mediated support of metastasis: Implication for bone invasion
Zengfeng Xin, Luying Qin, and Yang Tang contributed equally to this work.
Abstract
Bone is a common organ affected by metastasis in various advanced cancers, including lung, breast, prostate, colorectal, and melanoma. Once a patient is diagnosed with bone metastasis, the patient's quality of life and overall survival are significantly reduced owing to a wide range of morbidities and the increasing difficulty of treatment. Many studies have shown that bone metastasis is closely related to bone microenvironment, especially bone immune microenvironment. However, the effects of various immune cells in the bone microenvironment on bone metastasis remain unclear. Here, we described the changes in various immune cells during bone metastasis and discussed their related mechanisms. Osteoblasts, adipocytes, and other non-immune cells closely related to bone metastasis were also included. This review also summarized the existing treatment methods and potential therapeutic targets, and provided insights for future studies of cancer bone metastasis.
List of abbreviations
-
- AKT
-
- protein kinase B
-
- BHLHE22
-
- basic helix loop helix family member e22
-
- BME
-
- bone microenvironment
-
- BMP
-
- bone matrix protein
-
- CAF
-
- cancer-associated fibroblast
-
- CCL
-
- C-C motif ligand
-
- CCR
-
- C-C-motif receptor
-
- CD137L
-
- CD137 ligand
-
- cGAS
-
- cyclic GMP-AMP synthase
-
- CNP/siCCR2
-
- SicCr2-encapsulated cationic nanoparticle
-
- COL1A1
-
- Collagen Type I Alpha 1
-
- COX2-I
-
- cyclooxygenase-2 inhibitor
-
- CpG
-
- Cytosine phosphoric acid Guanine
-
- CST6
-
- cystatin 6
-
- CTL
-
- lymphocyte
-
- CTLA-4
-
- cytotoxic T-lymphocyte-associated antigen 4
-
- CTNND1
-
- catenin delta 1
-
- CTSB
-
- cathepsin B
-
- CXCL
-
- C-X-C motif chemokine ligand
-
- CXCR
-
- C-X-C motif receptor
-
- DC
-
- dendritic cell
-
- DKK1
-
- dickkopf-1
-
- EMT
-
- epithelial-mesenchymal transformation
-
- ER+
-
- estrogen receptor-positive
-
- GM-CSF
-
- granulocyte-macrophage colony stimulating factor
-
- GO
-
- graphene oxide
-
- HIF-1
-
- hypoxia-inducible factor 1
-
- IBSP
-
- integrin-binding sialoproteins
-
- IFN-γ
-
- Interferon γ
-
- IL
-
- interleukin
-
- IL-R
-
- interleukin receptor
-
- JNK
-
- c-Jun N-terminal kinase
-
- LGR4
-
- leucine-rich repeat-containing G protein-coupled receptor 4
-
- LIF
-
- leukaemia inhibitory factor
-
- LRP5
-
- LDL receptor-associated protein 5
-
- MCP-1
-
- monocyte chemoattractant protein-1
-
- M-CSF
-
- macrophage colony-stimulating factor
-
- MDSC
-
- myeloid-derived suppressor cell
-
- MHC
-
- major histocompatibility complex
-
- miRNA
-
- microRNA
-
- MMP
-
- matrix metalloprotease
-
- NET
-
- neutrophils extracellular trap
-
- NK
-
- natural killer
-
- NO
-
- nitric oxide
-
- NOS
-
- nitric oxide synthase
-
- NSCLC
-
- non-small cell lung cancer
-
- ODN
-
- oligodeoxynucleotide
-
- PD-1
-
- programmed cell death protein 1
-
- pDC
-
- derived dendritic cell
-
- PD-L1
-
- programmed death-ligand 1
-
- PGE2
-
- prostaglandin E2
-
- PI3K
-
- phosphoinositide 3-kinase
-
- PR3
-
- protease 3
-
- PRMT5
-
- protein targeting methyltransferase 5
-
- PSA
-
- prostate specific antigen
-
- PTEN
-
- phosphatase and tensin homolog
-
- PTHrP
-
- parathyroid hormone-related protein
-
- RAGE
-
- glycation end products
-
- RANKL
-
- NF-kB ligand
-
- ROS
-
- reactive oxygen species
-
- RSPO2
-
- R reactive protein 2
-
- SCID
-
- severely combined immunodeficient
-
- SCLC
-
- small cell lung cancer
-
- SMAD2
-
- SMAD family member 2
-
- SPHK1
-
- sphingosine kinase 1
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- STING
-
- stimulator of interferon genes
-
- TAM
-
- tumor-associated macrophage
-
- TGF-β
-
- transforming growth factor β
-
- TGFβR1
-
- transforming growth factor β receptor 1
-
- TLR
-
- toll-like receptor
-
- TME
-
- tumor microenvironment
-
- TNF
-
- tumor necrosis factor
-
- TRAIL
-
- tumor necrosis factor-related apoptosis-inducing ligand
-
- Treg
-
- regulatory T cell
1 BACKGROUND
Bone metastasis is a serious complication of advanced cancer and is frequently observed in various types of tumors. Breast and prostate cancers, which are the most prevalent malignancies among women and men, respectively, display a high propensity for bone metastasis [1, 2]. The treatment of bone metastasis is often challenging. In addition to the clinical symptoms of the primary tumor, bone metastasis can cause pathological fractures, bone pain, hypercalcemia, and other complications, significantly reducing quality of life [3, 4].
The occurrence and treatment difficulty of bone metastases are related to the particularities of the bone. The bone microenvironment (BME) is in a state of hypoxia, and the partial pressure of oxygen in bone is significantly lower than that in other tissues and organs [5]. Hypoxia increases the expression of hypoxia-inducible factor 1 (HIF-1) [6]. It has been found that high expression of HIF-1 can promote bone metastasis of tumor cells [6]. Hypoxia and metabolism of tumor cells can easily cause the BME to be in an acidotic state. Acidosis can enhance osteoclast activity, inhibit osteoblast activity, cause bone damage, and provide space for tumor cell growth [7, 8]. The lack of oxygen in the bone is difficult to overcome, and dormant tumor cells can develop resistance to radiotherapy and chemotherapy [9]. These factors complicate the treatment of bone metastasis.
Bone metastasis can be classified into osteolytic, osteogenic, and mixed types based on the bone changes at the metastatic site [10]. It is now understood that the occurrence of bone metastasis involves multiple steps, including the colonization of circulating cancer cells to the bone, the adaptation of tumor cells to the BME in a dormant state, the transformation of tumor cells from a dormant state to a proliferative state, and finally the reconstruction of bone structure and function by tumor cells [11, 12]. Epithelial-mesenchymal transformation (EMT) is an event that tumors must undergo to acquire the ability to metastasize [13]. After tumor cells acquire the characteristics of mesenchymal cells, their migration ability is enhanced, and metastasis is more likely [13]. Interleukin 6 (IL-6), IL-1β, tumor necrosis factor (TNF), and other inflammatory factors in the microenvironment can effectively induce the occurrence of EMT [14]. The recovery and proliferation of tumor cells in BME are closely related to inflammatory factors. The origin of these inflammatory factors is related to the interaction between immune and tumor cells in BME [15, 16]. The occurrence of bone metastasis is closely related to the interaction between tumor cells and BME.
BME contains cellular components such as osteocytes, macrophages, lymphocytes, granulocytes, dendritic cells (DCs), adipocytes, and extracellular matrix [17]. The immune microenvironment of the bone is unique compared to that of other organs. There are many immature immune and immunosuppressive cells in the bone, making it an area of low immune function, and disseminated cancer cells proliferate easily in the bone [18, 19]. Under normal conditions, cytokines released by immune cells in the bone immune microenvironment promote the differentiation of osteoclast precursors into osteoclasts. Osteocytes can also regulate immune cells, and their interaction maintains the dynamic balance of the BME [20]. The presence of tumor cells can increase this balance. Tumor cells secrete parathyroid hormone-related protein (PTHrP), which enhances the differentiation and maturation of osteoclasts and aggravates bone destruction [21]. Some growth factors deposited in the bone, such as transforming growth factor β (TGF-β), are released, which in turn can promote tumor cell growth [21, 22].
