Disitamab vedotin, a HER2-directed antibody-drug conjugate, in patients with HER2-overexpression and HER2-low advanced breast cancer: a phase I/Ib study
Trial registration: NCT02881138 (C001 CNACER); NCT03052634 (C003 CNACER)
Abstract
Background
Disitamab vedotin (DV; RC48-ADC) is an antibody-drug conjugate comprising a human epidermal growth factor receptor 2 (HER2)-directed antibody, linker and monomethyl auristatin E. Preclinical studies have shown that DV demonstrated potent antitumor activity in preclinical models of breast, gastric, and ovarian cancers with different levels of HER2 expression. In this pooled analysis, we report the safety and efficacy of DV in patients with HER2-overexpression and HER2-low advanced breast cancer (ABC).
Methods
In the phase I dose-escalation study (C001 CANCER), HER2-overexpression ABC patients received DV at doses of 0.5-2.5 mg/kg once every two weeks (Q2W) until unacceptable toxicity or progressive disease. The dose range, safety, and pharmacokinetics (PK) were determined. The phase Ib dose-range and expansion study (C003 CANCER) enrolled two cohorts: HER2-overexpression ABC patients receiving DV at doses of 1.5-2.5 mg/kg Q2W, with the recommended phase 2 dose (RP2D) determined, and HER2-low ABC patients receiving DV at doses of 2.0 mg/kg Q2W to explore the efficacy and safety of DV in HER2-low ABC.
Results
Twenty-four patients with HER2-overexpression ABC in C001 CANCER, 46 patients with HER2-overexpression ABC and 66 patients with HER2-low ABC in C003 CANCER were enrolled. At 2.0 mg/kg RP2D Q2W, the confirmed objective response rates were 42.9% (9/21; 95% confidence interval [CI]: 21.8%-66.0%) and 33.3% (22/66; 95% CI: 22.2%-46.0%), with median progression-free survival (PFS) of 5.7 months (95% CI: 5.3-8.4 months) and 5.1 months (95% CI: 4.1-6.6 months) for HER2-overexpression and HER2-low ABC, respectively. Common (≥5%) grade 3 or higher treatment-emergent adverse events included neutrophil count decreased (17.6%), gamma-glutamyl transferase increased (13.2%), asthenia (11.0%), white blood cell count decreased (9.6%), peripheral neuropathy such as hypoesthesia (5.9%) and neurotoxicity (0.7%), and pain (5.9%).
Conclusion
DV demonstrated promising efficacy in HER2-overexpression and HER2-low ABC, with a favorable safety profile at 2.0 mg/kg Q2W.
List of abbreviations
-
- ABC
-
- Advanced breast cancer
-
- ADC
-
- Antibody-drug conjugate
-
- AEs
-
- Adverse events
-
- AUC
-
- Area under the concentration-time curve
-
- CBR
-
- Clinical benefit rate
-
- CI
-
- Confidence interval
-
- Cmax
-
- Peak plasma concentration
-
- CT
-
- Computed tomography
-
- CTCAE
-
- Common Terminology Criteria for Adverse Events
-
- DCR
-
- Disease control rate
-
- DLT
-
- Dose-limiting toxicity
-
- DOR
-
- Duration of response
-
- DV
-
- Disitamab vedotin
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- FISH
-
- Fluorescence in situ hybridization
-
- HER2
-
- Human epidermal growth factor receptor 2
-
- HR
-
- Hormone receptor
-
- IHC
-
- Immunohistochemistry
-
- ILD
-
- Interstitial lung disease
-
- LVEF
-
- Left ventricular ejection fraction
-
- MRI
-
- Magnetic resonance imaging
-
- MTD
-
- Maximum tolerated dose
-
- MMAE
-
- Microtubule inhibitor monomethyl auristatin E
-
- NCA
-
- Noncompartmental analysis
-
- NCI
-
- National Cancer Institute
-
- ORR
-
- Objective response rate
-
- OS
-
- overall survival
-
- PS
-
- Performance status
-
- AJCC
-
- American Joint Committee on Cancer
-
- TNM
-
- Tumor Node Metastasis
-
- PK
-
- Pharmacokinetics
-
- PD
-
- Progressive disease
-
- PFS
-
- Progression-free survival
-
- Q2W
-
- Every two weeks
-
- Ra(AUC)
-
- Accumulation ratio for AUC
-
- RECIST
-
- Response Evaluation Criteria in Solid Tumors
-
- RP2D
-
- Recommended phase 2 dose
-
- t1/2
-
- half-life
-
- TAb
-
- Total antibody
-
- T-DXd
-
- Trastuzumab deruxtecan
-
- SG
-
- sacituzumab govitecan
-
- TEAEs
-
- Treatment-emergent adverse events
-
- tmax
-
- time to Cmax
-
- VC
-
- Valine-citrulline
-
- CDK
-
- cyclin-dependent kinase
-
- MBC
-
- Metastatic breast cancer.
