“Find Me” and “Eat Me” signals: tools to drive phagocytic processes for modulating antitumor immunity
Abstract
Phagocytosis, a vital defense mechanism, involves the recognition and elimination of foreign substances by cells. Phagocytes, such as neutrophils and macrophages, rapidly respond to invaders; macrophages are especially important in later stages of the immune response. They detect “find me” signals to locate apoptotic cells and migrate toward them. Apoptotic cells then send “eat me” signals that are recognized by phagocytes via specific receptors. “Find me” and “eat me” signals can be strategically harnessed to modulate antitumor immunity in support of cancer therapy. These signals, such as calreticulin and phosphatidylserine, mediate potent pro-phagocytic effects, thereby promoting the engulfment of dying cells or their remnants by macrophages, neutrophils, and dendritic cells and inducing tumor cell death. This review summarizes the phagocytic “find me” and “eat me” signals, including their concepts, signaling mechanisms, involved ligands, and functions. Furthermore, we delineate the relationships between “find me” and “eat me” signaling molecules and tumors, especially the roles of these molecules in tumor initiation, progression, diagnosis, and patient prognosis. The interplay of these signals with tumor biology is elucidated, and specific approaches to modulate “find me” and “eat me” signals and enhance antitumor immunity are explored. Additionally, novel therapeutic strategies that combine “find me” and “eat me” signals to better bridge innate and adaptive immunity in the treatment of cancer patients are discussed.
List of abbreviations
-
- A2AR
-
- Adenosine A2A receptor
-
- ABC
-
- ATP-binding cassette
-
- ABCA1
-
- ATP-binding cassette transporter A1
-
- ACKR2
-
- Atypical chemokine receptor 2
-
- ADCC
-
- Antibody-dependent cell-mediated cytotoxicity
-
- ADP
-
- Adenosine diphosphate
-
- AMP
-
- Adenosine monophosphate
-
- APMAP
-
- Adipocyte plasma membrane-associated protein
-
- αVβ3/5
-
- Integrin alpha V beta 3/alpha V beta 5
-
- ATP
-
- Adenosine triphosphate
-
- ATP8A
-
- ATPase phospholipid transporting 8A
-
- B7-H4
-
- B7 homolog 4
-
- BAI1
-
- Brain-specific angiogenesis inhibitor 1
-
- BAP31
-
- B-cell receptor-associated protein 31
-
- Bax
-
- Bcl-2-associated X protein
-
- Bak
-
- Bcl-2 homologous antagonist/killer
-
- BiTNs
-
- Bispecific tumour-transforming nanoparticles
-
- BLNK
-
- B cell linker protein
-
- Btk
-
- Bruton tyrosine kinase
-
- C3bi
-
- inactivated complement-3b
-
- CAR
-
- Chimeric antigen receptor
-
- CALR
-
- Calreticulin
-
- CCL1
-
- C-C motif chemokine ligand 1
-
- CCR2
-
- C-C motif chemokine receptor 2
-
- CED-8
-
- Cell death abnormality-8
-
- cGAMP
-
- Cyclic GMP-AMP
-
- cGAS
-
- Cyclic GMP-AMP Synthase
-
- CR3
-
- Complement receptor 3
-
- CSF-1R
-
- Colony-stimulating factor 1 receptor
-
- CSK
-
- C-terminal Src kinase
-
- CX3CL1
-
- C-X3-C motif chemokine ligand 1
-
- CX3CR1
-
- CX3C chemokine receptor 1
-
- DAMPs
-
- Damage-associated molecular patterns
-
- DAP12
-
- DNAX-activating protein of 12 kDa
-
- DLBCL
-
- Diffuse large B-cell lymphoma
-
- DOCK180
-
- Dedicator of cytokinesis protein 1
-
- E2F1
-
- E2F transcription factor 1
-
- EAT-2
-
- EWS/FLI1 activated transcript 2
-
- eATP
-
- Extracellular ATP
-
- EGFR
-
- Epidermal growth factor receptor
-
- eIF2α
-
- Eukaryotic translation initiation factor 2 subunit alpha
-
- ELMO
-
- Engulfment and cell motility protein
-
- ERK
-
- Extracellular signal-regulated kinase
-
- ERp57
-
- Endoplasmic reticulum protein 57
-
- FAK
-
- Focal adhesion kinase
-
- Fc
-
- Fragment crystallizable
-
- Fgl2
-
- Fibrinogen-like protein 2
-
- G2A
-
- G-protein coupled receptor 2A
-
- GAS6
-
- Growth arrest-specific protein 6
-
- GPCR
-
- G-protein-coupled receptor
-
- GPR4
-
- G-protein coupled receptor 4
-
- HDL
-
- High-density lipoprotein
-
- HER2
-
- Human epidermal growth factor receptor 2
-
- HSP70
-
- Heat shock protein 70
-
- HSP90
-
- Heat shock protein 90
-
- IFNγ
-
- Interferon gamma
-
- IgM
-
- Immunoglobulin M
-
- IGRT
-
- Image-guided radiation therapy
-
- IL4R
-
- Interleukin 4 receptor
-
- PLA2
-
- phospholipase A2
-
- IMRT
-
- Intensity-modulated radiation therapy
-
- Itk
-
- Interleukin-2-inducible T-cell kinase
-
- KRAS
-
- Kirsten rat sarcoma viral oncogene homolog
-
- LDL
-
- Low-density lipoprotein
-
- LILRB1/2/4
-
- Leukocyte immunoglobulin-like receptor subfamily B Member 1/2/4
-
- LOX-1
-
- Lectin-like oxidized low-density lipoprotein receptor 1
-
- LPA
-
- Lysophosphatidic acid
-
- LPC
-
- Lysophosphatidylcholine
-
- LRP1
-
- Low-density lipoprotein receptor-related protein 1
-
- LTB-4
-
- Leukotriene B4
-
- Mac-1
-
- Macrophage antigen complex 1
-
- MDSCs
-
- Myeloid-derived suppressor cells
-
- MFG-E8
-
- Milk fat globule epidermal growth factor 8
-
- Mfsd2b
-
- Major facilitator superfamily domain-containing 2b
-
- MGL1/2
-
- Macrophage galactose-type lectin 1/2
-
- MHC
-
- Major histocompatibility complex
-
- MIP1α
-
- Macrophage inflammatory protein 1 alpha
-
- MM
-
- Multiple myeloma
-
- MPL
-
- Myeloproliferative leukemia protein
-
- mTOR
-
- Mammalian target of rapamycin
-
- NSG
-
- Non-obese diabetic (NOD)-scid IL2rγnull
-
- ORRs
-
- Overall response rates
-
- OVs
-
- Oncolytic viruses
-
- P2X/P2Y
-
- Purinergic receptors
-
- PAF
-
- Platelet-activating factor
-
- PANX1
-
- Pannexin-1
-
- PAT-2
-
- Phosphatidylserine aminophospholipid translocase 2
-
- PC
-
- Phosphatidylcholine
-
- PD-1
-
- Programmed cell death protein 1
-
- PE
-
- Phosphatidylethanolamine
-
- PERK
-
- Protein kinase R (PKR)-like endoplasmic reticulum kinase
-
- PI3K
-
- Phosphoinositide 3-kinase
-
- PIP2
-
- Phosphatidylinositol 4,5-bisphosphate
-
- PKC
-
- Protein kinase C
-
- PLCγ
-
- Phospholipase C gamma
-
- PILRα
-
- Paired immunoglobulin-like type 2 receptor alpha
-
- PPARγ
-
- Peroxisome proliferator-activated receptor gamma
-
- PRRs
-
- Pattern recognition receptors
-
- PS
-
- Phosphatidylserine
-
- PSD
-
- Phosphatidylserine decarboxylase
-
- PSR
-
- Phosphatidylserine receptor
-
- PSS1
-
- Phosphatidylserine synthase 1
-
- RAGE
-
- Receptor for advanced glycation end products
-
- ROS
-
- Reactive oxygen species
-
- RP S19
-
- Ribosomal protein S19
-
- S1P
-
- Sphingosine-1-phosphate
-
- S1PR
-
- Sphingosine-1-phosphate receptor
-
- SAP
-
- SLAM-associated protein
-
- SFRs
-
- SLAM family receptors
-
- SHP-1
-
- Src homology region 2 domain-containing phosphatase-1
-
- Siglec-10
-
- Sialic acid-binding immunoglobulin-like lectins 10
-
- SLAMF7
-
- Signaling lymphocytic activation molecule family member 7
-
- SLP-76
-
- SH2 domain containing leukocyte phosphoprotein of 76-kDa
-
- SNARE
-
- Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
-
- SPHK1/2
-
- Sphingosine kinase 1/2
-
- Src
-
- Src kinase
-
- STC1
-
- Stanniocalcin-1
-
- Syk
-
- Spleen tyrosine kinase
-
- TAM
-
- Tyro3/Axl/Mer
-
- TAMs
-
- Tumor-associated macrophages
-
- TAT-1
-
- Transbilayer amphipath transporter-1
-
- Tec
-
- Tec kinase
-
- TG2
-
- Transglutaminase 2
-
- TIM
-
- T cell immunoglobulin and mucin domain
-
- TLR
-
- Toll-like receptor
-
- TME
-
- Tumor microenvironment
-
- TMEM16F
-
- Transmembrane protein 16F
-
- Treg
-
- Regulatory T cell
-
- TyrRS
-
- Tyrosyl-tRNA synthetase
-
- UTP
-
- Uridine triphosphate
-
- VAV1
-
- Vav guanine nucleotide exchange factor 1
-
- VCAM-1
-
- Vascular cell adhesion molecule 1
-
- VISTA44
-
- V-domain Ig suppressor of T cell activation
-
- VTCN1
-
- V-Set domain containing T-cell activation inhibitor 1
-
- WSP-1
-
- Wiskott-aldrich syndrome protein 1
-
- Xk
-
- Kell blood group complex subunit-related family
-
- Xkr8
-
- Xk-related protein 8
-
- XRCC4
-
- X-Ray repair cross-complementing 4
-
- ZO-1
-
- Zonula occludens-1
1 BACKGROUND
Phagocytes, including mononuclear phagocytes and neutrophils [1-3], comprise two major cell types that play a significant role in immune responses by binding and engulfing dying cells, thereby enhancing or suppressing inflammation (Table 1). Phagocytes execute their functions through three primary steps: “find me”, “eat me”, and “digest me” [4-6].