Currently, approved therapeutic drugs for bone metastasis include bisphosphonates and monoclonal antibodies [17]. It has been reported that diphosphate can induce apoptosis of osteoclasts [23], and denosumab can target receptor activators of NF-κB ligand (RANKL) to inhibit the formation of bone destruction [24]. Although these treatment methods can improve the quality of life to a certain extent, the survival rate of patients with bone metastases has not significantly improved, and current treatment methods fall short of meeting treatment expectations. Immune checkpoint therapy has been shown to be effective in the treatment of multiple tumors, but it is usually not effective in the treatment of tumors with bone metastases. Many clinical studies have shown that the presence of bone metastases can impair the efficacy of immunotherapy for advanced prostate [25] cancer and non-small cell lung cancer (NSCLC) [26]. This may be related to the unique immune microenvironment of the bone.
Therefore, a comprehensive understanding of the mechanisms governing bone metastasis and targeted therapies aimed at the interaction between various components of BME and tumor cells is important. This article reviewed the mechanisms of action of various immune cells, including macrophages, osteoclasts, DCs, myeloid-derived suppressor cells (MDSCs), granulocytes, lymphocytes, and natural killer (NK) cells, in bone metastasis and discussed the therapeutic strategies for these cells. Considering that some non-immune cells in BME also play an important role in bone metastasis, this paper briefly discussed the role of osteoblasts, osteocytes, and adipocytes in bone metastasis.
2 IMMUNE CELLS AND BONE METASTASIS
2.1 Macrophages and bone metastasis
Macrophages are immune cells that exhibit high heterogeneity and plasticity. The direction of macrophage differentiation is determined by signals in the microenvironment. Generally, macrophages are categorized into M1 (activated by interferon γ [IFN-γ] and lipopolysaccharide) and M2 (activated by cytokines such as IL-4 and IL-13) phenotypes [27]. M1 macrophages secrete pro-inflammatory mediators, such as IL-12, TNF-α, IFN-γ, and nitric oxide synthase (NOS), which are believed to promote inflammation and combat pathogen invasion [28, 29]. In contrast, M2 macrophages secrete anti-inflammatory mediators, including IL-10, IL-13, IL-4, CD206 (the mannose receptor), and vascular endothelial growth factor A (VEGFA), which promote angiogenesis and tissue repair [28, 30].
Macrophages that reside within the tumor microenvironment (TME) are called tumor-associated macrophages (TAMs) and are the main white blood cells in the TME [31]. TAMs are mainly composed of tumor-promoting macrophages similar to M2 [32]. Many studies have suggested that interactions between TAMs and other cells in the TME constitute a complex immune regulatory network. For instance, lactate produced by anaerobic respiration of tumor cells can promote the polarization of TAMs toward an M2-like state [33]. TAMs secrete IL-1β, IL-8, TNF-α, TGF-β, matrix metalloprotease 9 (MMP-9), and MMP-2 to participate in the epithelial-mesenchymal transformation of tumors and promote the occurrence of tumor metastasis [34]. IL-1β secreted by TAMs can induce EMT in tumor cells and promote metastasis of tumor cells, which has been reported in different cancers such as breast [35] and colorectal cancers [36]. Some studies also found that the M1 gene was expressed in TAMs [37, 38]. Therefore, the traditional M1/M2 classification could not describe macrophages in the complex environment of the TME well. The functional classification of TAMs requires a new perspective [15] (Figure 1). Currently, most studies have suggested the promoting role of macrophages in tumor bone metastasis [39].
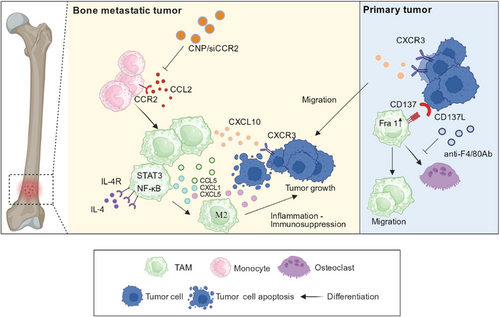
Tumor cells transferred to bone tissue can upregulate C-X-C motif chemokine ligand 10 (CXCL10) expression in macrophages in the BME, which can promote metastasis to bone tissue by binding to C-X-C motif receptor 3 (CXCR3) on tumor cells [40]. Macrophages can not only directly release CXCL10, but also indirectly increase CXCL10 levels by releasing TNF-α. The released TNF-α can be activated by NF-κB signal to promote CXCL10 expression [41]. High levels of CXCL10 can not only recruit tumor cells expressing CXCR3 but also improve the survival rate of tumor cells by enhancing their binding ability to type I collagen, the most abundant extracellular matrix molecule in bone. In a mouse model, neutralizing CXCL10 with antibodies significantly reduces the number and size of bone metastases [40]. Thus, CXCL10 may be a potential therapeutic target for bone metastasis [40] (Figure 1).
C-C motif ligand 2 (CCL2) is a member of the CC chemokine family that enhances monocyte chemotaxis to the site of inflammation [42]. CCL2 is a tumor-derived chemokine [43]. One study found that bone marrow endothelial cells augment the recruitment of prostate cancer cells to bone tissue by secreting high levels of CCL2 [42]. Mechanically, CCL2 binds to C-C-motif receptor 2 (CCR2) expressed on macrophages, inducing macrophage infiltration into the tumor tissue, and further promoting tumor cell proliferation. Thus, targeting the CCL2/CCR2 pathway can decrease macrophage infiltration, thereby reducing metastasis [42, 44]. Shen et al. [43] showed that siCCR2-encapsulated cationic nanoparticle (CNP/siCCR2) blocked the CCL2/CCR2 pathway by downregulating the expression of CCR2 in macrophages (Figure 1). The application of CCL2 antibodies can also be used to treat bone metastasis.
Jiang et al. [45] showed that CD137 is expressed on macrophages, combined with CD137 ligand (CD137L) on tumor cells, leading to the upregulation of Fra1. Fra1 signaling promotes the migration of mononuclear cells into the BME and the differentiation of mononuclear cells into osteoclasts, which is conducive to bone customization of tumor cells and bone metastasis in breast cancer [46]. Notably, a novel F4/80-targeted liposomal nanoparticle encapsulating the anti-CD137 blocking antibody (NP-αCD137 Ab-F4/80) was found to effectively inhibit bone metastasis in breast cancer [45] (Figure 1).
In addition to the macrophage recruitment pathway mentioned above, we focused on the role of M2 polarization in bone metastasis. Immunophenotyping has revealed that macrophages associated with bone metastasis highly express the IL-4 receptor (IL-4R), which aids macrophage differentiation toward the M2 phenotype [47]. Ma et al. [47] reported that the incidence of bone metastasis in breast cancer was reduced after conditional IL-4R knockout in macrophages and concluded that macrophages dependent on the expression of IL-4R promoted the occurrence of bone metastasis. In addition to the regulation of macrophage polarization in the BME, previous studies have shown that tumor cells in the primary foci can regulate the polarization of macrophages toward the M2 type by secreting immunosuppressive cytokines, such as milk fat globule-EGF factor 8 (MFGE8) [48, 49]. This process is mediated by the phagocytosis of macrophages [49]. At the site of bone metastasis, bone macrophages engulf apoptotic tumor cells through phagocytosis, activate intracellular NF-κB and signal transducer and activator of transcription 3 (STAT3) signaling pathways, and secrete large amounts of pro-inflammatory factors, such as CCL5, CXCL1, and CXCL5 [50]. These pro-inflammatory factors in turn promote the polarization of M2. M2 macrophages, together with these pro-inflammatory factors, form a sustained inflammatory immunosuppressive BME and promote the development of bone metastasis [51, 52] (Figure 1).