1 BACKGROUND
Breast cancer emerged as the most prevalent cancer in 2020, with an estimated 2.3 million new cases and 685,000 deaths globally [1]. Human epidermal growth factor receptor 2 (HER2) overexpression, which is seen in 15%-20% of breast cancers, is associated with aggressive disease and poor prognosis [2]. The level of expression of HER2 in biopsy samples was determined by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) [3]. At present, trastuzumab deruxtecan (T-DXd) has become the standard second-line treatment for HER2-overexpression advanced breast cancer (ABC) [4]. However, there is no standard treatment after the failure of T-DXd, which means there are still unmet clinical needs.
HER2-low breast cancer, defined as IHC 1+ or IHC 2+ and FISH-, represents 45%-55% of all breast cancers [3]. Chemotherapy remains the main treatment option for patients with metastatic disease, especially in China [5, 6]. However, novel antibody-drug conjugates (ADCs) have shown promising antitumor activity in various tumor types [7], including breast cancer [8]. T-DXd, an ADC targeting HER2, has become the standard treatment for HER2-low breast cancer after first-line chemotherapy [5]. After the failure of second-line chemotherapy, there is no standard treatment available, and sacituzumab govitecan (SG) can be used for patients with triple-negative breast cancer but not for Hormone receptor (HR)+/HER2- ABC in China [9, 10]. Therefore, there are still clinical needs to be met after the failure of standard treatment for patients with HER2-low ABC.
One such ADC is disitamab vedotin (DV), also known as RC48-ADC. DV is composed of a fully humanized anti-HER2 antibody linked to the microtubule inhibitor monomethyl auristatin E (MMAE) via a protease-cleavable vedotin linker [11, 12]. Preclinical studies have shown that DV has a higher affinity to immobilized HER2 than trastuzumab [11]. When DV binds to HER2 on tumor cells, it is internalized, and MMAE is released through proteolytic cleavage of the valine-citrulline (vc) MMAE drug linker. MMAE can then exert a bystander effect on adjacent tumor cells regardless of their HER2 status [13]. DV has demonstrated potent antitumor activity in preclinical models of breast [11], gastric [14], and ovarian cancers [15] with different levels of HER2 expression.
The phase I study C001 CANCER evaluated the safety, pharmacokinetics (PK), and efficacy of DV in patients with HER2-overexpression ABC. The trial included five different dose groups, ranging from 0.5 to 2.5 mg/kg, administered once every two weeks (Q2W). Previous results demonstrated good tolerability and preliminary antitumor activity of DV in HER2-overexpression ABC [16]. Since responses were observed in HER2-overexpression ABC patients receiving DV of 1.5, 2.0, and 2.5 mg/kg in the C001 CANCER, the efficacy and safety of DV in HER2-overexpression ABC at these different dose levels were further explored in one cohort of HER2-overexpression ABC in the phase Ib study C003 CANCER. After the 2.0 mg/kg dose was determined to be the recommended phase 2 dose (RP2D), another cohort of HER2-low ABC patients was included to explore the efficacy and safety of DV in HER2-low ABC. The updated data validated the efficacy offigure DV in ABC patients with different HER2 statuses [17]. Herein, we presented additional data from the phase I/Ib trial and performed a pooled analysis to assess the safety and efficacy of DV in patients with HER2-overexpression and HER2-low ABC.