| Cell type | Subtype | Identification basis | Receptors | Functions | Impact in cancer immunotherapy | References | |
|---|---|---|---|---|---|---|---|
| Mononuclear phagocyte system (MPS) | Monocytes | Classical monocyte | CD14hi CD16− | FcR-I, C3b, CD14, CCR2, CD16, CX3CR1, CCR2, SIRPα, TREM1, MARCO, CLEVER1, LILRB2, LILRB4, LAIR1, FcγR-IIa, FcγR-IIb, TLRs, MAC-1, Siglec-10 | Host's pro-inflammatory defense mechanisms | Regulation of tumor-Associated macrophage polarization and function | [7-17] |
| Intermediate monocyte | CD14hi CD16+ | Attuned toward antigen presentation | |||||
| non-Classical monocyte | CD14−/low CD16+ | Vascular patrolling and surveillance | |||||
| Dendritic Cells | Conventional DC 1 (cDC1) | XCR1hiCD172low | FcγR-IIa, FcγR-IIb, SIRPa, TREM1, TREM2, MR, DC-SIGN, SR, DEC-205, TLRs, CLR, FPR, FcR, HK, CD86, CD80, MHC-I, HVEM, CD40, PD-L1/2, MHC-II, IL12, FcγR-IIa | Cross-priming |
(1) Antigen presentation, (2) Activation of T cell responses against tumors |
[14, 15] | |
| Conventional DC 1 (cDC2) | XCR1lowCD172hi | CD4+ T cell priming | |||||
| MoDCs | CD11c+, Ly6C+, CD103 | Inflammation | |||||
| Plasmacytoid DC (pDC) | 120G8+, B220+, CD11c+, LY6C+, CD11b− |
(1) Type I interferons (2) Antigen presentation (3) T cell priming |
|||||
| Macrophages | M1 (classically activated macrophage) | CD11b+, F4/80+, CD86+ | FcR-II, and -III, SIRPa, TREM1, TREM2, Siglec-10, MARCO, CLEVER1, LILRB2, LILRB4, LAIR1, Siglec-15, CLR, FcγR-IIa, FcγR-IIb, CR1/3/4, CD300b, BAI1, TIM4, TYRO3, AXL, MER, αVβ3/αVβ5, Stabilin2, LILRB1, MARCO, TREM2, Siglec-7/9, CD169, CD163, CD206, CD86, Clever 1, PSGL1, PD-1, TLRs, LOX-1, G2A, S1PR1-5, Siglec-10, CD200R, MGL1, MGL2, CD163, CD206, IL4R, CD81, VCAM-1, MHC-II |
(1) Secrete pro-inflammatory cytokines and chemokines (2) Actively present antigens (3) Participate in positive immune responses (4) Serve immune surveillance functions |
Aid cancer immunotherapy by promoting inflammation and antigen presentation, | [7, 9, 12, 14, 15, 17, 18–23-25–39] | |
| M2 (alternatively activated macrophage) | CD11b+, F4/80+, CD206+ |
(1) Have weaker antigen presentation capabilities (2) Secrete inhibitory cytokines such as IL-10 or TGF-β (3) Play important roles in immune regulation (4) Downregulate immune responses |
Hinder treatment by fostering immunosuppression and angiogenesis | ||||
| Neutrophils | Pre-neutrophils) |
Mouse: LCS− cKitint Ly6C+CD11b+ Ly6GloCXCR2− CXCR4hi Human: CD15+CD66b+CD11b+CD49dintCD101− |
FcR-I, -II, and -III, complement receptors (i.e., receptors for C5a and C3b) and C3bi, C3b, PAF, LTB-4, SIRPa, TREM1, TREM2, FcγR-IIIb, FcγR-IIa, CD200R, LILRB2, PILRα, VISTA, PDL1, CD86, 4-1BBL, FcγR, FcαR-I, OX40L, MAC-1, IL-8R, CD300ld, CXCR1, CXCR2, ACKR2, P2Y1, P2Y2, TLRs |
(1) High proliferation, low motility, low effector functions (2) Expand in BM and spleen during emergency granulopoiesis |
(1) Promoting tumor progression through angiogenesis, matrix remodeling, metastasis, and immunosuppression (2) Participating in T-cell-mediated anti-tumor responses and directly killing tumor cells. |
[12, 14, 15, 18, 24, 28, 40–51] | |
| Immature neutrophils) |
Mouse: LCS− cKitlo Ly6CloCD11b+ Ly6GintCXCR2− CXCR4lo Human: CD15+CD66b+CD11b+CD49d−CD101+CD16intCD10− |
Intermediate proliferation, motility and effector functions | |||||
| Mature neutrophils |
Mouse: LCS− cKit− Ly6CloCD11b+ Ly6GhiCXCR2+ CXCR4− Human: CD15+CD66b+CD11b+CD49d−CD101+CD16hiCD10+ |
Low proliferation, high motility, high effector functions | |||||
- Abbreviations: 4-1BBL, 4-1BB ligand; ACKR2, atypical chemokine receptor 2; C3b, complement component 3b; C5a, complement component 5a; CCR2, C-C motif chemokine receptor 2; CD14, cluster of differentiation 14; CD16, cluster of differentiation 16; CD163, cluster of differentiation 163; CD200R, cluster of differentiation 200 receptor; CD206, cluster of differentiation 206; CD300ld, cluster of differentiation 300ld; CD81, cluster of differentiation 81; CLEVER1, common lymphatic endothelial and vascular endothelial receptor 1; CX3CR1, CX3C chemokine receptor 1; CXCR1, C-X-C motif chemokine receptor 1; CXCR2, C-X-C motif chemokine receptor 2; FcR-I, Fc receptor I; FcR-I, -II, and -III, Fc receptors I, II, and III; FcαRI, Fc alpha receptor I; FcγR, Fc gamma receptor; FcγR-IIa, Fc fragment of IgG receptor IIa; FcγR-IIb, Fc fragment of IgG receptor IIb; IL-4R, interleukin-4 receptor; IL-8R, interleukin-8 receptor; LAIR1, leukocyte-associated immunoglobulin-like receptor 1; LILRB2, leukocyte immunoglobulin-like receptor subfamily B member 2; LILRB4, leukocyte immunoglobulin-like receptor subfamily B member 4; LTB-4, leukotriene B4; MAC-1, macrophage-1 antigen; MARCO, macrophage receptor with collagenous structure; MGL1, macrophage galactose-type lectin 1; MGL2, macrophage galactose-type lectin 2; OX40L, OX40 ligand; P2Y1, purinergic receptor P2Y1; P2Y2, purinergic receptor P2Y2; PAF, platelet-activating factor; PILRα, paired immunoglobulin-like type 2 receptor alpha; Siglec-10, sialic acid-binding immunoglobulin-like lectins 10; SIRPα, signal regulatory protein alpha; TLRs, Toll-like receptors; TREM1, triggering receptor expressed on myeloid cells 1; VCAM-1, vascular cell adhesion molecule 1; VISTA44, V-domain Ig suppressor of T cell activation.
The “find me” signals recognized by phagocytes include nucleotides such as adenosine triphosphate (ATP) and uridine triphosphate (UTP), membrane lipids like phosphatidylserine (PS), chemokines such as C-X3-C motif chemokine ligand 1 (CX3CL1) [4], polyamines [52], and others. These signals not only attract phagocytes but also prepare them for action, such as increasing the expression of phagocytic receptors and factors involved in digestive processes. On the other hand, the “eat me” signals recognized by phagocytes include calreticulin (CALR), signaling lymphocytic activation molecule family member 7 (SLAMF7), Fc receptors (FcRs), and PS. Once phagocytes migrate to the vicinity of apoptotic cells, their surface receptors recognize and bind these “eat me” signals, enabling phagocytosis.
The recognition of “find me” and “eat me” signals by phagocytes is crucial for efficient phagocytic activity, enabling timely signaling for effective clearance of apoptotic cells by phagocytes. “Find me” signals attract immune cells to the location of apoptotic cells, while “eat me” signals tag apoptotic cells for phagocytosis and clearance, facilitating cell clearance and tissue repair. The “find me” and “eat me” signals have immune regulatory functions; these signals can enhance the phagocytic activity of macrophages and mediate anti-inflammatory effects by regulating the production of cytokines, which can mediate macrophage phagocytosis of tumors and provide ideas for cancer treatment [53]. Therefore, a comprehensive understanding of the mechanisms by which phagocytes recognize “eat me” and “find me” signals from apoptotic cells has practical significance for cancer therapy. This review summarizes the roles of “eat me” and “find me” signals in tumor development and explores how modulating these signals, in combination with other therapeutic approaches, can enhance antitumor immune responses.
2 “FIND ME” AND “EAT ME” SIGNALS WITH THE ABILITY TO SUPPORT PHAGOCYTIC FUNCTION
2.1 “Find me” signals
“Find me” signals are chemoattractants that guide phagocytes to sites of cell death. We briefly describe the concept of “find me” signaling molecules, the reasons for signal release, the relevant ligands, and their functions (Figures 1, 2, Table 2).
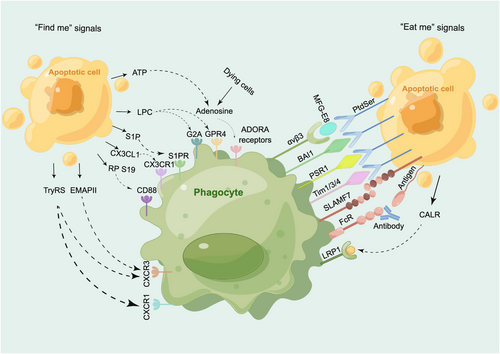
“Find me” and “eat me” signals and their receptors. The primary “find me” signals, including ATP, LPC, S1P, CX3CL1, RP S19, LPC, TryRS, and EMAPII, interact with receptors such as adenosine, G2A/GPR4, S1PR, CX3CR1, CD88, CXCR3, and CXCR1. Similarly, the main “eat me” signals, consisting of CALR, SLAMF7, Fc, and PtdSer, engage with receptors such as LRP1, SLAMF7, FcR, αVβ3, and BAI1/PSR1/TIM1/3/4. ADORA receptors, adenosine A receptors; ATP, adenosine triphosphate; BAI1, brain-specific angiogenesis inhibitor 1; CALR, calreticulin; CX3CL1, C-X3-C motif chemokine ligand 1; CX3CR1, CX3C chemokine receptor1; CXCR1, CXC-chemokinereceptor1; CXCR3, CX3C chemokine receptor 1; EMAPII, endothelial monocyte activating polypeptide II; G2A, G-protein coupled receptor 2A; GPR4, G-protein coupled receptor 4; LPC, lysophosphatidylcholine; LRP1, low-density lipoprotein receptor-related protein 1; MFG-E8, milk fat globule-epidermal growth factor 8; PSR1, phosphatidylserine receptor 1; PtdSer, phosphatidylserine; RP S19, ribosomal protein S19; S1P, Sphingosine-1-phosphate; S1PR, sphingosine-1-phosphate receptor; SLAMF7, signaling lymphocytic activation molecule family member 7; TIM1/3/4, T cell immunoglobulin and mucin domain 1/3/4; TryRS, tyrosyl tRNA synthetase; αVβ3, integrin alpha V beta 3.
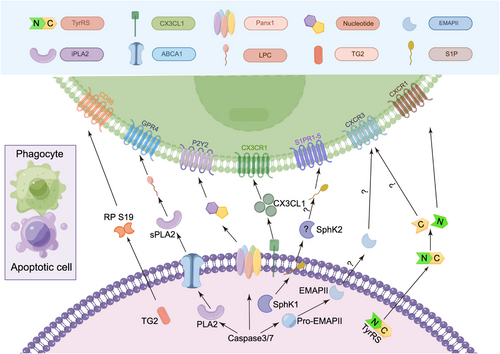
“Find me” signals. The different “find me” signals released from apoptotic cells, their known or putative mechanisms of release, and the possible receptors on phagocytes that can regulate chemotaxis. Apoptotic cells release “find me” signals, such as nucleotides, LPC, S1P, CX3CL1, RP S19, TryRS, and EMAPII, to the extracellular space; these signals can interact with P2Y2, GPR4, S1PR1-5, CD88, CXCR1/CXCR3, and CXCR3 on macrophages, respectively. Pannexin channels, activated by caspase-3/7 during apoptosis, release “find me” signals like nucleotides. The released ATP induces phagocyte migration via P2Y2 receptors. ABCA1-transported PLA2 transforms into sPLA2, hydrolyzing phospholipids to generate LPC. LPC binds GPR4, inducing macrophage migration. TG2 acts as a chemoattractant for macrophages by cross-linking RP S19 monomers. CD88 senses RP S19, mediating monocyte migration. CX3CL1's release mechanism is unclear, but its chemotactic effect on phagocyte relies on CX3CR1. Post-proteolysis, EMAPI and TyrRS exhibit chemotactic properties. EMAPI may result from caspase-7 cleavage, while elastase from neutrophils generates TyrRS. TyrRS stimulates phagocyte migration through CXCR1 and CXCR3. EMAPII promotes endothelial progenitor cell migration via CXCR3, which is unrelated to apoptotic cell clearance. Intracellular S1P, synthesized by SphKs, is released through Mfsd2b. The extracellular SphK1 levels remain stable, indicating that mainly intracellular S1P is produced. During apoptosis, SphK2 can be secreted; this mechanism explains why extracellular S1P production primarily depends on SphK2. Released S1P can bind to S1PR1-5. ABCA1, ATP-binding cassette transporter A1; CD88, cluster of differentiation 88; CX3CL1, C-X3-C motif chemokine ligand 1; CX3CR1, CX3C chemokine receptor 1; CXCR1, C-X-C motif chemokine receptor 1; CXCR3, C-X-C motif chemokine receptor 3; EMAPII, endothelial monocyte-activating polypeptide II; GPR4, G-protein coupled receptor 4; iPLA2, independent phospholipase A2; LPC, lysophosphatidylcholine; P2Y2, P2Y purinoceptor 2; PANX1, pannexin-1; PLA2, phospholipase A2; RP S19, ribosomal protein S19; S1P, sphingosine-1-phosphate; S1PR1-5, sphingosine-1-phosphate receptors 1-5; SphK1, sphingosine kinase 1; sPLA2, secretory phospholipase A2; TG2, transglutaminase 2; TyrRS, tyrosyl-tRNA synthetase.
| “Find me” signal | Major dependencies for release/exposure | Receptors | Downstream biological effects | References |
|---|---|---|---|---|
| LPC | Caspase3 | G2A | Mediates migration of macrophages to apoptotic cells | [16, 54, 55] |
| S1P | SphK | GPCR | Inhibits apoptosis, induces cell proliferation and/or migration and increases drug resistance via inhibition of BAX-caspase-3 signaling, induces survival autophagy and/or inhibition of serine/threonine-protein phosphatase 2A (PP2A) | [17, 56] |
| Nucleotides (ATP, UTP) | PANX1 | P2Y2, P2RX7 | Amplifies chemotactic signals and directs cell orientation via feedback | [25, 57–59] |
| CX3CL1 | Caspase and Bcl-2 | CX3CR1 | The CX3CL1-CX3CR1 axis activates G proteins and the MAPK and AKT signaling pathways involved in tumor biology, and it plays an antitumor role by recruiting immune cells to control tumor growth; however, it can also stimulate a pro-tumor response | [26, 58, 60, 61] |
| RP S19 | Unknown | C5aR (CD88) | L131DR, I134AGQVAAAN, and K143KH in the RP S19 C-terminus contribute to C5aR binding, plasma membrane penetration, and interactions with molecules like delta lactoferrin or annexin A3, respectively, to activate the p38 MAPK pathway in macrophages | [18, 27] |
| TryRS | Unknown | CXCR3 | Unknown | [62, 63] |
| EMAPII | Caspase-7 | CXCR1, CXCR3 | Unknown | [64, 65] |
- Abbreviations: ATP, adenosine triphosphate; Bcl-2, B-cell lymphoma 2; C5aR (CD88), C5a receptor (Cluster of Differentiation 88); CX3CL1, C-X3-C motif chemokine ligand 1; CX3CR1, CX3C chemokine receptor 1; CXCR1, C-X-C motif chemokine receptor 1; CXCR3, C-X-C motif chemokine receptor 3; EMAPII, endothelial monocyte-activating polypeptide II; G2A, G-protein coupled receptor 2A; GPCR, G protein-coupled receptor; LPC, lysophosphatidylcholine; P2RX7, P2X purinoceptor 7; P2Y2, P2Y purinoceptor 2; PANX1, pannexin-1; RP S19, ribosomal protein S19; S1P, sphingosine-1-phosphate; SphK, sphingosine kinase; TryRS, tyrosyl-tRNA synthetase; UTP, uridine triphosphate.