Considering the opposite effects of M1 and M2 macrophages in the TME, the transformation from the M2 to the M1 phenotype represents a potential approach for treating bone metastases. For example, zoledronic acid, a drug with a high affinity for bone tissue, can act as a tumor killer by shifting the balance from the M2 to M1 phenotype [53]. These findings strongly support zoledronic acid as a therapeutic drug for bone metastasis [53, 54]. A stable nanocomplex (gal-C-dextran + oligonucleotides [GDO]) formed by the combination of galactosylated ionic dextran (gal-C-dextran) and Cytosine phosphoric acid Guanine (CpG) oligodeoxynucleotide (CpG ODN), developed by Huang et al. [55], can also be used as a tool for reprogramming M2 cells into the M1 phenotype. It is now understood that GDO releases ODN under the action of H+ in the TME. When ODN enters TAMs, they can increase the expression of IL-12, reduce the expression of IL-10, and play an antitumor role [55]. Although Huang et al. [55] only demonstrated the role of GDO in a mouse liver tumor model, TAMs also exhibited significant infiltration in the BME. Therefore, the application of GDO for the treatment of bone metastasis is worth investigating.
Most studies suggest the need to reduce M2 in the treatment of tumors [56, 57], however, there are also reports that M2 can be used to promote angiogenesis in the treatment of bone metastasis in tumors [58]. Graphene oxide (GO) nanoparticles, hydrated CePO4 nanorods, and bioactive chitosan (CS) composite scaffolds (CePO4/CS/GO scaffolds) constructed by Ge et al. [58] have been reported to bear multiple aspects of bone metastasis. CePO4 in composite scaffolds can promote M2 polarization and harness the ability of M2 macrophages to promote angiogenesis and osteoblast mineralization to alleviate osteolytic damage caused by bone metastasis in breast cancer. Ce3+ ions also promote bone tissue regeneration. In addition, GO in the CePO4/CS/GO scaffold can kill tumor cells through photothermal effects [58]. This study also proposes new treatment methods targeting macrophage polarization.
In addition to the mechanisms mentioned above, macrophage subtypes are also associated with the dormant state of tumors in bone metastasis [59]. Several studies have shown that breast cancer cells can enter a dormant state after spreading to bone tissue, and tumor cells can survive in a dormant state for a long time [60, 61]. Some studies have found that macrophages of the M2 type in the BME can form intercellular communications with tumor cells and maintain the static state of tumor cells [62, 63]. M1 macrophages can secrete exosomes and activate the NK pathway in tumor cells to reverse tumor dormancy [62]. Injecting M1-type macrophages into mice carrying dormant tumor cells “woke up” the tumor cells and extended the mice's lives [62]. The findings mentioned above altogether highlight the role of macrophages in bone metastasis, and suggest potential therapeutic targets in clinical treatment.
2.2 Osteoclasts and bone metastasis
Osteoclasts are the only cells in the human body that dissolve bone tissue and play an important role in regulating bone homeostasis [64]. Osteoclasts form a closed space on the bone surface, secreting substances such as H+ and tissue protease K, which degrade the mineral and organic components of the bone tissue [65]. Differentiation and maturation of osteoclasts are complex processes. Hematopoietic stem cells first differentiate into macrophages. Mononuclear macrophages subsequently become osteoclast precursors after acquiring positive tartrate-resistant acid phosphatase and calcitonin receptors. Ultimately, the osteoclast precursors fuse to form mature osteoclasts [66]. Macrophage colony-stimulating factor (M-CSF) and RANKL can stimulate osteoclast differentiation and maturation, while osteoprotegerin yields the opposite effects [67, 68]. PTHrP is involved in osteoclast formation and promotes osteoclast differentiation and maturation by increasing RANKL expression and reducing osteoprotegerin expression [69].
An increasing number of studies have indicated that specific environmental components in metastatic organs can affect the occurrence of tumor metastasis, and changes in the bone marrow niche lead to a preference for tumor metastasis to the bone tissue [70-72]. The establishment of a pre-metastatic niche is favorable for bone metastasis, and abnormal bone remodeling caused by an abnormal number and activity of osteoclasts promotes the formation of a pre-metastatic niche [73]. Metastatic tumor cells secrete substances, such as RANKL and PTHrP, which directly or indirectly activate osteoclasts [12]. Activated osteoclasts can destroy bone tissue, release growth factors deposited in the bone matrix, promote tumor cell growth, and form a “vicious cycle” of bone metastasis [66, 74]. The overactivation of osteoclasts is an important mechanism that leads to osteolytic bone metastasis and cancerous bone pain [75].
Tumor cells that metastasize to the bone tissue activate osteoclasts through various pathways. It is widely believed that within the TME of bone metastasis, programmed death-ligand 1 (PD-L1) expressed by tumor cells is upregulated [76]. Upregulation of PD-L1 binding to programmed cell death protein 1(PD-1) on osteoclast precursors can induce RANKL-dependent osteoclast production by activating c-Jun N-terminal kinase (JNK) in osteoclast precursors and promoting the secretion of CCL2, thereby increasing the number of osteoclasts [77]. The CCL2/CCR2 signaling pathway is involved in the pathogenesis of cancerous bone pain [78, 79]. Additionally, immunotherapy blocking the PD-1 pathway inhibits osteoclast formation and alleviates bone pain [76]; CCR2 antagonists also alleviate pain [80]. Yue et al. [81] revealed that in the mouse breast cancer model, tumor cells can promote the formation of bone metastasis by secreting R reactive protein 2 (RSPO2) and RANKL. RSPO2 and RANKL, through interaction with their receptor leucine-rich repeat-containing G protein-coupled receptor 4 (LGR4), activate Gαq, and β-catenin signal promotes the release of dickkopf-1(DKK1) in breast cancer cells. DKK1 can bind to LDL receptor-associated protein 5 (LRP5) on osteoclast precursors, recruiting osteoclast precursors by inhibiting the Wnt signaling pathway and upregulating Rnasek expression. The application of anti-DKK1 antibodies and soluble LGR4 extracellular domain proteins in mice has been shown to effectively retard bone metastasis and reduce the occurrence of osteolytic bone metastasis, indicating that the RSPO2/RANKL-LGR4 axis may be targeted to reduce bone metastasis [81]. Recombinant cystatin 6 (CST6) is a protease inhibitor that can inhibit the activity of cysteine proteases, such as cathepsin B (CTSB) [82]. A previous study suggested that CST6 can stabilize sphingosine kinase 1 (SPHK1) by inhibiting the activity of CTSB, whereas SPHK1 inhibits osteoclast formation through p38. Current evidence suggests that CST6 expression is significantly reduced in tumor cells [83, 84]. Therefore, CST6-like polypeptides can affect the generation and differentiation of osteoclasts by influencing the CST6-CTSB-SPHK1 axis and represent candidate drugs for the treatment of bone metastasis [85] (Figure 2).
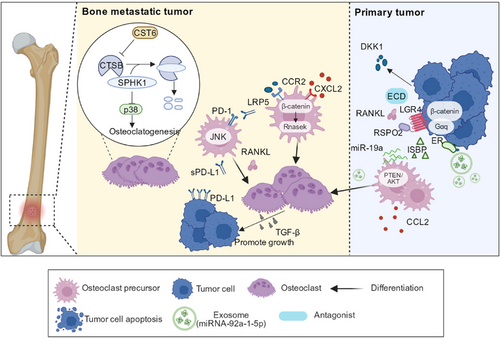
Additionally, tumor cells can affect osteoclasts via exosome secretion. Exosomes are small membrane vesicles that range from 30 to 150 nm in size and are secreted by cells through exocytosis. Their contents include proteins, mRNAs, and microRNAs (miRNAs) [86]. There is consensus that estrogen receptor-positive (ER+) breast cancer has a higher tendency for bone metastasis [87]. Studies have shown that ER+ breast cancer cells secrete high levels of integrin-binding sialoprotein (IBSP) and miR-19a [88, 89]. IBSP recruits osteoclast precursors, and miR-19a promotes osteoclast differentiation and maturation through phosphatase and tensin homolog/protein kinase B (PTEN/AKT) [89]. The effects of IBSP and miR-19a on osteoclasts provide an optimal BME for bone metastasis because activated osteoclasts can absorb bone tissue and provide space for tumor growth [89]. Moreover, the bone destruction caused by osteoclasts further accelerates the release of embedded bone matrix growth factors, such as TGF- β, which can stimulate the growth of tumor cells [90]. Wu et al. [89] identified chlorogenic acid as an inhibitor of the ISBP that can prevent bone metastasis to a certain extent. In addition to metastatic cells, osteogenic tumors produce exosomes that promote the differentiation and maturation of osteoblasts. The highest content of miRNA-92a-1-5p in exosomes secreted by prostate tumor cells directly targets Collagen Type I Alpha 1 (COL1A1), promoting osteoclast differentiation while also causing the degradation of type I collagen and inhibiting osteogenesis [74] (Figure 2). In general, understanding the underlying interplay between the action of extracellular vesicles and bone metastasis can help explore new methods for the targeted treatment of bone metastasis to a great extent.