2 METHODS
2.1 Study design and participants
A phase I/Ib, open-label, single-arm study was conducted in China (Figure 1). The phase I dose-escalation study (C001 CANCER) using a standard 3 + 3 design determined the maximal tolerance dose (MTD), safety, and PK of DV in patients with HER2-overexpression ABC. Participants received initial intravenous doses of DV of 0.5, 1.0, 1.5, 2.0, and 2.5 mg/kg Q2W, and dose-limiting toxicities (DLTs) were assessed over a 28-day cycle. Since the responses were observed at 1.5, 2.0, and 2.5 mg/kg dose levels in the C001 CANCER study, meanwhile one cohort of the phase Ib study (C003 CANCER) was designed to establish the RP2D of DV and analyze its PK and efficacy in HER2-overexpression ABC with these three dose levels, 15 patients were planned to be enrolled in each group, and the higher dose group was started successively after the lower dose group was enrolled. After the 2.0 mg/kg dose was determined to be RP2D, another cohort of HER2-low ABC patients was included to explore the efficacy and safety of DV.
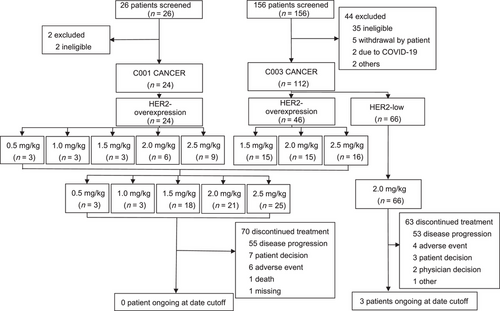
Eligible patients were at least 18 years old with a life expectancy of more than 12 weeks, had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0-1, had histologically or cytologically confirmed invasive breast cancer which was in the locally advanced or metastatic stage as determined by the 7th edition of American Joint Committee on Cancer (AJCC) Tumor Node Metastasis (TNM) staging system, were refractory to standard of care or unable to receive standard of care, had at least one measurable disease based on Response Evaluation Criteria in Solid Tumors (RECIST) criteria, version 1.1 [18], and had adequate organ functions (see the Supplementary Material for full eligibility criteria). Patients were excluded if they had brain or meningeal metastases. HER2 status was determined by local pathology laboratory results according to the 2014 Chinese breast cancer HER2 detection guideline [19]. HER2-overexpression was defined as IHC 3+, IHC 2+/FISH+. HER2-low was defined as IHC 2+/FISH-, IHC 1+/FISH-, or IHC 1+/FISH untested.
All patients provided written informed consent, and the study protocol was approved by independent ethics committees or institutional review boards at each site (approval No. 15-112/1039 [C001 CANCER] and 16-180/1259 [C003 CANCER]). The pooled analyses are in accordance with the two study protocols, the guidelines for Good Clinical Practice and the Declaration of Helsinki.
2.2 Treatment and assessments
Eligible patients received DV (RemeGen Co., Ltd., Yantai, Shandong, China) via intravenous infusion Q2W until progressive disease (PD), unacceptable toxicity, or withdrawal of consent. The dose-escalation phase involved evaluating doses of 0.5, 1.0, 1.5, 2.0, and 2.5 mg/kg Q2W using a 3 + 3 design. The DLTs were grade 4 neutropenia that was observed but effectively managed, grade 3 neutropenia concurrent with infection, and grade 3 nonhematological toxicity. Three doses (1.5, 2.0, and 2.5 mg/kg Q2W) were chosen for the phase Ib study in patients with HER2-overexpression ABC, and only 2.0 mg/kg Q2W was tested in patients with HER2-low ABC.
Spiral computed tomography or magnetic resonance imaging was performed at baseline, every 6 weeks for 24 weeks, and every 12 weeks thereafter. RECIST version 1.1 criteria were used to evaluate disease response and progression. Laboratory assessments were performed Q2W during treatment and 4 weeks after the last treatment.
The safety of DV was assessed by evaluating adverse events (AEs), changes in vital signs and laboratory tests in patients who received at least one dose of DV. The classification of treatment-emergent adverse events (TEAEs) was based on the Medical Dictionary for Regulatory Activities, version 23.0 (www.meddra.org) [20], and they were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0) [21]. In the C003 CANCER study, dexamethasone was administered before DV infusion after the dose-escalation phase to explore its potential in preventing peripheral neuropathy.