2.1.1 Lysophosphatidylcholine (LPC)
One of the first identified recruitment signals for the phagocytosis of apoptotic cells is LPC [66], which is a major phospholipid component of oxidized low-density lipoprotein (LDL) [67]. LPC has been described as a surface target for natural immunoglobulin M (IgM) antibodies and may function as a dual “find me” and “eat me” signal [68]. LPC release from apoptotic cells may involve caspase-3-mediated phospholipase A2 (PLA2) activation. PLA2 is an enzyme that hydrolyzes SN-2 acyl groups of phospholipids, releasing lysophospholipids and polyunsaturated fatty acids, which promote monocyte migration [54, 69]. Elevated PLA2 levels during apoptosis lead to more LPC release. IgM antibodies recognize LPC, triggering complement activation, which attracts phagocytes to apoptotic cells [70, 71]. ATP-binding cassette transporter A1 (ABCA1) dysfunction causes cholesterol buildup in macrophages, promoting the formation of foam cells. Knockout of ABCA1 reduces macrophage chemotaxis toward apoptotic cells, but the effect of decreased LPC levels in apoptotic cell supernatants remains uncertain [72]. Notably, G protein-coupled receptor 4 (GPR4) and G2 accumulation protein (G2A) are receptors associated with the recognition of LPC by phagocytes, and G2A exhibits higher affinity than GPR4 [55]. Reducing G2A expression may decrease the ability of phagocytes to migrate toward apoptotic cells [66].
2.1.2 Nucleotides (ATP and UTP)
Another group of “find me” signals that play crucial roles in various biological processes are nucleotides, such as ATP and UTP [73]. Nucleotides play vital roles in fundamental biological processes such as genetics, development, and growth and influence activities such as cell migration, chemotaxis, cytokine release, maturation, and cytotoxicity [74].
Nucleotides can be released from cells through passive leakage and active secretion [75]. Passive release occurs from dying and damaged cells [76, 77], while active release relies on pathways such as exocytosis of secretory vesicles derived from the outer embryonic layer [78], plasma membrane-derived microvesicles [78], ATP-binding cassette (ABC) transporters [79, 80], and others. Cystic fibrosis transmembrane conductance regulator (CFTR) has also been considered to participate in an ATP release pathway [81], but a subsequent study has failed to confirm this finding [82]. Currently, five channel families have been proven to mediate the physiological and pathological release of ATP, namely, connexin hemichannels [78], pannexin-1 (PANX1) [78], calcium homeostasis modulator 1 [83], volume-regulated anion channels [84], and maxi-anion channels [85]. In addition, the purinergic P2×7 receptor (P2×7R), an ATP-gated ion channel, also plays a role in ATP release [86]. However, nucleotide release is primarily dependent on the PANX1 channel, a complex structure that spans the cell membrane and opens during cell apoptosis, leading to nucleotide release and promoting phagocyte chemotaxis to enable the phagocytosis and digestion of apoptotic cells [57]. Early apoptotic cells release less than 2% of the total cellular ATP through PANX1, while the loss of membrane integrity caused by cell damage may result in the release of more nucleotides [87]. During apoptosis, caspase-3 and caspase-7 cleave the C-terminus of PANX1, leading to channel opening and thus allowing nucleotides to be released through the channel to the extracellular space [52]. Despite the presence of higher intracellular ATP levels, the change in extracellular ATP and UTP concentrations is not substantial. Further research is needed to ascertain whether PANX1 favors UTP and promotes UTP release or if ATP undergoes metabolic degradation during apoptosis.
The release of ATP and UTP from early apoptotic cells can effectively attract monocytes both in vitro and in vivo. Removing ATP and UTP (through apyrase or ecto-CD39 expression) weakens the ability of apoptotic cells to recruit monocytes to both extracellular and intracellular environments [88]. Released ATP induces phagocyte migration through the purinergic receptor P2Y [58], UTP is degraded by extracellular nucleotidases to UDP, and UDP released from damaged microglia induces upregulation of P2Y in the hippocampus, contributing to the clearance of damaged cells [89]. Nucleotides also promote phagocytosis by inducing the binding of CD11b and integrin α5β3 [90, 91].
2.1.3 Sphingosine-1-phosphate (S1P)
S1P is a multifunctional lysophospholipid that serves as a “find me” signal which is derived from the sphingolipid metabolic pathway; S1P plays a critical role in regulating cellular processes such as lymphocyte migration, vascular integrity maintenance, and cytokine and chemokine generation [92]. S1P is synthesized through the phosphorylation of sphingosine by intracellular sphingosine kinases (SphKs) and can be released into the extracellular space through transport proteins [56]. S1P is exported by the major transport protein major facilitator superfamily domain-containing 2b (Mfsd2b), and the plasma levels of S1P are significantly reduced in Mfsd2b-deficient mice [93]. S1P released from dying cells activates pro-erythrocyte signaling in macrophages, thereby promoting the clearance of apoptotic cells and immune tolerance [94, 95]. There are five related G-protein-coupled receptors (GPCRs), known as S1PR1-5, that are involved in S1P-induced chemotaxis. S1P activates downstream signaling pathways by binding to S1PR1-5. Monocytic phagocytes express all known S1PR family members, making it difficult to determine which S1PR is most important [96]. Among them, S1PR1 has been a focus of research due to its role in regulating T-cell and B-cell migration, making it a key drug development target [97].
2.1.4 CX3CL1
CX3CL1 (also known as Fractalkine), a chemokine and intercellular adhesion molecule, is released rapidly from apoptotic lymphocytes via caspase- and Bcl-2-regulated mechanisms to attract macrophages [60]. CX3CL1 is the sole member of the CX3C chemokine subfamily. It is composed of 373 amino acids and is a transmembrane glycoprotein characterized by the insertion of three amino acids between two cysteine residues [98, 99]. CX3CL1 exists in two forms: membrane-bound and soluble. Membrane-bound CX3CL1 is a membrane-associated protein that contains a cytoplasmic domain, transmembrane domain, mucin-like stalk, and chemokine domain. Soluble CX3CL1 is generated via the cleavage of membrane-bound CX3CL1 by A disintegrin and metalloproteinase (ADAM) [100, 101]. CX3CL1 release can be spontaneous or induced by various factors. Under normal conditions, ADAM10 naturally cleaves membrane-bound CX3CL1 to form soluble CX3CL1. Inflammatory agents such as lipopolysaccharide and interleukin-1 beta (IL-1β) can enhance this release through ADAM17. Membrane-bound CX3CL1 promotes monocyte adhesion to endothelial cells [98], while soluble CX3CL1 chiefly attracts monocytes [98], T cells [98], and natural killer (NK) cells [102, 103]. CX3CL1 is rapidly released from apoptotic lymphocytes through caspase and Bcl-2 regulatory mechanisms to induce macrophage chemotaxis. CX3CR1 is the receptor for CX3CL1 [98] and is a seven-transmembrane G-protein-coupled receptor consisting of extracellular, transmembrane, and intracellular regions; it appears to be crucial for sensing intracellular and extracellular chemokines and inducing monocyte migration [104]. CX3CR1 is mainly expressed on monocyte, leukocyte, and platelet membranes and mediates cell migration and adhesion by binding with CX3CL1. CX3CR1 has stable adhesion ability and exerts strong adhesive effects on circulating monocytes, T cells [105], and NK cells [106], and CX3CR1-expressing T cells exhibit enhanced cytotoxicity [98, 99, 107]. The absence of CX3CR1 leads to reduced chemotaxis of macrophages toward the germinal centers of apoptotic B cells [58].
2.1.5 Other “find me” signals
In addition to the aforementioned “find me” signals, there may be other undiscovered “find me” signals. To date, researchers have reported several factors that induce phagocyte chemotaxis, such as the ribosomal protein S19 (RP S19) dimer [18], two cell cytokine activity fragments generated from the cleavage of human tyrosyl-transfer RNA synthetase (TyrRS) (N-terminal fragment and C-terminal fragment) [62, 63], and endothelial monocyte-activating polypeptide II [64, 65]. Future research could elucidate whether these “find me” signals indeed impact phagocyte chemotaxis.
2.2 “Eat me” signals
“Eat me” signals are a class of signaling molecules that can bind to phagocyte membrane receptors and induce phagocytes to perform their engulfment function. In this section, the known “eat me” signals, including CALR, SLAMF7, and FcR, will be discussed in terms of concept, structure, reasons for signal release, ligands, and functions (Figures 1 & 3, Table 3)
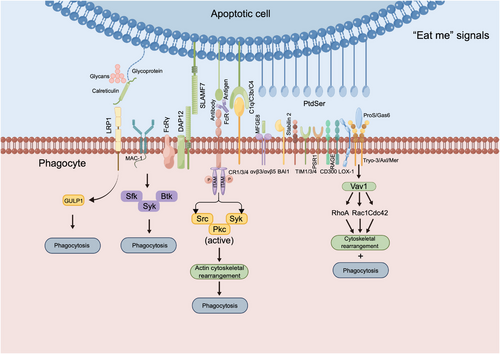
“Eat me” signals and downstream responses upon binding to phagocyte receptors. Stress-induced and dying tumor cells expose CALR on the cell surface, which binds to LRP1 on phagocytes, possibly recruiting GULP1 to regulate phagocytosis. SLAMF7 on macrophages binds to MAC-1, which, in turn, recruits FCRγ and DAP12, activating Syk and Btk kinases to promote phagocytosis. Macrophages express Fcγ receptors (FcγR-IIb, FcγR-I, FcγR-IIIa, and FcγR-IIa). Crosslinking these receptors with IgG complexes triggers ITAM phosphorylation, activating the Syk, Src, and Pkc pathways, and leading to actin remodeling, which is crucial for the phagocytosis of IgG immune complexes. BAI1, brain-specific angiogenesis inhibitor 1; Btk, Bruton's tyrosine kinase; C1q/C3b/C4, complement components 1q/3b/4; CD300, cluster of differentiation 300; Cdc42, cell division control protein 42; CR1/3/4, complement receptors 1/3/4; DAP12, DNAX-activating protein of 12 kDa; FcRγ, Fc receptor gamma chain; Gas6, growth arrest-specific protein 6; GULP1, Engulfment adaptor PTB domain-containing 1; GULP1, engulfment adaptor PTB domain-containing 1; LRP1, low-density lipoprotein receptor-related protein 1; MAC-1, macrophage-1 antigen; MFG-E8, milk fat globule epidermal growth factor 8; Pkc, protein kinase C; ProS, Protein S; PSR1, phosphatidylserine receptor 1; PtdSer, Phosphatidylserine; Rac1, Ras-related C3 botulinum toxin substrate 1; RAGE, receptor for advanced glycation end products; RhoA, Ras homolog family member A; Sfk, Src family kinases; SLAMF7, signaling lymphocytic activation molecule family member 7; Src, Src kinase; Syk, spleen tyrosine kinase; TAM, Tryo3/Axl/Mer; TIM1/3/4, T cell immunoglobulin and mucin domain 1/3/4; Vav1, vav guanine nucleotide exchange factor 1; αVβ3/αVβ5, integrin alpha V beta 3/ alpha V beta 5.