Admittedly, the influences of tumor cells and osteoclasts are mutual. In addition to the osteoclast activation by tumor cells, osteoclasts promote tumor development. For example, after osteoclasts cause osteolysis, growth factors such as insulin-like growth factors, bone morphogenetic proteins, and TGF-β are released from the bone matrix [11]. These growth factors can promote the growth of tumor cells that metastasize to the bone tissue. TGF-β is the growth factor with the highest content in the bone matrix, and the classic TGF-β signaling pathway can activate SMAD family member 2 (SMAD2) and SMAD3 in tumor cells [91]. SMAD2 and SMAD3 form complexes with transcriptional coactivators or co-inhibitors in the nucleus, regulate gene expression in tumor cells, and promote tumor cell growth [91]. TGF-β has been proved to play an important role in the formation of bone metastasis in breast cancer, prostate cancer, melanoma, and other tumors [92, 93]. Therefore, blocking TGF is a good method to prevent bone metastasis. Previous studies have shown that the application of the small-molecule inhibitor SD208 to the transforming growth factor β receptor 1 (TGFβR1) kinase in a mouse model of bone metastasis reduced the occurrence of osteolytic bone metastasis and improved the survival rate of mice [91, 94, 95] (Figure 2).
In addition to osteolytic lesions, osteoclasts play an important role in osteogenic bone metastasis. Prostate cancer-associated bone metastasis, which is typically osteogenic, is characterized by osteoblast activation [96]. Previous studies have observed that osteoblasts are distributed around prostate cancer cells that metastasize to bone tissue [11, 97, 98]. Prostate tumor cells secrete factors such as VEGF, endothelin-1, and plasminogen activator urokinase to promote the differentiation and maturation of osteoblasts [99]. New bone formation can also be observed at the bone metastasis site of prostate cancer; however, it forms loose woven bone with lower strength than normal layered bone, making patients prone to pathological fractures [99, 100]. Another reason for pathological fractures is that prostate cancer with bone metastasis undergoes resorption while forming new bone. Tumor cells that metastasize to the bone tissue first enter a dormant state, and osteoclasts can activate dormant tumor cells [101]. For example, vascular cell adhesion molecule 1 secreted by breast cancer cells can combine with integrins to recruit osteoclast precursors [102]. Osteoclasts in multiple myeloma “shut down” the dormant state of tumor cells by altering the ecological niche of the endometrium [101].
Dormant tumor cells are resistant to conventional treatment methods [103]. Thus, inhibiting osteoclast differentiation could be a solution to eliminate dormant tumor cells. The use of bisphosphonates and denosumab, which directly inhibit bone resorption, reduced the number of osteoclasts and tumor volume in a mouse model of bone metastasis [23, 24]. Considering the particularities of the skeletal environment, bone-targeted nanomedicine delivery systems have great potential for development. Nanocarriers coated with hyaluronic acid/alendronate on zeolitic imidazolate framework-8 containing NF-κB inhibitor were found to promote monocytes to differentiate into macrophages with tumor-killing effect instead of osteoclasts [104].
Overall, osteoclasts play a promoting role in both osteolytic and osteogenic bone metastases. The mechanism by which it functions can activate dormant tumor cells or may be an important member of the “vicious cycle” model [12]. The presence of osteoclasts can provide a suitable growth environment for tumors, and the targeted treatment of osteoclasts has great potential in the treatment of bone metastases.
2.3 DCs and bone metastasis
DCs are antigen-presenting cells (APCs) that bridge the innate and adaptive immunity. When DCs are stimulated and activated, chemokine receptors (such as CCR7), adhesion molecules, costimulatory molecules (CD54, CD80, and CD86), and major histocompatibility complex class I (MHC I) and MHC II molecules are upregulated, leading to a decrease in phagocytic ability and an increased ability to process and present antigens, affecting the occurrence of specific and non-specific immunity [105, 106]. Multiple studies have shown that DCs located in the TME impair antigen cross-presentation, which may account for antitumor immune failure [107].
To date, limited research has been conducted on the role of DCs in bone metastasis. Multiple myeloma, a bone-destructive disease, may provide insights into the connection between DCs and bone metastasis. The maturation of DCs is of great significance for their immune function, and immature DCs have been shown to cause bone destruction in multiple myeloma [108, 109]. Cytokines and adhesion factors in myeloma TME inhibit DCs maturation. These immature DCs can undergo osteoclast-like transformation through the activation of the RANK-RANKL and CD47 platelet reactive protein-I axis receptor agonist-activated receptor, which exacerbates bone destruction, suggesting a potential role for DCs in bone metastasis [109].
Based on the antigen presentation function of DCs, their use as vaccines is a feasible strategy to treat cancer. Clinical trials have shown that antigen-loaded DCs have a favorable effect on antitumor responses as cancer vaccines [110, 111]. In the phase I clinical trial of Fuessel et al. [112], a peptide cocktail-loaded DCS vaccine was administered to eight patients with hormone-refractory prostate cancer, and four out of eight patients showed a decrease in serum prostate-specific antigen (PSA). However, simple administration of DC vaccines rarely has clinical effects, and the injected DCs may quickly decompose or be inhibited by the TME [113]. Therefore, DC vaccines combined with other therapies may hold tremendous potential for the treatment of bone metastasis. Strontium-89 radiation has been used to treat bone metastases because of its ability to kill cancer directly and promote antigen presentation [114]. Fragments of tumor cells killed by radiotherapy may serve as specific antigens for uptake by DCs, which present antigens to T cells and initiate tumorigenic effects. Therefore, the combination of Sr radiotherapy and DC vaccines can effectively combat bone metastasis [115]. In this regard, De Silva et al. [116] found that the combination of h11c, IFN-γ, and cyclooxygenase-2 inhibitor (COX2-I) could effectively inhibit bone metastasis in SCC. Among these, h11c is a new synthetic lipopeptide with a toll-like receptor 2 (TLR2) ligand and DC-targeting peptide (ATPEDNGRSFS). This DC-based combination therapy may be applied to prevent bone metastasis [116, 117]. The GVAX® vaccine, which involves granulocyte-macrophage colony-stimulating factor (GM-CSF) gene-transduced irradiated prostate cancer vaccine cells, contains DCs loaded with antigens from tumor bone metastases. DCs play a crucial role in presenting antigens and initiating immune responses against bone metastases in prostate cancer [118] (Figure 3).
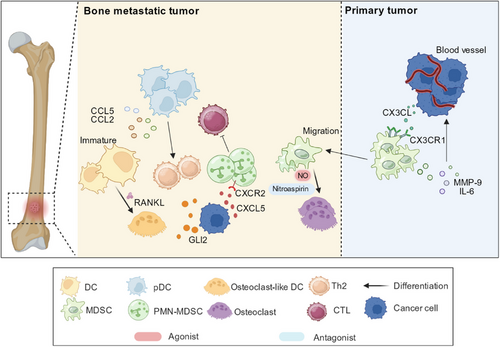
Plasma-derived DCs (pDCs) can produce IFN-α and theoretically have anti-tumor activity. Further research has found that the role of pDCs is closely related to tumor background. For example, they can exert anti-tumor effects in melanoma and basal cell carcinoma [119, 120], while in leukemia, tumor-like amplification occurs, leading to impaired secretion of IFN-α [121]. pDCs are associated with the promotion of bone metastasis in breast cancer. After breast cancer spreads to bone, it causes macrophage infiltration, produces inflammatory response, and triggers a pDC response [122]. The expression levels of cytokines related to macrophage growth and differentiation are elevated, such as CCL5 [123], monocyte chemoattractant protein-1 (MCP-1) [124], etc. After depletion of pDCs, the expression levels of these pro-inflammatory factors significantly decreased [122]. In addition, pDCs undergo macrophage-dependent aggregation, which triggers a pro-inflammatory response characterized by Th2 cells and exacerbates osteolysis [122, 125]. In the mouse model of bone metastasis of breast cancer used in the study of Sawant et al. [122], it was found that after depletion of pDCs, the activity of CD8+ T cells in the BME increased, and bone metastasis in mice was significantly reduced (Figure 3). Consequently, pDCs depletion in the BME represents a potential method for treating bone metastasis in breast cancer.