2.3 PK analysis
PK analysis of DV involved collecting blood samples during the first three doses at predetermined times up to 336 h post-dose. Phoenix WinNonLin version 8.1 (Pharsight Corp., Mountain View, CA, USA) was used to perform non-compartmental analysis. This analysis determined the peak plasma concentration (Cmax), the time to Cmax (tmax), the area under the concentration-time curve (AUC), the elimination half-life (t1/2) and the accumulation ratio (Ra(AUC)).
2.4 Statistical analysis
The pooled analyses were conducted on the full analysis set and safety set using data from the C001 CANCER and C003 CANCER studies. The full analysis set included patients who had provided informed consent, were enrolled in the phase I/Ib study, and received at least one dose of DV. The safety set consisted of patients who received at least one dose of DV and had available posttreatment safety evaluation data. Clinical activity endpoints were calculated separately for investigator-assessed data.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The efficacy results were analyzed based on the full analysis set, and the safety results were analyzed based on the safety set. Clinical activity endpoints included objective response rate (ORR), disease control rate (DCR), clinical benefit rate (CBR), overall survival (OS), progression-free survival (PFS), percent change of target lesions, and duration of response (DOR). ORR was defined as complete response (CR) plus partial response (PR). DCR was defined as CR, PR, or stable disease (SD) for a minimum of 6 weeks from the first dosing date. CBR was defined as CR, PR, or SD for a minimum of 6 months from the first dosing date. OS was defined as the time from the date of the first dose to the date of death from any cause. PFS was defined as the time from the date of the first dose to the first objective documentation of radiographic PD or death due to any cause, whichever was earlier. DOR was measured from the time at which CR or PR criteria were first met to the first date of objectively documented PD or death due to any cause. PD was defined as an increase in the sum of diameters of target lesions of at least 20% and an absolute increase of at least 5 mm, or the appearance of ≥1 new lesion. The ORR, DCR and CBR were reported with point estimates and two-sided 95% confidence intervals (CIs) using the Clopper–Pearson method. Time-to-event statistics were calculated using the Kaplan-Meier method. No statistical tests or P values were provided in efficacy analyses. Safety evaluations included all AEs and serious AEs observed before October 30, 2021. TEAEs were summarized by grade and dose level. Fisher's exact test was employed to compare the proportions of patients with peripheral neuropathy between groups (with or without dexamethasone pretreatment), and the log-rank test was used to compare the first occurrence time of peripheral neuropathy between the groups. Descriptive statistics summarized the best percent change in the sum of the diameters of measurable tumors, demographic characteristics, and safety data.
3 RESULTS
3.1 Patient disposition and baseline characteristics
Between March 2016 and March 2018, 24 patients with HER2-overexpression ABC received DV at 5 dose levels (0.5, 1.0, 1.5, 2.0, and 2.5 mg/kg) in the C001 CANCER study (Figure 1). In the C003 CANCER study, 46 patients in the HER2-overexpression cohort received DV at 3 doses (1.5, 2.0, and 2.5 mg/kg) between December 2016 and October 2018, and 66 patients in the HER2-low cohort received DV at a single dose level of 2.0 mg/kg between October 2018 and June 2021.
Pooled analysis of the two studies included 136 patients with HER2-overexpression (n = 70) and HER2-low ABC (n = 66). Of these, 87 patients received the recommended dose of 2.0 mg/kg. The data cutoff date was October 30, 2021. The median follow-up was 5.1 months (range, 0-24.1 months) for patients with HER2-overexpression ABC and 4.3 months (range, 0-27.4 months) for patients with HER2-low ABC, and only 3/136 patients (2.2%) continued to receive DV treatment at date cutoff, and all three patients with HER2-low. The primary reason for treatment termination was disease progression (HER2-overexpression: 55/70 [78.6%]; HER2-low: 53/66 [80.3%]), followed by AEs (HER2-overexpression: 6/70 [8.6%]; HER2-low: 4/66 [6.1%]) (Figure 1).