| “Eat me” signal | Major dependencies for release/exposure | Receptors | Downstream biological effects | References |
|---|---|---|---|---|
| CALR | Exocytic | LRP1 (CD91) | Recruits the adapter protein PTB domain-containing engulfment adapter protein 1 (GULP1) to regulate promote phagocytic processes | [108-113] |
| SLAMF7 | – | CRACC (CD319) | SLAMF7 on macrophages binds to MAC-1, which interacts with FCRγ and DAP12, recruiting the Src family kinases Syk and Btk to promote phagocytosis | [114-117] |
| Fc | – | FcR | Activated immune cells can clear pathogenic microorganisms through mechanisms like antibody-mediated phagocytosis and ADCC | [118-121] |
| Phosphatidylserine (PtdSer) and oxidized PtdSer | Phospholipid scramblase/amino-phospholipid translocase | BAI1 | Directly recruits a Rac-GEF complex to mediate the uptake of apoptotic cells | [28, 122, 123] |
| TIM1/3/4 | TIM-1 enhances T-cell activation in Th2 cells, while TIM-3 in Th1/Tc1 cells induces cell death and assists dendritic cells in phagocytosis and antigen presentation; TIM-4, which is found exclusively on antigen-presenting cells, supports phagocytosis and immune tolerance | [29–32, 124] | ||
| Stabilin-2 | Phosphatidylserine recognition on the cell surface activates signaling via the CrkII/DOCK180/ELMO or Gulp1 pathways, leading to actin rearrangement and apoptotic cell engulfment through CED-10/Rac1 | [33–35, 125] | ||
| CD300 | CD300b enhances engulfment by binding to F-actin at apoptotic cell contacts, and activation via DAP12 with a functional ITAM motif is essential; binding to apoptotic cells triggers the PI3K-Akt pathway, but silencing CD300b reduces it, impairing efferocytosis | [19, 36, 37] | ||
| MFG-E8-αVβ3 | Integrin activation triggers tyrosine kinase (FAK and Src) activation and signaling to Rho-GTPases (Rac and Cdc42), regulating the actin cytoskeleton | [20, 38, 39, 126] | ||
| Protein S/Gas6- Tyro3/Axl/Mer (TAM) | TAM receptors activate PI3K/Akt in macrophages through direct p85 binding or through Grb2 as a bridge; this leads to phosphorylation of Akt, suppressing NF-kB translocation, impacting gene transcription, and influencing macrophage function and phenotype | [40-43] | ||
| RAGE | Unknown | [127] | ||
| PSR-1 | TAT-1/ATP8A, PSR-1/PSR, and PAT-2/α-integrin start engulfment, activating CED-10/Rac1 via CED-2/CrkII, CED-5/DOCK180, and CED-12/ELMO; WSP-1/nWASp aids actin remodeling during phagocytosis | [128] | ||
| CD36 | Unknown | [44, 129] | ||
| LOX-1 | Unknown | [129] |
- Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity; Akt, protein kinase B; ATP8A, ATPase phospholipid transporting 8A; BAI1, brain-specific angiogenesis inhibitor 1; Btk, Bruton's tyrosine kinase; CALR, calreticulin; CD300, cluster of differentiation 300; CD300b, cluster of differentiation 300b; CD36, cluster of differentiation 36; CED-10, cell death abnormality-10; CED-12, cell death abnormality-12; CED-2, cell death abnormality-2; CED-5, cell death abnormality-5; CrkII, CT10 Oncogenic Gene Homologue II; DAP12, DNAX-activating protein of 12 kDa; DOCK180, dedicator of cytokinesis protein 1; ELMO, engulfment and cell motility protein; FAK, focal adhesion kinase; Fc, fragment crystallizable region; FcR, Fc receptor; Gas6, growth arrest-specific protein 6; GULP1, engulfment adapter protein 1; LOX-1, lectin-like oxidized low-density lipoprotein receptor 1; LRP1 (CD91), low-density lipoprotein receptor-related protein 1; MAC-1, macrophage-1 antigen; MFG-E8, milk fat globule-epidermal growth factor 8; NF-kB, Nuclear factor kappa-light-chain-enhancer of activated B cells; nWASp, neural Wiskott-Aldrich syndrome protein; PAT-2, phosphatidylserine aminophospholipid translocase 2; PI3K, phosphoinositide 3-kinase; PSR-1, phosphatidylserine receptor 1; PTB, phosphotyrosine binding domain; Rac1, Ras-related C3 botulinum toxin substrate 1; Rac-GEF, Rac GTPase-activating protein; RAGE, receptor for advanced glycation end products; Rho-GTPases, Rho guanosine triphosphatases; SLAMF7, signaling lymphocytic activation molecule family member 7; Src, Src kinase; Syk, spleen tyrosine kinase; TAM, Tyro3/Axl/Mer; TAT-1, transbilayer amphipath transporter-1; TIM1/3/4, T cell immunoglobulin and mucin domain 1/3/4; WSP-1, Wiskott-aldrich syndrome protein 1; αVβ3, integrin alpha V beta 3.
2.2.1 CALR
Cell surface-exposed CALR, functioning as an “eat me” signal, delivers potent pro-phagocytic signals to antigen-presenting cells (APCs), including dendritic cells (DCs) and their precursors [130, 131]. CALR is an endoplasmic reticulum (ER)-resident protein that consists of 417 amino acids (46 kDa) and is involved in various cellular processes, such as cell adhesion, migration, apoptosis, protein folding, and protein modification [132-134]. CALR is composed of three distinct domains: (1) an N-terminal lectin-like globular domain, (2) a central proline-rich domain, and (3) a highly acidic C-terminal region [135]. Additionally, there is a Lys-Asp-Glu-Leu-COO amino acid sequence (KEDL) at the C-terminus, which retains CALR in the ER [136]. CALR serves as a guide for macrophages to target live cancer cells [137]. CALR moves from the macrophage ER to the cell surface via ER stress molecules. Apoptotic triggers activate protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), which phosphorylates eukaryotic translation initiation factor 2 subunit alpha (eIF2α). Subsequent caspase-8 activation leads to the cleavage of B-cell receptor-associated protein 31 (BAP31), activating Bcl-2-associated X protein (Bax) and Bcl-2 homologous antagonist/killer (Bak). These activated proteins aid in the transfer of CALR from the ER to the Golgi, followed by soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent exocytosis to the cell membrane [133, 138]. However, PERK-mediated phosphorylation of eIF2α is inhibited by B7 homolog 4 (B7-H4), which is associated with poor T-cell infiltration and prognosis in cancer [139]. Additionally, research has revealed that CALR moves from the ER to the cytosol through the nucleus, interacting with various proteins along the way. In the cytosol, it is converted to CALR-Arg, and in the presence of inducible nitric oxide synthase, it can produce nitric oxide byproducts [140]. On the cell membrane, both citrullinated and arginylated CALR isoforms have been identified.
2.2.2 SLAMF7
The SLAMF receptor consists of a group of type I transmembrane receptors, including SLAMF4 (also known as 2B4), SLAMF3 (also known as Ly9), SLAMF7 (also known as CRACC), SLAMF2 (also known as CD48), SLAMF1 (also known as SLAM), SLAMF8 (also known as BLAME), SLAMF5 (also known as CD84), SLAMF9 (also known as SF2001), and SLAMF6 (also known as SF2000 in humans or Ly108/ CD352 in mice) [114, 115, 141–143]. Except for 2B4, which binds to CD48 as its ligand [144-146], all other SLAM family receptors are self-ligands, thus triggering downstream signaling pathways in heterotypic or homotypic cell-cell interactions [142, 147]. SLAMF signals are mediated via their cytoplasmic immunoreceptor tyrosine-based switch motifs (ITSMs), which recruit a series of adapter proteins containing only SH2 domains, including SLAM-associated protein (SAP) and its homologs EWS/FLI1 activated transcript 2 (EAT-2) and EAT-2-related transducer [146, 148, 149]. SLAMF3 and SLAMF4 have been identified as “do not eat me” receptors on macrophages [150]. They suppress “eat me” signals by impeding mammalian target of rapamycin (mTOR) and spleen tyrosine kinase (Syk) activation via low-density lipoprotein receptor-related protein 1 (LRP1). In parallel, they activate macrophages through SH2 domain-containing phosphatases, facilitating the engulfment of hematopoietic cells lacking SLAM family receptors (SFRs). While SFRs can work alongside the CD47 pathway, they independently reduce macrophage phagocytosis.
In the context of the SLAM receptor family, we focus on SLAMF7, a vital “eat me” signal critical for the phagocytosis of hematopoietic tumor cells via Mac-1 integrin [116]. SLAMF7 is a transmembrane receptor with three parts: the extracellular, transmembrane, and cytoplasmic regions. The cytoplasmic region contains ITSMs, including Y281 for activation and Y261 for inhibition of its signaling pathways [143]. SLAMF7 is expressed at low levels in CD4+ T cells, monocytes, macrophages, DCs, and B cells [116, 151, 152] and is highly expressed in normal plasma cells and malignant plasma cells in multiple myeloma [153]. Additionally, it can also be expressed on NK cells [154] and immune cell subsets (such as CD8+ T cells [155]). In contrast to that mediated by other members of the SLAM receptor family, the phagocytic activity mediated by SLAMF7 is independent of the SAP adapter [117]. SLAMF7 mediates its activation or inhibitory function through the EAT-2 bridging protein [156-163]. However, in the absence of EAT-2, SLAMF7 can recruit Src homology region 2 domain-containing phosphatase-1 (SHP-1), SHP-2, SH2 domain-containing inositol polyphosphate 5-phosphatase 1 (SHIP1), C-terminal Src kinase (Csk), and Fyn kinase (Fyn) to effectively inhibit NK cell function [164, 165].
The phagocytic function of SLAMF7 is dependent on immunoreceptor tyrosine activation motifs (ITAMs), which mediate immune cell activation through the Src kinase (Src), Syk, and Bruton's tyrosine kinase (Btk) kinases [166], as well as the interaction of Mac-1 with FcRγ and DNAX-activating protein of 12 kDa (DAP12). Mac-1 [also known as complement receptor 3 (CR3)] is an antigen composed of the integrins CD11b and CD18, which form a heterodimer [167]. Mac-1 at a site of infection can link phagocytes to target cells containing inactivated complement-3b (C3bi) and/or β-glucan or lipopolysaccharide (LPS), thereby facilitating phagocytosis [168]. SLAMF7-dependent phagocytosis of tumor cells requires the expression of Mac-1 on macrophages, but Mac-1 and C3bi have not been demonstrated to interact with SLAMF7 during phagocytosis [116].
2.2.3 Fc
Fc is a very strong “eat me” signal for macrophages. FcRs are cell surface proteins that specifically bind to the Fc portion of antibodies. Various cells express different FcRs with different specificities. FcRs differ in their ability to bind to antibodies of various structural types; for example, FcγRs bind to IgG, FcαRs bind to IgA, FcεRs bind to IgE, FcμRs bind to IgM, and FcδRs bind to IgD [118, 119, 169], thereby inducing different immune responses.
Based on their functionality, FcRs can be classified into two categories: those capable of eliciting cellular activation and those incapable of doing so [120, 121]. The former typically possesses ITAMs [170-172], while the latter lacks ITAMs [173]. Among the most prevalent FcRs in white blood cells are FcγRs, including FcγR-I, FcγR-III, and FcγR-IV in mice and FcγR-Ia, FcγR-IIa, and FcγR-IIIa in humans [174]. For example, FcγR-IIIA activates lymphocyte-specific protein tyrosine kinase (Lck) in NK cells [175], while FcγR-IIA and FcγR-IIIA activate Lck/Yes-related novel protein tyrosine kinase (Lyn) and hematopoietic cell kinase (Hck) in monocytes and macrophages [176]. Similarly, Syk is activated in macrophages and mast cells, while Zeta-chain-associated protein kinase 70 (ZAP70) is activated in NK cells. FcγR activation is akin to the activation of other ITAM-containing receptors. Phosphoinositide 3-kinase (PI3K) is activated first and produces phosphatidylinositol 4,5-bisphosphate (PIP2) and recruits pleckstrin homology (PH) domain-containing molecules [such as phospholipase C gamma (PLCγ) and Tec kinase (Tec)] through PIP2-PH interactions. Various Tec kinases {such as Btk [177] and IL-2-inducible T-cell kinase (Itk) [178]} are activated by FcγR in myeloid cells. During FcR-dependent macrophage activation, SH2 domain containing leukocyte phosphoprotein of 76-kDa (SLP-76) and B cell linker protein (BLNK) adapters activate Syk and interact with Btk and PLCγ. Activated PLCγ degrades PIP2, yielding inositol trisphosphate (IP3) and diacylglycerol (DAG), which leads to calcium mobilization [173, 179–183]. The binding of FcγR-IIB to ITAM-containing receptors leads to tyrosine phosphorylation of ITIMs by Lyn kinase, which leads to the recruitment of SHIP and further inhibition of ITAM-triggered calcium mobilization and cell proliferation, as well as downstream phagocytic functions [169, 184]. Morris et al. [185] confirmed the intrinsic function of FcγR-IIB in inhibiting mouse and human CD8+ T-cell responses, regulated by fibrinogen-like protein 2 (Fgl2) as a functional ligand, which modulates the apoptotic signaling pathway of CD8+ T cells, challenging the previous notion that “T cells do not express FcRs” [186].
2.2.4 PS
PS, a key glycerophospholipid in eukaryotic cell membranes, consists of glycerol, two fatty acid chains, and a phosphate headgroup. It is derived from phosphatidylcholine (PC) and phosphatidylethanolamine (PE) via the enzymes phosphatidylserine synthase 1 (PSS1) and PSS2, and it can be converted to PE via phosphatidylserine decarboxylase (PSD) [187]. In higher mammals, PS synthesis is carried out by two homologous enzymes, phosphatidylserine synthase 1 (PTDSS1) and PTDSS2 [188]. PS is typically confined to the inner leaflet of the plasma membrane in healthy cells. However, the exposure of PS on the surface of apoptotic cells is common and is considered a typical “eat me” signal for apoptosis [189, 190].
PS is typically kept within the inner layer of the plasma membrane by a protein called Flippase. During apoptosis, a different enzyme, scramblase, is activated, rapidly exposing PS on the cell's surface [191, 192]. The Kell blood group complex subunit (Xk)-related protein 8 (Xkr8) and cell death abnormality-8 (CED-8) mediate PS exposure in response to apoptotic stimuli [193-196]. X-ray repair cross-complementing protein 4 (XRCC4), when cleaved by caspase-3, can be activated, and its C-terminal fragment translocates from the nucleus to the cell membrane. Under apoptotic stimulation, XRCC4 can regulate the activity of Xkr4, thereby promoting membrane perturbation and PS exposure [194]. Transmembrane protein 16F (TMEM16F) is a Ca2+-dependent lipid scramblase that, in the presence of Ca2+, moves phosphoserine from the inner leaflet of the membrane to the cell surface, changing the membrane structure and possibly aiding in cell membrane repair [197].
PS can directly bind to PS receptors on the surface of macrophages, such as brain-specific angiogenesis inhibitor 1 (BAI1) [122], T cell immunoglobulin and mucin domain (Tim)-1/3/4 [124], Stabilin-2 [125], CD300 (CD300a, CD300b and CD300f) [19], lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1) [129], receptor for advanced glycation end products (RAGE) [127], and phosphatidylserine receptor 1 (PSR-1) [128], or indirectly bind through soluble bridging molecules, such as milk fat globule-EGF factor 8 (MFG-E8)-integrin alphaV beta (αVβ3) [20] and protein S (ProS)/growth arrest-specific protein 6 (Gas6)-Tyro3/Axl/Mer (TAM) [123, 198, 199]. Both proteins that directly bind to PS and those that bind to PS through bridging proteins possess immune-regulatory activity. PS signaling can suppress local immunity, providing tumors with a means to evade detection [200]. PS exposure is not limited to apoptotic cells and is observed in various cell types, including immune cells [myeloid-derived suppressor cells (MDSCs), monocytes, macrophages, activated B cells, and T cells] and cancer cells [201].