DCs, which are immune cells that play important roles in both specific and nonspecific immunity, are closely related to their type and activity in bone metastasis. DCs can affect BME by influencing osteoclasts and other immune cells. The combination of DC vaccines and other treatment methods may be a research direction for targeted treatment of bone metastases.
2.4 MDSCs and bone metastasis
Under normal conditions, immature bone marrow cells differentiate into granulocytes and macrophages. In pathological conditions, such as cancer, trauma, and chronic inflammation, immature bone marrow cell differentiation is partially blocked, resulting in MDSCs [126]. MDSCs are subdivided into two main groups: granulocytic/polymorphonuclear cells (G-MDSCs/PMN-MDSCs) and monocytic cells (M-MDSCs). The former is similar to granulocytes in terms of morphology and function, whereas the latter are similar to monocytes [127]. Many studies have corroborated that MDSCs have a significant inhibitory effect on both innate and adaptive immunity [128-130]. MDSCs inhibit the proliferation and activity of effector T cells, promote the activation of Tregs, and secrete cytokines that promote angiogenesis [131]. It has been revealed that MDSCs aggregate in both primary and metastatic tumors [132]. Furthermore, MDSCs promote bone metastasis in both an immune- and non-immune-dependent manner.
MDSCs are key factors in the formation of the ecological niche of the bone before metastasis. Pre-transfer ecological niche theory divides the formation of pre-transfer ecological niches into three important steps. Initially, the primary tumor releases cytokines to the metastatic organ, followed by the modulation of stromal cells within the metastatic site. Subsequently, hematopoietic stem cells are recruited and differentiated into MDSCs, which exert immunosuppressive effects [133]. In contrast, bone marrow-derived cells in BME do not require stromal cell modulation. Thus, the concept of a bone ecological niche before metastasis has been proposed, using MDSCs as a vital component [133, 134].
MDSCs are responsible for enhanced cell migration and immune escape at primary tumor sites [135]. MDSCs are recruited to the TME via CX3CL-CX3CR1 interaction between tumor cells and MDSCs. In hypoxic environments, the expression of CX3CL in tumor cells. CX3CL specifically binds to CX3CR1 on MDSCs, causing them to infiltrate the tumor site [136]. MDSCs also secrete MMP-9, IL-6, and other cytokines to induce EMT of tumor cells, which is associated with angiogenesis and metastasis [137, 138] (Figure 3).
MDSCs also promote tumor growth at the site of bone metastasis. CXCL5/CXCR2 signaling is considered important for PMN-MDSC recruitment [139]. Research has indicated higher levels of CXCL5 expression in bone metastases of prostate cancer, leading to increased PMN-MDSC infiltration in bone metastatic foci compared to primary lesions [139]. PMN-MDSCs have been identified as the main MDSC subgroup in prostate cancer and are responsible for immune evasion and bone metastasis by effectively inhibiting T cells [140]. Some studies have suggested that MDSCs from bone metastases can differentiate into osteoclasts under the action of nitric oxide (NO), causing bone damage and facilitating osteolytic bone metastasis [141, 142] (Figure 3). Notably, although a large number of MDSCs can be observed in primary tumors, only MDSCs isolated from bone metastatic foci can differentiate into osteoclasts, suggesting that interactions between tumor cells and MDSCs in the BME may account for the differentiation of MDSCs [141].
In summary, our review of MDSCs suggested possible avenues for the treatment of bone metastases. Actually, it has been reported that nitroaspirin can inhibit the inducible NOS in MDSCs, thereby inhibiting the differentiation of MDSCs into osteoclasts and reducing the osteolytic damage of bone metastases [143]. Gemcitabine, a chemotherapeutic agent, effectively reduces the number of MDSCs, thereby alleviating immunosuppression [143]. Therefore, gemcitabine could reduce bone damage and serve as a potential drug for the treatment of bone metastasis [143, 144].
2.5 Neutrophils and bone metastasis
Neutrophils are the most abundant type of white blood cells, are closely related to innate immunity, and are considered the first line of defense against viruses [145]. Neutrophils protect the body by releasing antimicrobial peptides, enzymes, reactive oxygen species (ROS), and neutrophils extracellular traps (NETs) to eliminate pathogens [146, 147]. Neutrophils are important hubs in the body's immune network because they can recruit other immune cells by secreting various cytokines and chemokines [148]. Neutrophils associated with tumors can also be classified into two categories: N1 with anti-tumor effects and N2 with tumor growth-promoting effects [149]. Differentiation between N1 and N2 depends on the environment. IFN-β promotes the differentiation and maturation of N1 [150], while TGF-β supports N2 [151]. The role of neutrophils in tumors is complicated and may vary in different types or stages of tumors.
Tumor cells recruit neutrophils by secreting various signaling molecules. For example, tumor cells enhance neutrophil infiltration by releasing IL-8 (a neutrophil chemokine), which is beneficial for metastasis [152]. Previous studies have found a positive correlation between IL-8 expression and the metastasis of various tumor cells, such as breast cancer and melanoma [152, 153]. Neutrophils can reduce the adhesion between tumor cells and the ECM by releasing matrix metalloproteinases and ROS, reshaping the tumor ECM, and providing a favorable environment for tumor cell metastasis [154, 155]. Through high-throughput sequencing, Lin et al. [156] found that catenin delta 1 (CTNND1) was significantly downregulated in bone metastasis tissues of breast cancer, and tumor cells with low CTNND1 expression could accelerate neutrophil infiltration by upregulating GM-CSF and IL-8. Recruited neutrophils can inhibit cytotoxic T-cells, providing a suitable growth environment for tumor cells [156] (Figure 4). Blocking the infiltration of neutrophils at the tumor site directly by blocking IL-8 is a good treatment method.
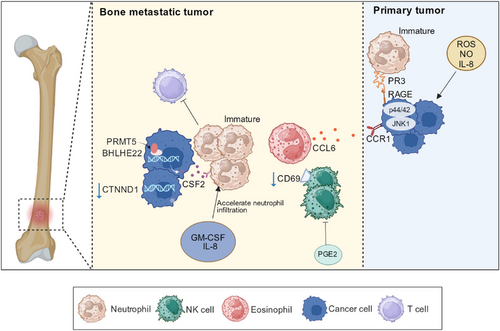
Neutrophils are generally believed to promote tumor metastasis. Several studies have shown that immature neutrophils promote bone metastasis. Neutrophils expressing protease 3 (PR3) can induce prostate cancer cell migration to BME. PR3 is secreted by myeloid cells and distributed on the surface of neutrophils; it targets cytoplasmic antibodies in neutrophils. High expression of the receptor for advanced glycation end products (RAGE) in malignant prostate cancer cells. PR3 and RAGE combine to activate the p44/42 and JNK1 pathways in prostate cancer cells, promote cancer cells to move to the BME, and induce bone metastasis [157]. Therefore, bone metastasis can be inhibited by blocking the binding of RAGE to its corresponding ligands [158]. Immature neutrophils and monocytes in BME can cause T cell failure, induce immunosuppression, and increase the difficulty of immunotherapy for bone metastasis. A study found that after prostate cancer bone metastasis, the expression of basic helix loop helix family member e22 (BHLHE22) increased in the BME, and the transcription complex formed by BHLHE22 and protein targeting methyltransferase 5 (PRMT5) increased the expression level of colony-stimulating factor 2 (CSF2), and high levels of CSF2 increased the number of immunosuppressive neutrophils [159] (Figure 4). Based on this mechanism, targeted inhibition of PRMT5 and CSF2 could be used to treat bone metastasis [159].