Baseline demographic and clinical characteristics are presented in Table 1. All patients were female, with a median age of 54 years (range, 26-69 years), and a majority of patients (120/136; 88.2%) had visceral metastases. In the HER2-overexpression ABC group, 49/70 (70.0%) of patients had previously received trastuzumab. For the HER2-low ABC group, there were 35/66 (53.0%) patients with IHC 2+/FISH- and 31/66 (47.0%) patients with IHC 1+. HR positivity was observed in 60/66 (90.9%) patients with HER2-low ABC, with 49/66(74.2%) of patients receiving endocrine therapy.
| Characteristics | HER2- overexpression breast cancer IHC3+, IHC2+/FISH+ | HER2-low breast cancer IHC 2+/FISH-, IHC1+ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All Patients | 0.5-1.0 mg/kg (n = 6) | 1.5 mg/kg (n = 18) | 2.0 mg/kg (n = 21) | 2.5 mg/kg (n = 25) | Total (n = 70) | 2.0 mg/kg (n = 35) | 2.0 mg/kg (n = 31) | Total (n = 66) | (n = 136) |
| Median age (range), years | 57.5 (40-65) | 49.5 (26-62) | 58.0 (35-62) | 53.0 (26-66) | 53.0 (26-66) | 55.0 (29-69) | 53.0 (27-69) | 54.5 (27-69) | 54.0 (26-69) |
| Sex, n (%) Female | 6 (100%) | 18 (100%) | 21 (100%) | 25 (100%) | 70 (100%) | 35 (100%) | 31 (100%) | 66 (100%) | 136 (100%) |
| Median time from initial diagnosis (range), years | 3.60 (2.6-6.8) | 2.65 (0.8-11.7) | 3.80 (1.5-9.3) | 3.40 (1.1-24.0) | 3.25 (0.8-24.0) | 4.00 (0.5-16.0) | 6.00 (0.7-25.0) | 4.90 (0.5-25.0) | 3.80 (0.5-25.0) |
| ECOG performance status score, n (%) | |||||||||
| 0 | 0 | 5 (27.8%) | 9 (42.9%) | 18 (72.0%) | 32 (45.7%) | 27 (77.1%) | 18 (58.1%) | 45 (68.2%) | 77 (56.6%) |
| 1 | 6 (100%) | 13 (72.2%) | 12 (57.1%) | 7 (28.0%) | 38 (54.3%) | 8 (22.9%) | 13 (41.9%) | 21 (31.8%) | 59 (43.4%) |
| Most common metastasis sites of diseasea, n (%) | |||||||||
| Visceral | 6 (100%) | 16 (88.9%) | 19 (90.5%) | 23 (92.0%) | 64 (91.4%) | 32 (91.4%) | 24 (77.4%) | 56 (84.8%) | 120 (88.2%) |
| Liver | 4 (66.7%) | 12 (66.7%) | 13 (61.9%) | 17 (68.0%) | 46 (65.7%) | 25 (71.4%) | 12 (38.7%) | 37 (56.1%) | 83 (61.0%) |
| Lung | 6 (100%) | 9 (50.0%) | 9 (42.9%) | 11 (44.0%) | 35 (50.0%) | 19 (54.3%) | 11 (35.5%) | 30 (45.5%) | 65 (47.8%) |
| HR status, n (%) | |||||||||
| HR + | 1 (16.7%) | 9 (50.0%) | 7 (33.3%) | 12 (48.0%) | 29 (41.4%) | 33 (94.3%) | 27 (87.1%) | 60 (90.9%) | 89 (65.4%) |
| HR - | 1 (16.7%) | 8 (44.4%) | 8 (38.1%) | 7 (28.0%) | 24 (34.3%) | 2 (5.7%) | 4 (12.9%) | 6 (9.1%) | 30 (22.1%) |
| Missing | 4 (66.7%) | 1 (5.6%) | 6 (28.6%) | 6 (24.0%) | 17 (24.3%) | 0 | 0 | 0 | 17 (12.5%) |
| HER2 expression, n (%) | |||||||||
| IHC 1+ | 0 | 0 | 0 | 0 | 0 | 0 | 31(100%) | 31 (47.0%) | 31 (22.8%) |
| IHC 2+/FISH- | 0 | 0 | 0 | 0 | 0 | 35(100%) | 0 | 35 (53.0%) | 35 (25.7%) |
| IHC 2+/FISH+ or IHC 3+ | 6(100%) | 18(100%) | 21(100%) | 25(100%) | 70(100%) | 0 | 0 | 0 | 70 (51.5%) |