2.2.5 Other “eat me” signals
In addition to the well-studied “eat me” signals mentioned above, there are other “eat me” signals that have not been studied systematically, such as ARS1620-KRASG12C covalent complex, galectin-3, lactate dehydrogenase C (LDHC), and ATP synthase subunit alpha.
3 “FIND ME” AND “EAT ME” SIGNALS ARE ASSOCIATED WITH TUMORS
Phagocytosis is a fundamental component of the immune system's response to cancer. “Find me” and “eat me” are two important signals that mediate phagocytosis. “Find me” and “eat me” signaling molecules play important roles in antitumor immunity. However, under specific circumstances, certain “find me” and “eat me” signaling molecules may promote tumor development, recurrence, and metastasis. This section focuses on the relationship between “find me” and “eat me” signaling molecules and the occurrence, development, diagnosis (stage, malignancy, metastasis, and recurrence), and prognosis of tumors.
3.1 Role of “find me” signals in tumor occurrence, development, diagnosis, and prognosis
3.1.1 LPC
LPC plays a complex role in cancer biology. LPC exerts a potent chemotactic effect on spleen lymphocytes from thymic lymphoma-bearing mice, as well as on NK T cells [202, 203]. Notably, LPC also plays a crucial role as a ligand for CD1d-restricted T cells, with subsequent initiation of an interaction that leads to the release of IL-13. This cytokine, in turn, promotes tumor growth [204]. LPC is a potential biomarker for various tumors, such as pancreatic cancer, colorectal cancer (CRC), and ovarian cancer. A diminished level of LPC (16:0) has been detected in patients with intrahepatic cholangiocarcinoma [205], ovarian cancer [206], and CRC [207]. Related studies have reported significantly downregulated expression of various lipids, including LPC, in the serum of patients with pancreatic ductal adenocarcinoma, possibly associated with KRAS-driven metabolic switches [208]. Zhao et al. [207] evaluated LPC in the plasma as a potential biomarker for CRC, and the levels of several LPC species, including LPC (18:1) and LPC (18:2), were significantly reduced in CRC patient plasma. Additionally, Zeleznik et al. [209] confirmed the associations of LPC, phosphatidylcholine, ceramide, and sphingomyelin with overall and histotype-specific ovarian cancer risks. LPC (18:0) levels are inversely linked to susceptibility to breast, prostate, and colorectal cancers [210], and melanoma patients have lower LPC (18:0) levels than healthy individuals [211]. Squamous cervical cancer is associated with higher LPC levels than uterine fibroids [212] and lymphangioleiomyomatosis [213]. LPC in cholangiocytes induces senescence marker expression, reactive oxygen species (ROS) production, DNA damage, and carcinogenesis [214].
The impact of LPC extends to tumor progression and recurrence. Lower LPC levels are associated with colorectal surgery complications [215] and recurrence after prostate surgery [216]. LPC hinders metastasis; LPC (18:0) disrupts the protein kinase C (PKC) delta pathway, thereby curbing melanoma invasion [211]. LPC reduces vascular cell adhesion molecule-1 (VCAM-1) and P-selectin, thereby curbing adhesion and lung invasion [217]. Tumors convert LPC to rigidifying fatty acids that suppress invasiveness [218]. LPC limits invasion by blocking lysophosphatidic acid (LPA) conversion via autotaxin [219]. LPC and lipid metabolism have promise as targets for cancer treatment.
3.1.2 ATP
In 1983, Rapaport [220] first demonstrated the antitumor activity of ATP. Exposure to ATP inhibits cancer cell growth by blocking the cell cycle in the S phase. Furthermore, intraperitoneal injection of ATP (50 mmol/L) effectively reduces tumor size. Shabbir et al. [221] performed an in vivo study using human prostate cancer xenografts and showed that daily intraperitoneal administration of extracellular ATP (eATP) (25 mmol/L) led to significant tumor regression. Moreover, clinical studies have reported good tolerance to intravenous ATP in cancer patients, with improvements in tumor-related cachexia and overall health status, suggesting that combining eATP with other therapeutic approaches may not only help reduce tumor size but also minimize adverse systemic effects [222].
ATP released from apoptotic cells, which is converted into adenosine, reduces inflammation, promotes anti-inflammatory gene expression, activates APCs, enhances APC function, and stimulates T cells. Additionally, it induces IL-18 secretion, activating immune cells within the tumor microenvironment (TME) [223]. ATP can activate the purinergic receptor P2X, ligand-gated ion channel 7 (P2RX7) receptor expressed in macrophages, DCs, granulocytes, T cells, and B cells, thereby triggering the NOD-like receptor protein 3 (NLRP3)/apoptosis-associated speck-like protein containing a CARD (ASC)/caspase-1 inflammasome and driving the secretion of IL-1β [224]. P2 receptors in immune cells trigger diverse responses. For instance, human neutrophils release ATP from their surface, amplifying chemotactic signals via the purinergic receptor P2Y, G-protein coupled 2 (P2Y2) receptor. Neutrophils promptly convert ATP to adenosine, which, through the A3 adenosine receptor, promotes cell migration [59, 225]. Additionally, ATP can activate the P2RX7 receptor on DCs, leading to NLRP3/ASC/caspase-1 inflammasome activation, driving IL-1β secretion, and inducing polarization of CD8+ T cells toward interferon gamma (IFNγ) production, exerting antitumor effects [224, 226, 227].
Notably, although it has some antitumor effects, extracellular ATP also exhibits tumorigenic properties. High ATP concentrations can promote regulatory T cells (Treg) cell proliferation, leading to immunosuppression. Extracellular ATP can also enhance the migration of tumor cells [228]. Additionally, ATP released by activated platelets induced by tumor cells can open the endothelial barrier, allowing tumor cells to migrate through the endothelium and promoting cancer cell extravasation [229]. Zhang et al. [230] discovered that extracellular ATP boosts filopodium and pseudopodium formation in prostate cancer cells by activating birdie triphosphatase and increasing metalloproteinase expression. This enhances cancer cell migration and invasion capacity. In colon cancer organoids, chemotherapy causes cancer cell death, leading to ATP release. This ATP binds to nearby cancer cells via P2×4 receptors, triggering mTOR-dependent pro-survival mechanisms. This protection from chemotherapy-induced cell death helps cancer cells resist treatment and promotes tumor development [230]. Whether increasing extracellular ATP concentrations can achieve antitumor immune effects requires further investigation.
3.1.3 S1P
S1P, produced from sphingosine by SphKs, acts as a “find me” signal for apoptotic cells but hinders antitumor immunity. It influences lymphocyte function and regulates Tregs through S1PR1 signaling. S1P deficiency limits peroxisome proliferator-activated receptor gamma (PPARγ) activity, blocking T-cell differentiation into Tregs [231].
However, S1P is beneficial for cancer therapy in certain situations. The progression of cancer is influenced by tumor angiogenesis [232], and S1P and its receptors play a critical role in this process. In tumor tissues, blood vessels are often irregular and leaky. When S1PR1-5 levels are increased on the endothelial cell membranes of tumor blood vessels, S1P signaling helps normalize the vasculature, reducing leakage, and promoting a more normal blood vessel structure [233]. This normalization enhances the effectiveness of chemotherapy and immune checkpoint inhibitor therapy in shrinking tumors. In contrast, mice lacking S1PRs have larger tumors and increased metastasis [234]. This research suggests that S1P plays a complex role in tumor angiogenesis and progression. We need to reassess its role in the immune system and find ways to utilize S1P and its receptors for improved cancer immunotherapy.
3.1.4 CX3CL1
The role of CX3CL1 in cancer progression is debated. Some studies have suggested that CX3CL1 inhibits metastasis in glioma cells, while others have linked high CX3CL1 expression to reduced survival in oligodendroglioma, astrocytoma, and glioblastoma [235, 236]. Lee et al. [237] demonstrated that CX3CR1 signaling enhances tumor-associated microglia/macrophage functions and angiogenesis, affecting the malignant transformation of low-grade gliomas. Additionally, CX3CL1 promotes breast cancer via activation of the epidermal growth factor pathway [238]. In the MΦ tumor cell system, IL-10 drives the upregulation of C-C motif chemokine receptor 2 (CCR2) and CX3CR1, and C-C motif chemokine ligand 1 (CCL1), colony-stimulating factor, and macrophage inflammatory protein 1 alpha (MIP1α) are needed for the upregulation of CCL2 and CX3CL1. In vivo, depletion of MΦ and genetic ablation of CCR2 and CX3CR1 inhibit the growth and metastasis of LLC1 tumors, induce MΦ polarization toward the M1 phenotype, suppress tumor angiogenesis, and improve survival rates [239].
3.2 Role of “eat me” signals in tumor occurrence, development, diagnosis, and prognosis
3.2.1 CALR
When CALR is exposed on the surface of stressed or dying tumor cells prior to apoptosis, it facilitates the engulfment of apoptotic cells by professional phagocytes and DCs, thereby contributing to the initiation of antitumor immunity [108, 109, 240–243]. Surface exposure of CALR is crucial for determining the immunogenicity of tumor cells and nonimmunogenic cell death [243, 244]. Blocking or depleting CALR using small-interfering RNA (siRNA) can prevent immunogenic cell death [240]. CALR, along with endoplasmic reticulum protein 57 (ERp57) [240, 243], heat shock protein 70 (HSP70), HSP90, and other ER chaperones exposed on the membrane of cells undergoing immunogenic cell death (ICD), act as “eat me” signals to promote the uptake of cell bodies and fragments by APCs [245]. Macrophages also have CALR on their surface or release it, making it a crucial player in the recognition and engulfment of neighboring tumor cells. Activation of Toll-like receptor (TLR) pathways in macrophages triggers Btk phosphorylation, leading to CALR exposure on the cell surface [137], thus aiding in the programmed removal of tumor cells. CALR on the tumor cell surface forms a bridge complex with LRP1 (also known as CD91) expressed on phagocytes [108, 110, 111, 246], initiating the clearance of tumor cells via phagocytes [109, 112, 113]. However, the phagocytic capacity of macrophages lacking LRP1 is somewhat limited. CALR acts as a bridge for interaction with specific sialylated glycoproteins (modified by neuraminidases NEU2 and NEU4 and sialyltransferases ST3GAL1 and ST3GAL6) [247] or exposed PS molecules on the cell surface [248]. Other proteins that bind to CALR include thrombospondin 1 [249], complement C1q A chain [250], and mannose-binding lectin family members [251, 252]. Indeed, tumor cells also express “do not eat me” signals (such as CD47 and CD24) to evade phagocytosis by APCs [253-255]. CALR signaling counteracts the CD47-signal regulatory protein alpha (SIRPα) axis and acts as a prophagocytic signal in CD47 blockade-mediated phagocytosis.
Cancer cells evade CALR signals via several strategies. First, cancer cells often have CALR mutations. These mutations frequently occur in Janus kinase 2 (JAK2)-unmutated myeloproliferative neoplasms. Common mutations involve exon 9, leading to a 52-bp deletion (CALRdel52, type 1 mutations) or a 5-bp insertion between residues 1,154 and 1,155 (CALRins5, type 2 mutations), leading to loss of CALR's C-terminal KDEL motif [256]. Mutated CALR can escape from the ER and interact with polyprenol on myeloproliferative leukemia protein (MPL) residue N117, forming a stable complex with MPL and activating the thrombopoietin receptor (TpoR/MPL) [257-261], thereby blocking the classic Golgi apparatus-dependent secretion of the CALR protein [262]. Second, soluble CALR protein acts as a bait receptor, preventing DCs from engulfing cells exposed to CALR, thus mediating significant immunosuppressive effects [260]. Third, tumor stanniocalcin-1 (STC1) interacts with CALR, reducing its membrane exposure [263]. High expression of STC1 induces CALR retention in mitochondria, minimizing membrane CALR levels and inhibiting APC phagocytic function [247, 263]. Disrupting the intracellular STC1-CALR interaction is a challenging endeavor, and researchers are still in search of compounds for effective intervention.
3.2.2 SLAMF7
SLAMF7 induces cytotoxicity in activated NK cells and antibody-dependent cell-mediated cytotoxicity (ADCC) [115, 152]. In most cases, the ADCC effect is primarily achieved through the binding of antibodies to FcRs, which activate NK cells and enhance the antitumor response. The binding of SLAMF7 receptors on NK cells or between NK cells and target cells to SLAMF7 ligands can regulate the PI3K and PLCγ1/PLCγ2 signaling pathways, induce the activation of NK cells, promote cytotoxicity, and stimulate IFNγ production [146, 157, 164, 264]. M1 macrophages are classically activated macrophages with the ability to kill tumor cells and inhibit tumor angiogenesis and lymphangiogenesis, while M2 macrophages are believed to contribute to the immunosuppressive TME [265]. Compared to M2 tumor-associated macrophages (TAMs), M1 macrophages show higher expression of SLAMF7 [266]. In the context of SIRPα-CD47 blockade, SLAMF7 has been demonstrated to play a critical role in macrophage-mediated phagocytosis of tumor cells [116, 267]. During SIRPα-CD47 blockade, the SLAMF7 interaction enhances tumor cell phagocytosis by macrophages, both in vitro and in vivo. SLAMF7 acts as an “eat me” signal on APCs, promoting cancer cell phagocytosis via homodimeric complexes. In hematological malignancies, SLAMF7 expression influences the response to immunotherapy [116]. Targeting SLAMF7 surface expression in solid tumors may mimic hematological malignancies, potentially improving immunotherapy efficacy [268]. However, He et al. [267] found that many diffuse large B-cell lymphoma (DLBCL) cell lines and primary cells lack SLAMF7 expression, and CD47-mediated phagocytosis does not depend on SLAMF7 expression in cancer cells. Recently, it was discovered that soluble SLAMF7 (sSLAMF7) is present in multiple myeloma (MM) patient serum, but its role in MM biology is unclear. Researchers observed that sSLAMF7 can bind to surface-expressed SLAMF7, promoting MM cell growth via the SHP-2 and extracellular signal-regulated kinase (ERK) signaling pathways [269]. Therefore, there is currently no definitive conclusion regarding whether SLAMF7 plays a role as an “eat me” signal in phagocytosis. Furthermore, other members of the SLAM receptor family, such as SLAMF3 and SLAMF4, have been identified as “do not eat me” signal receptors on macrophages, inhibiting macrophage phagocytosis of hematopoietic tumors [150].