In addition, neutrophils are associated with the activation of dormant tumor cells. Elastase and MMP-9 in neutrophil NET can cleave mucosal proteins, which can activate integrins to transform tumor cells from dormant to proliferative states [160]. In an experiment by Albrengues et al. [160], persistent chronic inflammation in the lungs induced neutrophil NETs to transform dormant cancer cells into invasive lung metastases. Neutrophils may play a similar role at bone metastasis sites.
Although neutrophils are believed to be associated with the promotion of tumor metastasis, they still hold a place in the field of antitumor activity. A previous study suggested that neutrophils can directly induce tumor cell apoptosis by producing tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [161]. Another study found that neutrophils exert antitumor effects by inhibiting STAT5 in the early stages of prostate cancer bone metastasis [162]. However, during tumor development, neutrophil maturity is reduced through unknown mechanisms, resulting in decreased cytotoxicity [162].
Multiple studies have shown that the ratio of neutrophils to lymphocytes can serve as a biomarker for bone metastasis, predicting the occurrence of bone metastasis, early detection and diagnosis, and improving prognosis [163, 164]. Therefore, reducing the polarization of neutrophils toward N2 by blocking TGF-β directly inhibits the secretion of tumor-promoting factors by neutrophils [148, 151].
2.6 Eosinophils and bone metastasis
Eosinophils are granulocytes containing large eosinophilic particles rich in cationic granule proteins that are believed to play an important role in parasitic infections and allergic reactions [165, 166]. Recent studies have revealed their involvement in tumor progression, making them a part of the TME. Eosinophil infiltration has been observed in many tumors, and the interaction between eosinophils and lymphocytes is crucial for shaping the TME [167, 168].
Previous studies have indicated an association between eosinophils and tumor metastasis. IL-5, a cytokine promoting eosinophil differentiation and activation, has been found to promote lung metastasis by recruiting eosinophils [169, 170]. It has been established that Cd3 δ- Il-5 transgenic (Il-5Tg) mice contain high levels of eosinophils. In an experiment conducted by Li et al. [171], mice with high eosinophil levels (Il-5Tg mice) were found to be more prone to bone metastasis than wild-type mice. After excluding the influence of stromal cells, they inferred that eosinophil proliferation was crucial for increased bone metastasis in Il-5 transgenic mice. Eosinophils secrete CCL6, which binds to CCR1 on tumor cells, attracting them and promoting bone metastasis [171] (Figure 4).
2.7 Basophils and bone metastasis
Basophils, a type of granulocyte, are typically found in the blood in very low quantities, usually accounting for less than 1%. However, research on basophils and their roles in cancer is limited. It is widely believed that their abundance significantly increases in the late stage of chronic myeloid leukemia, where they promote tumor development [172]. Some studies have associated basophils with solid tumors, such as colorectal cancer and lung metastasis of breast cancer. The serum basophil count has been linked to cancer prognosis, inversely correlating with the incidence and mortality of colorectal cancer [173] and the lung metastasis rate of breast cancer [174]. However, other studies have suggested that basophils can play an antitumor role in melanoma, where they secrete CCL3 and CCL4, and recruit CD8+ T cells to kill tumor cells [175]. Therefore, the role of basophils in bone metastasis warrants further study.
2.8 T cell and bone metastasis
T cells are crucial immune cells in the human immune system and different T cell subsets play different roles in bone metastasis. CD8+ T cells, also known as cytotoxic T lymphocytes (CTLs), have cytotoxic potential through the production of perforin, granzyme, and IFN-γ [176]. CD4+ T cells exhibit high heterogeneity and include Th1, Th2, Th17, and others, secreting various cytokines and serving auxiliary regulatory functions. Regulatory T cells (Tregs) are an important subgroup of CD4+ T cells with significant immunosuppressive effects. Tregs have been found to be highly infiltrated in various tumors, inhibiting the proliferation and the activity of CD8+ T cells through direct contact inhibition by secretion of TGF-β [177] and IL-10 [178]. The versatility of T cells dictates their diverse roles in bone metastasis. Tregs and Th17 cells are pro-metastatic [179], whereas Th1, Th2, and CD8+ T cells [180, 181] are anti-metastatic by suppressing osteoclast formation during bone metastasis. Escaping immune surveillance is a prerequisite for tumor cells to undergo bone metastasis, and the immunosuppressive environment at the bone metastasis site supports tumor growth.
Before breast cancer metastasis, T cells regulate bone tissue prior to tumor cells, forming a pre-metastatic ecological niche. In 4T1 breast cancer, after tumor-specific CD4+ T cells recognize the antigen of the primary tumor, they activate osteoclasts to cause bone loss by producing RANKL, which provides favorable conditions for bone metastasis [182]. Another study suggested that the CD8+ T cell phenotype derived from 67NR tumors could interfere with osteoclast activity in the distant bone marrow [183]. 67NR non-metastatic tumor cells, a sibling of the 4T1 tumor cell line, regulate bone homeostasis through specific CD8+ T cells. These CD8+ T cells further inhibit bone resorption, thereby preventing bone metastasis by producing cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and IFN-γ. Therefore, they are potential biomarkers of bone metastasis [183].
In addition to forming a premetastatic ecological niche, T cells affect the activity of osteoclasts at the site of bone metastasis. For instance, Th17 cells activate osteoclasts by secreting RANKL, which indirectly promotes osteolytic bone metastasis [184]. Immature osteoclasts can, in turn, stimulate the proliferation of Th17 cells, leading to excessive IL-17 production and osteoclast formation [109] (Figure 5). Therefore, inhibiting Th17 cells at the site of bone metastasis is beneficial for the treatment of bone metastasis.
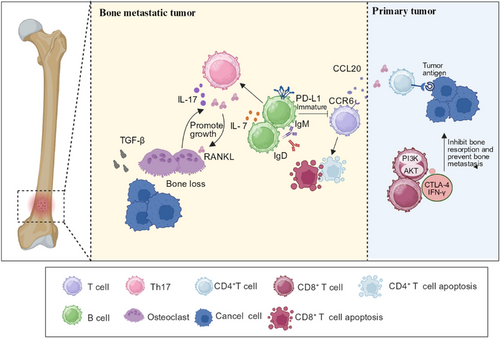
T cells create an immunosuppressive microenvironment at the site of bone metastasis. In the context of prostate cancer bone metastasis, the overexpression of CCR6 in T cells and CCL20 overexpression in TAMs and tumor inflammatory monocytes have been observed [185]. CCL20 binds to CCR6, leading to depletion and dysfunction of infiltrating T cells. Disruption of the CCL20/CCR6 axes can relieve T cell exhaustion and extend survival [185]. NSCLC is particularly prone to bone metastasis in advanced stages, with the spine being more susceptible [186, 187]. High expression of Siglec15 has been observed in the metastatic foci of NSCLC. During in vitro T cell killing tests, it was discovered that reducing Siglec15 expression could enhance the cytotoxicity and proliferative capacity of CD8+ T cells by downregulating genes associated with the cell cycle and cellular senescence. This also upregulated the phosphoinositide 3-kinase -AKT (PI3K-AKT) signaling pathway and increased the expression of IFN-γ in T cells (Figure 5). Notably, elevated expression of Siglec15 aids in the establishment of immunosuppressive BME in bone metastases [188, 189]. Consequently, targeting Siglec15 may serve as a novel strategy for treating breast cancer metastasis [188, 189].