Although it is difficult to elucidate the “eat me” role of SLAMF7, it can serve as a clinical auxiliary diagnostic marker and an indicator of disease progression and prognosis. SLAMF7 is highly expressed in almost all MM cells, is not expressed in the vast majority of solid tumors, and has extremely low expression in normal immune cells [270, 271], making it a novel biomarker for auxiliary diagnosis of MM.
3.2.3 Fc
From the perspective of antibody mechanisms of action, antibodies consist of an antigen-binding fragment (Fab) and a crystallizable fragment (Fc) [173, 272]. The Fab region can specifically bind to a particular antigen, determining the antibody's specificity and affinity. Similarly, the Fc region can bind to FcRs (FcγR-I, FcγR-II, and FcγR-III) expressed on immune cells and complement protein C1q, thereby activating immune effector cells to clear foreign substances. The structure of an antibody determines its mechanism of action [273-276]. The Fab region attaches to specific antigens, determining specificity and affinity, while the Fc region determines the effector functions of the antibody, including ADCC, antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC) [277]. By engineering modifications to increase the affinity of the antibody's Fc region for FcγRs with activating effects, the efficacy of ADCC/ADCP can be enhanced [278].
In adaptive immune responses, the activation of type I FcRs on DCs, follicular DCs, and macrophages is crucial. Bone marrow chimera experiments have shown that DCs and macrophages make the most significant contributions to initiating antibody responses [279]. DCs internalize immune complexes through the type I FcR pathway and efficiently process and present antigens on both major histocompatibility complex class I (MHC-I) and class II (MHC-II) molecules, which is a core process for inducing adaptive cellular immune responses [280]. When antigens are internalized as immune complexes through the activation of type I FcRs, the activation of DCs and the initiation of immune responses mediated by CD4+ and CD8+ T cells are significantly enhanced [280-282]. IgG can activate macrophages via binding of its Fc region to FcRs on the macrophage surface [279, 283]. Activation of the FcR signaling pathway forms a “phagocytic synapse” between tumor cells and phagocytes, and three events occur sequentially in the phagocytic synapse: contact between tumor cells and phagocytes, macrophage pseudopod engulfment of tumor cells, and phagocytosis of tumor cells [186]. In various mouse models, it has been demonstrated that anti-CD20 antibodies require interaction with activating type I FcRs expressed on monocytes/macrophages to deplete CD20+ cells [284, 285]. In vivo animal models require the activation of FcγRs to prevent the growth of human epidermal growth factor receptor 2 (HER2)+ tumors [284]. Allelic variants of FcγR-IIa and FcγR-IIIa have also been shown to predict the efficacy of anti-epidermal growth factor receptor (EGFR) antibody (cetuximab) therapy in patients with CRC [286]. Therefore, many antitumor antibodies require interaction with activating type I FcRs on innate effector cells to activate ADCC and mediate therapeutic effects on malignant cells.
3.2.4 PS
In the TME, exposed PS can be found on tumor cells, secreted microvesicles, and tumor endothelial cells. Phagocytes can distinguish PS exposed on apoptotic cells from that on viable cells. Importantly, activation of PS receptors on immune cells, including MDSCs, CD4+ and CD8+ T cells, DCs, macrophages, B cells, and NK cells, creates an immunosuppressive environment, which tumor cells exploit as immune camouflage [200, 287, 288]. In this intricate ecosystem, cavity-resident macrophages play a crucial role by upregulating Tim-4, a receptor for PS. Elevated Tim-4 levels correlate with reduced numbers of tumor-reactive CD8+ T cells in cancer patients' pleural effusions and peritoneal ascites. Mechanistically, we discovered that PS levels were elevated in viable CD8+ T cells, making them susceptible to sequestration and proliferation suppression by Tim-4+ macrophages. However, blocking Tim-4 reverses this effect, enhancing the efficacy of anti-tumor therapies like anti-programmed cell death protein 1 (PD-1) treatment and adoptive T cell therapy in murine models [287].
To study the immune changes caused by PS flipping in tumors, researchers established tumor models with continuous outward flipping of intracellular PS (CDC50a-knockout, PSout) or inward flipping of apoptotic tumor cell PS (Xkr8-knockout, PSin) through CRISPR/Cas9 gene editing. By using these tumor models, the research team revealed that flipping intracellular PS to the outer leaflet of the membrane restricts the expression of MHC-I/II on TAMs, affecting tumor antigen presentation and promoting tumor development. Conversely, when PS exposed on the outer membrane of apoptotic cells is flipped to the inner membrane, it activates cyclic GMP-AMP synthase (cGAS) to produce cyclic GMP-AMP (cGAMP), subsequently activating the type I interferon signaling pathway in the TME. These immune cells, including TAMs and NK cells, work together to inhibit tumor growth, thereby suppressing tumor development [289].
4 REGULATING “FIND ME” AND “EAT ME” SIGNALS TO PROMOTE ANTITUMOR IMMUNITY
In the pursuit of enhancing antitumor immunity, the regulation of “find me” and “eat me” signals is a pivotal strategy. We will cover ways to influence “find me” signals, including LPC reduction, ATP elevation, sphingosine-1-phosphate (S1P) modulation, and CX3CL1 reduction. Furthermore, we'll explore methods to regulate “eat me” signals, including exposure of CALR on the membrane, regulation of SLAMF7, enhancement of Fc signaling, and modulation of PS flipping (Figure 4, Table 4).
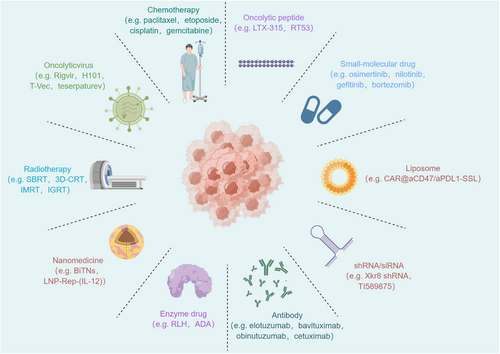
Related drug types and specific examples that regulate “find me” and “eat me” signals to exert anti-tumor immunity. These categories include chemotherapy, oncolytic peptides, small-molecule drugs, liposomes, shRNAs/siRNAs, antibodies, enzymes, nanomedicines, radiotherapy, oncolytic viruses, and others. Chemotherapy drugs such as paclitaxel, etoposide, cisplatin, and gemcitabine inhibit cancer cell growth and division. Oncolytic peptides such as LTX-315 and RT53 selectively target and kill cancer cells. Small-molecule drugs such as osimertinib, nilotinib, gefitinib, and bortezomib target molecular pathways in cancer cells. Liposomal formulations, including CAR@aCD47/aPD-L1-SSL, serve as effective drug delivery systems. Nucleic acid-based therapies like Xkr8 shRNA and TI589875 silence genes involved in cancer progression. Monoclonal antibodies such as elotuzumab, bavituximab, obinutuzumab, and cetuximab activate immune responses against tumors. Enzyme drugs such as RLH and ADA modulate immune responses and inhibit tumor growth. Nanoparticle-based therapies like BiTNs and LNP-Rep-(IL-12) deliver drugs specifically to cancer cells. Radiation therapy techniques like SBRT, 3D-CRT, IMRT, and IGRT precisely target and destroy cancer cells. Abbreviations: 3D-CRT, three-dimensional conformal radiation therapy; ADA, adenosine deaminase; IGRT, image-guided radiation therapy; IMRT, intensity-modulated radiation therapy; RLH, RIG-I-like helicases; SBRT, stereotactic body radiation therapy.
| “Find me” signals | Treatment | Intervention Types | Medicines | Biological Effects | Indications | Preclinical or clinical status | References/Clinical trial number |
|---|---|---|---|---|---|---|---|
| ATP | Induction of ATP release | Radiochemotherapy drugs | UV therapy | Induces ATP release, leading to DNA damage and activation of cellular stress response pathways. | Hodgkin lymphoma, parathyroid cancer | NDA | [290, 291] |
| Etoposide | Inhibits topoisomerase II, blocking DNA replication, leading to cell cycle arrest, apoptosis, and autophagy, along with the release of ATP. | Leukemia, solid tumors (such as testicular cancer, ovarian cancer, lung cancer and lymphoma) | Clinical application | [290, 291] | |||
| Ginsenosides | Regulates ATP release through their modulation of the Hippo-YAP/TAZ signaling pathway, which in turn affects innate immunity. | Non-small Cell Lung Cancer (NSCLC) | Clinical, phase II | NCT02714608 | |||
| Cadmium | Induces immunogenic cell death (ICD), promoting ATP release and activating signaling pathways associated with cell death modalities and immune recognition. | Unclear | Preclinical | [290, 291] | |||
| Inducers of cell death | Mitomycin C | Induces immunogenic cell death, triggering apoptosis pathways and promoting ATP release. | Bladder cancer, colorectal cancer, stomach cancer, esophagus cancer, liver cancer | Clinical application | [290, 291] | ||
| Staurosporine | Inhibits various protein kinases through competition with the ATP binding site. | Leukemia, non-small cell lung cancer, prostate cancer, adenocarcinoma | Preclinical | [290, 291] | |||
| Oxaliplatin | Induces cell death pathways and modulates ATP signals by cross-linking DNA, leading to cell cycle arrest and apoptosis. | Ovarian cancer, gastric cancer, esophageal cancer, colorectal cancer | Clinical application | ||||
| Cisplatin | Induces DNA cross-linking, triggering apoptosis in cancer cells and ATP release. | Ovarian cancer, bladder cancer, ovarian cancer, head and neck cancer (such as mouth, throat, and esophagus cancer) | Clinical application | [290, 291] | |||
| Thapsigargin | Induces endoplasmic reticulum stress and apoptosis in cancer cells, triggering ATP release to recruit immune cells to the site of cell death. | Prostate cancer, breast cancer, stomach cancer | Preclinical | [290, 291] | |||
| Mithramycin | Inhibits DNA transcription, leading to cell death, accompanied by the release of ATP. | Lung Cancer, esophageal cancer, mesothelioma, gastrointestinal neoplasms, breast cancer | Clinical, phase II | NCT01624090 | |||
| Doxorubicin | Interferes with DNA replication, inducing apoptosis, and subsequently triggering the release of ATP. | Breast cancer, ovarian cancer, lymphoma, lung cancer | Clinical application | [45] | |||
| Actinomycin | Inhibits RNA transcription, leading to apoptosis in cancer cells, and prompts the release of ATP. | Pediatric Wilms tumor, malignant soft tissue tumor, testicular tumor | Clinical application | [290, 291] | |||
| Hyperthermia | Induces heat shock response, leading to apoptosis or necrosis in cancer cells, and also triggers ATP release. | Head and neck cancer, breast cancer, lymphoma | Clinical application | [46] | |||
| Bromide | Induces DNA damage and apoptosis in cancer cells, leading to cellular stress that triggers ATP release. | Unclear | Preclinical | [47] | |||
| Ethidium | Interferes with DNA replication, inducing apoptosis in cancer cells, and triggers the release of ATP. | Unclear | Clinical application | ||||
| Shock wave | Mechanically disrupts tumor tissue, releasing ATP. | Advanced solid tumors | Clinical, phase II | ||||
| Inhibition of ATP hydrolysis | Anti-CD39 | TTX-030 | Inhibit CD39 activity, which converts ATP to adenosine in tumors. | Solid tumor, lymphoma | Clinical, phase I/Ib | NCT03884556 | |
| SRF617 | Gastric cancer and cancer patients resistant to checkpoint inhibitors | Clinical, phase I | NCT04336098 | ||||
| ES002 | Advanced or metastatic malignancy | Preclinical | [48] | ||||
| JS019 | Advanced solid tumors or lymphomas | Clinical, phase I | NCT05374226 | ||||
| ES014 | Advanced solid tumor | Clinical, phase I | NCT05717348 | ||||
| ES002023 | Advanced solid Tumor | Clinical, phase I | NCT05075564 | ||||
| IPH5201 | Advanced solid tumors | Clinical, phase I | NCT04261075 | ||||
| Antisense oligonucleotide targeting CD39 | Colorectal adenocarcinoma, breast cancer | Preclinical | [48] | ||||
| Anti-CD73 | MEDI9447 | Blocks CD73, reducing adenosine production. | Carcinoma, non-small-cell lung cancer | Clinical, phase III | NCT03381274 | ||
| Diclofenac | Pancreatic ductal adenocarcinoma | Preclinical | |||||
| PT199 | Metastatic cancer, refractory cancer, non-small cell lung cancer, pancreatic adenocarcinoma, pancreatic neoplasms, lung cancer | Clinical, phase I | NCT05431270 | ||||
| AK119 | Advanced or metastatic solid tumors | Clinical, phase I | NCT04572152 | ||||
| AB680 | Advanced pancreatic cancer | Clinical, phase II | NCT04104672 | ||||
| Others | Adenosine deaminase | Transforms adenosine (Ado) into inosine (INO), triggering a significant transcriptional shift toward a stem-like phenotype and bolstering CAR-T cell efficacy. | Diffuse midline gliomas | Preclinical | [292] | ||
| S1P | PF543 | SPHK1 inhibitor enhances T-cell-mediated cytotoxicity and modulates “find me” signals by blocking HDL-S1P-mediated cell migration. | Ovarian cancer | Preclinical | [293, 294] | ||
| Ex26 | A small-molecule antagonist that significantly inhibits HDL-S1P-mediated B16 cell migration. | Unclear | Unknown | [49] | |||
| CX3CL1 | Fasudil | Blocks Rho kinase (ROCK), disrupting cell migration and invasion, potentially modulating immune cell function and cytokine release. | Advanced solid tumor | Preclinical | [50] | ||
| Dasatinib | Inhibits Src family kinases and Bcr-Abl, inducing apoptosis in cancer cells and enhancing immune-mediated tumor cell killing, while also interfering with CX3CL1-induced tumor cell migration and metastasis. | Acute lymphoblastic leukemia, myeloma multiple, recurrent gastric cancer, etc. | Clinical, phase II | NCT00607594 | |||
| “Eat me” signals | |||||||
| CALR | ICD Inducers | Type I | Oxaliplatin | Induces immunogenicity by activating the unfolded protein response (UPR) pathway in the endoplasmic reticulum (ER) and upregulating calreticulin (CALR). | Carcinoma, transitional cell | Clinical, phase II | NCT04039867 |
| Mitoxantrone | Acute myeloid leukemia | Clinical, phase IV | NCT01587430 | ||||
| Cyclophosphamide |
Acute myeloid Leukemia Relapsed/refractory acute myeloid leukemia |
Clinical, phase I | NCT03318016 | ||||
| Shikonin | Breast cancer, liver cancer, pancreatic cancer | Clinical application | [51] | ||||
| Bortezomib | Multiple myeloma | Clinical, phase II | NCT01517724 | ||||
| Type II | High hydrostatic pressure (HHP) | Induces ER stress, leading to apoptosis, and upregulates CALR, enhancing cancer cell immunogenicity. | Osteosarcoma, pancreatic cancer | Clinical application | [295] | ||
| Radiotherapy | Head and neck cancer, esophageal cancer, nasopharyngeal cancer, etc. | Clinical application | [296] | ||||
| Hypericin-photodynamic therapy (HY-PDT) | Skin cancer, prostate cancer, etc. | Preclinical | [297, 298] | ||||
| Novel | RIG-I-like helicases | Induces immunogenic cell death of pancreatic cancer cells and sensitizes tumors toward killing by CD8+ T cells, while also upregulating CALR. | Melanoma, lung cancer, etc. | Preclinical | [299] | ||
| Pt (II)-NHC complex | Induces ROS-ERS-related DAMP balance to harness immunogenic cell death in hepatocellular carcinoma. | Ovarian cancer, esophageal cancer, etc. | Preclinical | [300] | |||
| Non-thermal plasma (NTP) | Stimulates cancer cells to release DAMP signals, leading to antigen presentation by APCs and initiation of adaptive immune responses. | Breast cancer, lung cancer, etc. | Preclinical | [301] | |||
| Nano-pulse stimulation | Stimulates caspase-3/7 activation and induced DAMP release. | Breast cancer, prostate cancer, etc. | Preclinical | [302] | |||
| SLAMF7 | SLAMF7-CAR T cell | Selectively kills normal lymphocytes with high expression of SLAMF7. | Multiple myeloma, lymphoma, etc. | Preclinical | [303, 304] | ||
| Elotuzumab | Targets SLAMF7's extracellular domain, activating NK cells through CD16 binding. | Multiple myeloma | Clinical, phase III | NCT05002816 | |||
| FcR | Fc glycosylation | Obinutuzumab | Target CD20 on B cells, activating cell death pathways and/or complement cascade, leading to B cell lysis. | Chronic lymphocytic leukemia | Clinical, phase II | NCT01868893 | |
| LFB-R603 | Targets CD20 on B cells. | Chronic lymphocytic leukemia | Clinical, phase I | NCT01098188 | |||
| Site mutation | MOR208 | CD19-targeting antibody with enhanced ADCC and ADCP, improving tumor cell killing. | Diffuse large B cell lymphoma | Clinical, phase II | NCT05222555 | ||
| BI 836826 | Fc-modified IgG1 class II anti-CD37 antibody that efficiently eliminates CLL cells in vivo. | Leukemia, chronic lymphocytic leukemia | Clinical, phase I | NCT02759016 | |||
| Fc multimerization | SAR442085 | Target CD38 with high affinity and effectively eliminate CD38-expressing tumor cells in vitro through ADCC and phagocytosis. | Plasma cell myeloma | Clinical, phase I | NCT04000282 | ||
| PS | Regulation of PS flipping | Xkr8 shRNA | Inhibit intracellular PS flipping, promoting tumor cell apoptosis and activating the cGAS-STING pathway. | Colon and pancreatic cancer, etc. | Preclinical | [305] | |
| PMBOP-CP | Improve tumor microenvironment and enhance antitumor activity in mouse models of colon and pancreatic cancer. | Colon and pancreatic cancer, etc. | Preclinical | [305] | |||
| Blocking the “do not eat me” signals | CD47/SIRPα axis blockade | Magrolimab | Interfere with SIRPα-CD47 interaction to block cancer cell's self-protection against macrophage phagocytosis. | Myelodysplastic syndromes, acute myeloid leukemia | Clinical, phase III | NCTO4313881 | |
| Letaplimab | Inhibit CD47-SIRPα binding enhances macrophage phagocytosis of tumor cells and promotes T cell cross-activation. | Acute myeloid leukemia | Clinical, phase I/II | NCT04485052 | |||
| RRX-001 | Immune checkpoint inhibitor that can downregulate. CD47 and SIRPα. | Small cell lung cancer | Clinical, phase III | NCT03699956 | |||
| AK117 | Bind to CD47 expressed on tumor cells, efficiently blocking the interaction between CD47 and its receptor SIRPα. | Triple-negative breast cancer (in situ advanced or metastatic) | Clinical, phase II | NCT0522766 | |||
| TTI-622 | SIRPα-Fc decoy receptor targets and binds to CD47, blocking its activity. | Oophoroma | Clinical, phase I/II | NCT05261490 | |||
| IMMO1 | Target human CD47 with an Fc fusion protein drug activates macrophages to engulf tumor cells, presenting the engulfed tumor antigens to T cells. | Myelodysplastic syndromes, Acute myeloid leukemia | Clinical, phase I/II | NCT05140811 | |||
| PD-1/PD- L1 axis blockade | ABSK043 | Potently inhibits the interaction of PD-1/PD-L1, relieving PD-L1-mediated inhibition of T cell activation. | Solid tumor | Clinical, phase I | NCT04964375 | ||
| MAX-10181 | Inhibiting PD-1/PD-L1 binding | Solid tumor | Clinical, phase I | NCT05196360 | |||
| Tomivosertib | MNK1/2 inhibitors can downregulate PD-L1 expression. | Breast cancer | Clinical, phase I | NCT04261218 | |||
| MHC-I/LILRB1 axis blockade | JTX-8064 | Binds LILRB2, stopping its interaction with ligands and MHC-I. | Cancer | Clinical, phase I/II | NCT04669899 | ||
| CD24/Siglec-10 axis blockade | ATG-031 | Block CD24 | Advanced solid tumors, B-cell non-Hodgkin lymphomas | Clinical, phase I | NCT06028373 |
- Abbreviations: ATP, adenosine triphosphate; CALR, calreticulin; CX3CL1, C-X3-C motif chemokine ligand 1; FcR, Fc receptor; PS, phosphatidylserine; S1P, sphingosine-1-phosphate; SLAMF7, signaling lymphocytic activation molecule family member 7.
4.1 Strategies to regulate “find me” signals
4.1.1 Reducing LPC levels
Currently, there are no drugs that directly target LPC. To reduce LPC levels during cancer therapy, practitioners should consider lipid-lowering medications such as statins, explore drugs targeting lipid metabolism, and recommend diets containing antioxidants.
4.1.2 Increasing ATP levels
Although some studies have indicated that extracellular ATP has clear tumorigenic effects, in most studies, increasing the extracellular ATP concentration was shown to favor antitumor immunity [224, 228].
Chemotherapy and radiotherapy can trigger ATP release. Various methods, including Fas crosslinking, ultraviolet (UV) treatment, and treatment with drugs such as etoposide, induce the release of nucleotides from cells such as Jurkat cells, primary thymocytes, MCF-7 cells, and lung epithelial cells [88]. Several agents that induce cell death, such as cadmium, etoposide, mitomycin C, oxaliplatin, cisplatin, staurosporine, thapsigargin, mitoxantrone, and doxorubicin, cause dying tumor cells to release ATP within 8-20 h after exposure in vitro [290, 291].
Another strategy is to increase the extracellular ATP concentration by inhibiting ATP hydrolysis. In the ATP-adenosine (ADO) pathway, CD39 is the rate-limiting enzyme responsible for hydrolyzing ATP into adenosine diphosphate (ADP) or adenosine monophosphate (AMP), while CD73 is a 5'-nucleotidase that converts AMP into ADO [306, 307]. Theoretically, CD39 suppresses ATP hydrolysis, leading to increased ATP levels in the TME, which promotes inflammation and cell proliferation while inhibiting the accumulation of ADO, counteracting ADO receptor-mediated immunosuppression, and preventing the long-term establishment of an immunosuppressive TME [308]. Zhang et al. [306] found that B cells release CD19+ extracellular vesicles containing the CD39 and CD73 enzymes, which convert ATP from chemotherapy-induced apoptotic tumor cells to ADO. ADO hinders CD8+ T-cell activation, reducing the antitumor impact of chemotherapy. By using mice lacking the Rab27a gene in B cells, the authors observed enhanced CD8+ T-cell activation after chemotherapy, resulting in tumor regression in some cases. However, Klysz et al. [292] indicated that knockout of CD39, CD73, or adenosine A2A receptor (A2AR) had minimal effects on exhausted chimeric antigen receptor (CAR) T cells. Conversely, the overexpression of adenosine deaminase, which metabolizes ADO into inosine, induced stemness features and significantly bolstered functionality.
4.1.3 Modulating S1P levels
S1P is highly expressed in cancer cells and can induce T-cell exhaustion. S1P can also upregulate the transcription of programmed death-ligand 1 (PD-L1) through E2F transcription factor 1 (E2F1) [309]. The sphingosine kinase 1 (SPHK1) inhibitor PF543 enhances T-cell-mediated cytotoxicity [309]. Furthermore, combining PF543 with anti-PD-1 antibodies in vivo more effectively reduces tumor burden and metastasis [309]. Janneh et al. [293] reported that the small-molecule antagonist Ex26-S1P significantly blocked high-density lipoprotein (HDL)-S1P-mediated B16 cell migration.
4.1.4 Reducing CX3CL1 levels
Microvascular barrier disruption is important for tumor cell metastasis. CX3CL1, a vital chemokine in vertebral bodies, is linked to this process. However, the precise role of CX3CL1 in guiding tumor cell migration to vertebral bodies remains uncertain. Yi et al. [310] found that CX3CL1 disrupts the microvascular endothelial cell barrier. This process involves stress fiber formation, cell contraction, damage to zonula occludens-1 (ZO-1) junctions, and increased permeability. The Src/P115 Rho guanine nucleotide exchange factor (P115-RhoGEF)/Rho-associated, coiled-coil containing protein kinase (ROCK) pathway is crucial for CX3CL1-induced barrier disruption and tumor cell migration across endothelial cells. Vincristine attracts CX3CR1+ monocytes to the sciatic nerve, causing pain. To reduce chemotherapy-induced pain, it is crucial to lower CX3CL1 levels. Old et al. [311] used CX3CR1 antagonists and CX3CL1 protein inhibitors targeting ADAM10/17 and/or cathepsin S to achieve this.
4.2 Strategies to regulate “eat me” signals
4.2.1 Increasing membrane exposure of CALR
When tumor cells die in response to external triggers, they can undergo a shift from nonimmunogenic to ICD. ICD involves the release of signaling molecules called damage-associated molecular patterns (DAMPs), which interact with DC receptors known as pattern recognition receptors (PRRs). This interaction initiates a chain reaction, activating innate and adaptive immune responses [312].
CALR becomes exposed on the cell membrane during ICD stimulation. Based on their mechanisms of action, ICD inducers can be classified into Type I and Type II [110]. Type I inducers indirectly trigger ER stress as a downstream effect. Type I inducers include well-known anticancer drugs such as anthracyclines (doxorubicin [313] and mitoxantrone [314]), taxanes (paclitaxel and docetaxel) [315], gemcitabine [316], cyclophosphamide [317], bortezomib [318], 5-fluorouracil [319], the third-generation platinum analog oxaliplatin [241], curcumin [320], cardiac glycosides [321] and the alkylating agent melphalan [322]. Type II inducers directly initiate ER stress, leading to cell death. Physical treatments such as photodynamic therapy [297] and near-infrared photoimmunotherapy [299, 323] are considered Type II inducers [110, 298, 324]. Both types of inducers initiate ER stress, activating pathways via ROS to release DAMPs, which interact with receptors such as LRP1/CD91, P2RX7/P2RY2, and innate immune cells (monocytes, neutrophils, macrophages, DCs) through TLR4 and other PRRs [325]. Several new therapies also induce ICD in tumor cells to promote antitumor immune responses. Studies have shown that oncolytic peptides such as LTX-315 [326] and RT53 [327] can induce ICD in tumor cells, leading to the release of a large number of DAMPs. DAMPs act as tumor vaccines, and nanoparticles (NPs) inhibit tumor growth by releasing DAMPs. Hyaluronic acid-conjugated polydopamine nanoparticles (HyPO) nanoparticles induce cell death in tumors. In a metastatic breast cancer model, HyPO nanoparticle therapy enhanced antitumor immunity, disrupted tumors and suppressed metastases [328]. Additionally, novel therapies that induce ICD in tumor cells include retinoic acid-inducible gene (RIG)-I-like helicases [299], oncolytic viruses (Ovs) [328], non-thermal plasma [301], and nanopulse stimulation [302].