PD-1 and PD-L1 immunotherapies have demonstrated strong therapeutic effects against different tumor types [190]. In a study by Zuo et al. [191], mice with a PD-L1 deletion in the bone marrow lineage exhibited inhibited bone metastasis of breast cancer and melanoma. The deficiency of PD-L1 in bone marrow reduces osteoclast development. Moreover, it significantly increased the total number of T cells, including CTLs and helper T cells, resulting in enhanced antitumor immunity. However, this phenomenon was not observed in mice with PD-L1 deficiency across the entire hematopoietic lineage. Thus, blocking PD-L1 in bone marrow cells may be a potent strategy for treating bone metastasis by inhibiting osteoclast formation and strengthening antitumor immunity [191] (Figure 5). In addition, immune checkpoint inhibitors can be used in combination with other anticancer drugs. Luminal B breast cancer shows a poor response to individual anti-PD-1 antibody [192]. Mehdi et al. [193] found that the combination of S-adenosylmethionine and an anti-PD-1 antibody increased the infiltration of CD8+ T cells, increased the cytotoxicity of CD8+ T cells, and reduced the area of diseased bone in a mouse tumor model. The therapeutic effect of the combination therapy was superior to that of individual therapy.
T cells play multiple roles in bone metastasis of tumors. T cells can affect the pre-metastatic niche, shape the immunosuppressive microenvironment, and indirectly affect bone metastasis by influencing osteoclasts. Therefore, targeted T cell therapy is highly feasible. Attempts have been made to block the corresponding signaling pathways. Current immunotherapies have proven effective in the treatment of multiple tumors [194, 195], and the combination of immunotherapy and other methods provides new treatment ideas for bone metastases that are ineffective with immunotherapy.
2.9 B cell and bone metastasis
B cells play important roles in both humoral and cellular immunity and directly or indirectly participate in tumor metastasis by secreting various antibodies and cytokines and exerting antigen presentation effects [196]. Heterogeneous B cells have been documented in the TME, including naïve B cells, memory B cells, and plasma cells [197]. Based on existing research, it is uncertain whether B cells promote or inhibit bone metastasis [198, 199].
B cells may orchestrate bone metastasis by interacting with T cells in the BME. In the context of advanced melanoma bone metastasis, Wu et al. [190] found that regulatory B cells, overexpressing PD-L1, are enriched in the metastatic focus. Generally, B cells with high PD-L1 expression presented higher IgM and IgD expressions, indicating an immature state. These immature B cells inhibit T cells via PD-L1, thereby creating an immunosuppressive environment [190]. B cells also play a role in promoting osteolytic bone metastasis by modulating the production of IL-7. Research has shown that levels of IL-7 produced by B cells in the BME are abnormally elevated in patients with osteolytic bone metastasis. The increase in IL-7 further activates T cells, leading to the production of RANKL and TNF-α, which induce osteolysis through cytokine signaling [200] (Figure 5). Therefore, attenuating the effects of B cells by blocking PD-L1 and IL-7 may be an effective therapeutic strategy.
Immunotherapy is likely to be an effective way to prevent and treat tumor metastasis in the future; however, currently, more research is focused on T cells. As an important member of the immune surveillance system, further research is required to determine the role of B cells.
2.10 NK cells and bone metastasis
NK cells are the main effector cells of natural immunity with cytotoxic effects similar to CTLs, exhibiting high heterogeneity in the TME [201]. As an important component of tumor immune detection, the decreased activity of NK cells has been found to be related to tumor susceptibility and metastasis [202]. NK cells kill tumor cells directly; however, they are also affected by tumor cells, which may trigger NK cell dysfunction. For example, prostaglandin E2 (PGE2) significantly inhibited NK cells in the TME. NK cells gradually become anergic or even immunosuppressive; thus, tumor cells can metastasize via immune escape [203, 204] (Figure 4). Notably, some studies have suggested an inhibitory role of NK cells in bone metastasis [205, 206]. The depletion of NK cells in severely combined immunodeficient (SCID) mice has been used to establish bone metastasis models for experimental research. This construction process is straightforward, yielding a high success rate, and the clinical characteristics of this mouse model closely resemble human small cell lung cancer (SCLC). It is a valuable tool for testing the anti-bone metastatic effects of various therapeutic drugs [207-209]. A study using this model effectively demonstrated that PTHrP promoted bone metastasis in SCLC but was not associated with visceral metastasis [207].
NK cells are modulated by many cytokines, such as type I IFN, which are indispensable for the development and maturation of NK cells. Loss of type I IFN signaling is believed to promote bone metastasis by impairing NK cell function [210]. In a breast cancer mouse model lacking type I IFN signaling, NK cell abundance and CD69 (a marker of NK cell activation) sharply decreased, contributing to a weakened NK cell-killing effect on breast cancer cells. Conversely, injecting activated NK cells to rescue these mice reduced the likelihood of bone metastasis [210]. The underlying mechanism for this outcome may be that depletion of type I IFN undermines antigen cross-presentation by NK cells [211]; however, further research is warranted to validate this mechanism (Figure 4). Therefore, restoring the function of NK cells is a challenge that must be overcome for the treatment of bone metastasis.
3 NON-IMMUNE CELLS AND BONE METASTASIS
3.1 Adipocytes and bone metastasis
Adipocytes and osteoblasts are derived from bone marrow-derived stem cells [212]. The number of adipocytes in the BME increases with age and changes in the disease status [213]. Many studies have shown that adipocytes can promote tumor growth and invasion [214, 215]. Studies have also shown that obesity is positively correlated with a high-fat diet, the growth of bone metastases, and bone destruction [216, 217]. Although adipocytes do not belong to the immune microenvironment of bone, they can affect the occurrence and development of bone metastasis by influencing various immune cells in the immune microenvironment of bone. Therefore, studying adipocytes in bone is also important.
Adipocytes promote osteoclast formation and enhance bone destruction during bone metastases. Using a high-fat diet-induced obese mouse model, Hardaway et al. [218] found that with an increase in the number of adipocytes in the bone marrow, the expression levels of CXCL1 and CXCL2 increased. CXCL1 and CXCL2 accelerate the differentiation of osteoclasts by binding to the receptor CXCR2, enhancing the function of osteoclasts, and contributing to the osteolysis of metastatic prostate cancer [218]. Adipocytes contribute to the immune escape from tumors. In a study by Sato et al. [219] on bone metastasis, the density of CD8+ T cells in adipose-rich bone marrow was significantly lower than that in bone marrow rich in hematopoietic stem cells in the control group. Fatty bone marrow resists immunotherapy. In addition, their study also found that adipocytes can differentiate into cancer-associated fibroblasts (CAFs), which have a strong ability to induce tumor invasion and rebuild the extracellular matrix [219] (Figure 6A).
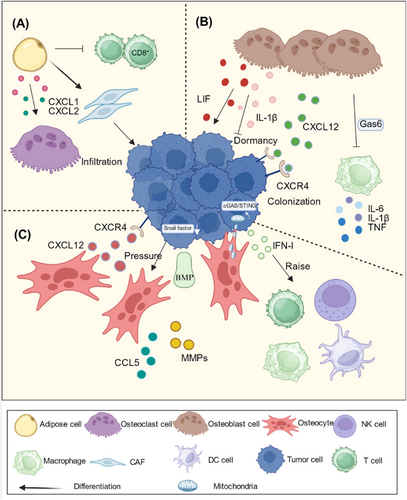
In addition to affecting the immune microenvironment, adipocytes and osteoblasts can secrete a variety of inflammatory cytokines, growth factors, chemokines, and other cytokines that directly act on tumor cells to enhance their proliferation and invasion abilities [220, 221]. Drugs that target adipocytes also have clinical significance for the treatment of bone metastasis.
3.2 Osteoblasts and bone metastasis
Osteoblasts secrete bone matrix proteins (BMP) after maturity and become osteocytes after bone formation. Osteocytes are the most abundant cells in the bone [222]. Previously, it was believed that osteoblasts activated osteoclasts mainly by secreting RANKL and M-CSF, which play indirect roles in bone metastasis [223]. Now, an increasing number of studies show that osteoblasts play an important role in the homing and dormancy of tumor cells [224].