Furthermore, the potency of CALR as an “eat me” signal can be strengthened through a strategy that restores CALR exposure. The pathways for the restoration of CALR exposure can be activated by eliminating or blocking inhibitory molecules (such as B7-H4 [139], PERK [329-331], ST3GAL1 [332], ST6GAL1 [262], and STC1 [262]), competitively disrupting inhibitory interactions (for example, between V-Set domain containing T Cell activation inhibitor 1 (VTCN1) and eIF2α or between CALR and STC1), providing favorable stress signals for CALR exposure, or using genetic engineering to create CALR variants that avoid STC1 retention and bind to the cell surface [262].
4.2.2 Regulating SLAMF7
Elotuzumab (Elo) is an IgG1 antibody targeting SLAMF7. SLAMF7 is expressed on tumor cells in 95% of multiple myeloma (MM) patients [271, 333]. Elo can activate NK cells through SLAMF7, inducing an FcγR-III-mediated ADCC antitumor immune response [186, 334]. Additionally, it participates in FcγR activation in TAMs to promote ADCP for tumor clearance [277]. Elo has shown effective responses in in vitro and in vivo preclinical studies of MM and has improved progression-free survival (PFS) in relapsed/refractory MM patients when used in combination with lenalidomide and dexamethasone [270].
Gogishvili et al. [303] utilized the huLuc63 antibody (Elo) to induce the expression of chimeric antigen receptors (CARs) on T cells to redirect their specificity to SLAMF7. They demonstrated that SLAMF7-CAR T cells from patients or donors showed antimyeloma effects. SLAMF7-CAR T cells selectively killed normal lymphocytes with high expression of SLAMF7. Through SLAMF7-CAR modification, both CD8+ and CD4+ T cells rapidly acquired and maintained the SLAMF7 phenotype. Importantly, the cytotoxicity induced by SLAMF7-CAR T cells preserved a fraction of cells in each cell population with a SLAMF7−/low phenotype, thus protecting functional lymphocytes, including virus-specific T cells.
Furthermore, some studies have suggested that high expression of SLAMF7 enhances the phagocytic activity of macrophages. Lu et al. [268] created a versatile nanobiologic platform called bispecific tumour-transforming nanoparticles (BiTNs), which were used for the treatment of HER2-expressing breast cancer. These nanoparticles combine anti-HER2 antibodies with recombinant SLAMF7, which is present on both cancer cells and phagocytes. In solid tumors, these BiTNs induce macrophage phagocytosis by targeting cells expressing SLAMF7 and blocking CD47 effectively. The study showed that SLAMF7-overexpressing cancer cells exhibited enhanced phagocytosis by macrophages upon CD47 blockade. Targeting HER2high breast cancer cells to convert them to the SLAMF7high phenotype was crucial for initiating macrophage phagocytosis.
4.2.3 Enhancing Fc signaling
The Fc region of IgG interacts with FcγRs on the surface of macrophages, providing an “eat me” signal that promotes macrophage anticancer activity. The main strategies for modifying the Fc fragment include Fc site-specific mutagenesis [335], glycosylation [173], and Fc multimerization [336].
Site-specific Fc mutagenesis boosts ADCC and ADCP. For example, single mutations such as I332E or double mutations such as S239D in trastuzumab's Fc region enhance the affinity of FcγR-IIIa-V158 by 10-fold and 100-fold, respectively [337]. Adding the A330L mutation to the double mutant increases effector FcγR-IIIa affinity while reducing inhibitory FcγR-IIb, resulting in 100- to 1000-fold ADCC enhancement [173].
Glycosylation of IgG Fc can alter its affinity for FcγRs, thereby regulating inflammatory responses and tumor cytotoxicity [173]. Changes in N-linked glycan modification of IgG influence its interaction with FcRs. Removing core fucose greatly enhances ADCC, boosting binding to FcγR-IIIa by 50-fold. Terminal sialic acid triggers inhibitory FcγR-IIb, giving antibodies anti-inflammatory properties. However, the sialylation of Fc reduces antibody binding to complement C1q, weakening CDC and making antibodies anti-inflammatory [273-276].
4.2.4 Regulating PS flipping
The flipping of PS provides a target for tumor treatment. Inactivation of the PS-promoting flippase Xkr8 in combination with an anti-PD-1 strategy allows complete tumor elimination in mice [289]. Although there are currently no small-molecule drugs targeting Xkr8, Wang et al. [289] used lentiviral particles to knock down Xkr8 in tumors using shRNA. Chen et al. [305] used modified nanoparticles to target tumors without affecting healthy tissues. These nanoparticles, called PMBOP-CPs, carried siXkr8 and FuOXP and were tested in mouse models of colon and pancreatic cancers. The combination of siXkr8 and FuOXP significantly improved the TME and enhanced antitumor activity.
4.2.5 Blocking “do not eat me” signals
Blocking the “do not eat me” signal serves to enhance the “eat me” signal, as it prevents the inhibition of the latter, thus amplifying its overall effectiveness within the signaling mechanism.
Almost all types of tumors have mechanisms to prevent phagocytes from engulfing them by expressing “do not eat me” signaling proteins on the surface of tumor cells [338]. In the late 2000s, the crosstalk between CD47 and SIRPα was recognized as the first checkpoint associated with tumor phagocytosis [21, 22, 339–341]. The PD-1/PD-L1 axis [23], MHC-I/leukocyte immunoglobulin-like receptor subfamily B member 1 (LILRB1) axis [342-344], and CD24/sialic acid-binding immunoglobulin-type lectin 10 (Siglec-10) axis [345] were successively discovered between 2017 and 2019. Subsequently, a range of monoclonal antibodies or fusion proteins targeting these four distinct macrophage phagocytosis-related checkpoints were developed [346].
CD47-SIRPα-directed therapy is currently being explored in multiple clinical trials in various tumor types. In numerous hematologic malignancies as well as solid tumors, there is a notable increase in the expression of CD47 [341, 347], with a significant positive correlation observed between high levels of CD47 expression and poor cancer prognosis [348-350]. Hence, inhibiting the CD47/SIRPα interaction could represent a promising strategy for cancer immunotherapy, either independently or in combination with other tumor-targeted therapies [351]. CD47-SIRPα-directed therapy is being investigated in clinical trials across multiple tumor types [352]. Magrolimab (Hu5F9-G4), an anti-CD47 monoclonal antibody, is under investigation alone or combined with other drugs [353, 354]. Trials in lymphoma patients showed that magrolimab combined with rituximab achieved overall response rates (ORRs) of 40% and 71% (NCT02953509) [353]. In CRC, treatment with magrolimab combined with cetuximab had a 6.7% ORR (NCT02953782). The use of magrolimab combined with avelumab for the treatment of ovarian cancer resulted in stable disease in 56% of patients (NCT03558139). CC-90002 is tested with rituximab for advanced cancers (NCT02367196). CC-95251, combined with rituximab, achieved a 41% ORR in non-Hodgkin lymphoma (NCT03783403) [355]. Recent studies have identified a novel regulator of ADCP in cancer cells and macrophages through genome-wide screening: adipocyte plasma membrane-associated protein (APMAP) [356]. Loss of APMAP expression, when combined with CD47 blockade monoclonal antibodies, enhances phagocytosis and suppresses tumor growth [356].
PD-1 is expressed in both murine and human TAMs, and its expression levels are correlated with cancer progression and advanced TNM stage [23, 357]. PD-1+ TAMs exhibit a phenotype resembling that of M2-like macrophages and demonstrate reduced phagocytic capacity toward cancer cells [358, 359]. The expression of PD-1 inhibits the function of various immune cells in the TME, including T cells [360], B cells [361], NK cells [362], and DCs [363]. Research on antibody-mediated blockade of the PD-1/PD-L1 axis has been reported in various cancer models, including melanoma [364], non-small cell lung cancer (NSCLC) [365], and renal cell carcinoma [366, 367]. The PD-1 inhibitor pembrolizumab (Keytruda) has received the US Food and Drug Administration (FDA) approval for the treatment of various cancers, including melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, urothelial carcinoma, gastric cancer, cervical cancer, primary mediastinal large B-cell lymphoma, and solid tumors with high microsatellite instability. The PD-L1-targeting antibody atezolizumab (Tecentriq) has demonstrated significant anti-tumor efficacy in preclinical research and clinical trials and received the US FDA approval for marketing in May 2016 [368, 369]. Data from a phase III clinical trial targeting PD-L1+ NSCLC patients suggest that pembrolizumab, a PD-1 antibody, demonstrates more significant efficacy compared to platinum-based doublet chemotherapy [370]. PD-1/PD-L1 inhibitors, including nivolumab, pembrolizumab, and camrelizumab, have also been applied clinically for the treatment of melanoma, NSCLC, renal cancer, Hodgkin lymphoma, and other diseases [7].
LILRB1 and LILRB2 are considered candidate regulators of MHC-I-mediated phagocytic suppression. The MHC-I/LILRB1 signaling axis represents a “do not eat me” signaling pathway, where inhibition of LILRB1 or MHC-I could significantly enhance the phagocytic activity against tumor cells [8, 9, 24]. LILRB1 monoclonal antibody enhances the anti-tumor activity of NK cells in multiple myeloma, leukemia, and lymphoma [10]. However, it remains unclear whether blocking LILRB2 would directly or indirectly promote the phagocytic activity of TAMs. In a previous study by Chen et al. [344], therapeutic antibody blockade of LILRB2 was shown to promote macrophage maturation and enhance their pro-inflammatory phenotype. Anti-LILRB2 monoclonal antibodies are currently under investigation in phase I clinical trials for malignant tumors (JTX-8064, INNATE).
CD24 constitutes a “do not eat me” signal and is defined as an immune checkpoint by its interaction with the inhibitory receptor Siglec-10 on macrophages within the TME [11, 12]. Accordingly, CD24 blockade using a monoclonal antibody induced macrophage-mediated phagocytosis of breast, ovarian, and pancreatic cancer cell lines in vitro and inhibited tumor growth of breast cancer MCF-7 cell xenograft in a non-obese diabetic (NOD)-scid IL2rγnull (NSG) mouse model.
Precisely modulating the functions of phagocytes, including the regulation of “find me,” “eat me,” and “do not eat me” signals, is pivotal in tumor immunology. However, the augmentation of phagocyte populations does not consistently enhance tumor immunity. In certain contexts, specific subsets of macrophages may elicit immune suppression, particularly in response to tumor antigens. For instance, in murine models with liver metastases, the expansion of hepatic CD11b+F4/80+ myeloid cells characterized by heightened expression of colony-stimulating factor 1 receptor (CSF-1R) has been associated with resistance to immunotherapy [13]. Yu et al. [288] demonstrated that targeted depletion of these myeloid cells using low-dose clodronate liposomes and anti-CSF-1 monoclonal antibody effectively mitigated intrahepatic antigen-specific T cell apoptosis, highlighting a potential strategy to overcome immunotherapy resistance.
5 CONCLUSIONS
The “find me” and “eat me” signals can directly and indirectly alter the behavior of phagocytes, suggesting significant implications for tumor immunotherapy. Researchers have identified various “find me” and “eat me” signals produced by tumor cells, such as PS and CALR. These signals can induce phagocytes in the immune system to recognize, locate, and engulf tumor cells, thereby promoting tumor clearance. However, it is intriguing that not all upregulation of “find me” and “eat me” signals benefits anti-tumor immunity. For instance, the upregulation of signals like LPC and CX3CL1 can accelerate tumor progression, but the underlying mechanisms remain unclear. Therefore, the effects of “eat me” and “do not eat me” signals in anti-tumor immunity should be considered dialectically. Exploring the mechanisms and molecules associated with these signals could pave the way for improving cancer treatment. Combining phagocytic signals targeting cancer with other interventions, such as oncolytic viruses, CAR-T cells, exosomes, and nanoparticles, can potentially enhance anti-tumor immune responses more effectively.
AUTHOR CONTRIBUTIONS
All authors have read and approved the article. JW and LX organized, wrote, and revised the manuscript. LZ and CG revised the manuscript. LX, QX, CJ and XG summarized the literature.
ACKNOWLEDGMENTS
The figures in this article were generated using FigDraw, and we thank the platform for its abundant resources. The research was supported by the Shandong Provincial Laboratory Project (SYS202202); the National Natural Science Foundation of China (81972888, 82272819); the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-202219B, JNL-202204A, JNL-2023017D); Jiangsu Provincial Key Research and Development Program (BE2022840); and Nanjing University of Chinese Medicine (2020YLXK007).
CONFLICTS OF INTEREST STATEMENTS
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable
Open Research
DATA AVAILABILITY STATEMENTS
Not applicable