Osteoblasts secrete CXCL12, which binds to CXCR4 on tumor cells to induce them to colonize bone [103, 225]. The dormancy of tumor cells is also related to the cytokines secreted by osteoblasts. The leukemia inhibitory factor (LIF) secreted by osteoblasts can induce the expression of dormancy-related genes in tumor cells and directly promote the dormancy of tumor cells [226]. Gas6, produced by osteoblasts, has a similar effect [227]. Osteoblasts also produce IL-1β, which can break the dormancy of tumor cells [228]. Cytokines produced by osteoblasts play a dual role in the dormant state of tumors [229] (Figure 6B).
In addition to their ability to interact directly with tumor cells, osteoblasts can influence the role of immune cells in BME. Gas6 secreted by osteoblasts can inhibit the production of IL-6, IL-1β, TNF, and other pro-inflammatory factors by macrophages [229]. Gas6 is a hub that connects inflammatory networks with tumor dormancy [230]. Osteoblasts can also be influenced by macrophages and play an important role in osteogenic bone metastasis. Wu et al. [231] found that reducing CD169+ macrophages in mouse models can reduce the formation of pathological woven bone in prostate cancer bone metastasis.
The mechanisms by which osteoblasts play a role in bone metastasis are diverse. The method of targeting osteoblasts to treat bone metastasis is worth trying. More research is needed in the future to make the mechanisms by which osteoblasts work more thorough.
3.3 Osteocytes and bone metastasis
Osteocytes are the most abundant cells in bone and develop from osteoblasts. As osteocytes mature, they form a strong network of dendrites that touch each other and can interact with other cells [232]. Simultaneously, the osteocytes sense physical changes in the environment and transmit mechanical stimulation signals [233]. The role of osteocytes as important regulators of bone metabolic balance in bone metastasis remains unclear.
In the early stages of bone metastasis, osteocytes produce CXCL12, which binds to CXCR4 on tumor cells and promotes the homing of tumor cells to bone [234, 235]. Osteocytes help tumor cells colonize the bone [235]. Liu et al. [236] found that osteocytes interact with tumor cells and that the BMP secreted by osteocytes can downregulate snail expression and promote the colonization of tumor cells by reversing EMT. In a mouse model of tibial prostate cancer, the researchers found that osteocytes respond to the physical forces generated by tumor cell growth by secreting CCL5 and MMPs to enhance the invasive ability of tumor cells and promote tumor cell growth [51]. However, some studies have shown that osteocytes can inhibit bone metastasis. Zhou et al. [237] found that osteocytes can transfer mitochondria to tumor cells, triggering cyclic GMP-AMP synthase/ stimulator of interferon genes (cGAS/STING) signaling, the upregulation of which can cause tumor cells to continue to secrete cytokines such as IFN-I, NK cells, DCs, macrophages, and T cells were recruited to enhance antitumor immunity [237] (Figure 6C). The mechanism of osteocyte action in bone metastasis deserves more research.
4 CONCLUSIONS
Overall, bone metastasis, the malignant progression of tumors, causes significant suffering in patients. Current treatments for bone metastasis do not fully meet the societal needs. BME is an essential participant in bone metastasis, with cellular components interacting with tumor cells through various growth factors. Understanding the mechanisms through which BME promotes or inhibits bone metastasis is of great significance. This review summarized the roles of immune cells, including macrophages, osteoclasts, DCs, MDSCs, granulocytes, lymphocytes, and NK cells, in the occurrence and development of bone metastasis. These cells alter the bone immune microenvironment through different mechanisms that promote or inhibit bone metastasis. They can also alter the pre-metastatic niche of the bone, affect bone remodeling by influencing osteoclasts, secrete various cytokines that affect tumor cell growth, and interact with each other through different signaling pathways. Non-immune osteoblasts, osteocytes, and adipocytes also play important roles in bone metastasis. They not only directly affect tumor cells through the secretion of growth factors and chemokines, but also indirectly affect tumor growth by affecting immune cells. In addition, this paper discussed targeted therapies for different immune cells. There are currently multiple clinical trials evaluating the role of different targeted drugs in the treatment of bone metastases (Table 1). Treatments targeting the specific mechanisms by which various cell types play a role can provide new ideas for preventing the occurrence of bone metastasis or reducing the harm caused by bone metastasis.
| Matrix, Cell | Target | Drug | Mechanism of drugs | Identifier |
|---|---|---|---|---|
| Osteoclast | RANKL | Denosumab | Inhibiting osteoclast activation | NCT02051218 |
| c-Src kinase | Tirbanibulin | NCT01074138 | ||
| FPPS | Bisphosphonates | NCT00104650 | ||
| miR-34a | MRX34 | Inhibiting osteoclast differentiation | NCT01829971 | |
| c-Met, VEGFR | Cabozantinib | NCT01599793 | ||
| mTOR |
Everolimus Temsirolimus |
NCT00466102 NCT01016015 |
||
| M-CSF | MCS110 | Inhibiting osteoclast differentiation and maturation | NCT00757757 | |
| ULK1 | Trametinib | NCT03878524 | ||
| RON | ASLAN002 | Suppressing osteolytic bone metastasis | NCT01721148 | |
| Cathepsin K | Odanacatib | NCT00399802 | ||
| PTHrP | CAL | Inhibiting osteoclast proliferation | NCT00051779 | |
| TOP-2 | Doxorubicin | Inhibiting osteoclast survival | NCT02306161 | |
| Osteoblast | Sclerostin | Romosozumab | Promoting osteoblast survival and proliferation | NCT04232657 |
| DKK-1 | BHQ880 | NCT01302886 | ||
| Bone matrix | TGF-β | Galinusertib | Promoting osseous tumor cell growth | NCT02452008 |
| Calcium |
Radium-223 Strontium-89 |
Inhibiting bone resorption by osteoclast, Inducing bone formation by osteoblast |
NCT03304418 NCT00002503 |
|
| c-Met, HGFR | Cabozantinib |
Inhibiting osteoclast differentiation, decreasing skeletal tumor burden and osteoblastic lesion formation |
NCT01599793 | |
| MDSC | GM-CSF | Regramostim | Inhibiting MDSC infiltration | NCT02499835 |
| CCR2, CXCR2 | MLN1202 | NCT01015560 |
- Abbreviations: CCR2/CXCR2, C-C-Motif Receptor 2/C-X-C motif receptor 2; c-Met, cellular-mesenchymal to epithelial transition factor; c-Src kinase, cellular proto-oncogene tyrosine-protein kinase Src; DKK-1, dickkopf 1; FPPS, farnesyl pyrophosphate synthase; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGFR, hepatocyte growth factor receptor; M-CSF, macrophage colony-stimulating factor; miR-34a, microRNA-34a; mTOR, mammalian target of rapamycin; NCT, clinicalTrials.gov identifier (ID) number; PTHrP, parathyroid hormone-related protein; RANKL, receptor activator of NF-κB ligand; RON, recepteur d'origine Nantais; TGF-β, transforming growth factors β; TOP-2, topoisomerase 2; ULK-1, UNC-51-like Kinase 1; VEGFR, vascular endothelial growth factor receptor.
AUTHOR CONTRIBUTIONS
Ting Zhang: Conceptualization; methodology; writing—review and editing. Zengfeng Xin: Writing—original draft preparation. Luying Qin: Writing—original draft preparation. Yang Tang: Writing—original draft preparation. Siyu Guo: Investigation. Fangfang Li: Investigation. Yuan Fang: Investigation. Gege Li: Investigation. Yihan Yao: Investigation. Binbin Zheng: Writing—review and editing. Bicheng Zhang: Writing—review and editing. Dang Wu: Writing—review and editing. Jie Xiao: Methodology; writing—review and editing. Chao Ni: Methodology; writing—review and editing. Qichun Wei: Methodology; writing—review and editing. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank Prof. Weixu Li and Yan Li from Department of Orthopedic Surgery, Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University for supporting our study. This work was supported by the grant from the National Natural Science Foundation of China (82173089, 82073332, and 82073142), Natural Science Foundation of Zhejiang Province (LY21H100004 and LY19H160050), Medical Science and Technology Project of Zhejiang Province (2021RC063), and Chinese Society of Clinical Oncology (Y-2020Sciclone/ms-0099 and Y-2019Sciclone-019).
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