| Median No. of prior anticancer regimens (range) | 2.5 (1-8) | 3 (0-9) | 3 (1-9) | 3 (1-5) | 3 (0-9) | 2 (0-12) | 4 (0-8) | 3 (0-12) | 3 (0-12) |
| Prior systemic anticancer therapyb, n (%) | |||||||||
| ≥3 systemic chemotherapy | 3 (50.0%) | 9 (50.0%) | 8 (38.1%) | 10 (40.0%) | 30 (42.9%) | 6 (17.1%) | 8 (25.8%) | 14 (21.2%) | 44 (32.4%) |
| Trastuzumab | 3 (50.0%) | 10 (55.6%) | 13 (61.9%) | 23 (92.0%) | 49 (70.0%) | 2 (5.7%) | 1 (3.2%) | 3 (4.5%)c | 52 (38.2%) |
| Pertuzumab | 0 | 0 | 0 | 1 (4.0%) | 1 (1.4%) | 0 | 0 | 0 | 1 (0.7%) |
| HER2-targeted TKI | 4 (66.7%) | 6 (33.3%) | 4 (19.0%) | 15 (60.0%) | 29 (41.4%) | 0 | 0 | 0 | 29 (21.3%) |
| Endocrine therapy | 1 (16.7%) | 3 (16.7%) | 3 (14.3%) | 5 (20.0%) | 12 (17.1%) | 26 (74.3%) | 23 (74.2%) | 49 (74.2%) | 61 (44.9%) |
| CDK 4/6 inhibitor | 0 | 0 | 0 | 1 (4.0%) | 1 (1.4%) | 5 (14.3%) | 9 (29.0%) | 14 (21.2%) | 15 (11.0%) |
| Other systemic therapy | 0 | 2 (11.1%) | 4 (19.0%) | 3 (12.0%) | 9 (12.9%) | 6 (17.1%) | 12 (38.7%) | 18 (27.3%) | 27 (19.9%) |
- Abbreviations: BC, breast cancer; CDK, cyclin-dependent kinase; ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; HR, hormone receptor; TKI, tyrosine kinase inhibitor.
- a Patients with advanced breast cancer may combine multiple site metastases.
- b These patients with advanced breast cancer who had received prior multiple lines therapy.
- c These patients were diagnosed with HER2-overexpression breast cancer at the time of surgery, received trastuzumab in the adjuvant stage, and had HER2-low breast cancer after recurrence and metastasis.
3.2 Safety
A total of 136 patients received DV. The median number of doses administered was 9 (range, 1-48), and the median treatment duration was 151 (range, 14-834) days. The number of treatment cycles and duration were similar across the 2.0 and 2.5 mg/kg dose groups.
In total, 133/136 (97.8%) of patients experienced at least one TEAE, and 68/136 (50.0%) had an AE grade of 3 or higher (Supplementary Table S1). The most common grade 3 or higher AEs were neutrophil count decreased (24/136, 17.6%), gamma-glutamyl transferase increased (18/136, 13.2%), asthenia (15/136, 11.0%), white blood cell count decreased (13/136, 9.6%), peripheral neuropathy including hypoesthesia (8/136, 5.9%) and neurotoxicity (1/136, 0.7%), and pain (8/136, 5.9%) (Table 2, Supplementary Table S2). Platelet count decreased occurred in 13/136 (9.6%) of patients, with 2/136 (1.5%) of patients experiencing grade 3 or higher. One patient (0.7%) in the 2.5 mg/kg dose group developed a grade 1 left ventricular ejection fraction decreased (Supplementary Table S2).
| Disitamab vedotin [cases (%)] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 mg/kg | 1.0 mg/kg | 1.5 mg/kg | 2.0 mg/kg | 2.5 mg/kg | Total | |||||||
| (n = 3) | (n = 3) | (n = 18) | (n = 87) | (n = 25) | (n = 136) | |||||||
| Adverse events | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 | All | Grade ≥3 |
| Hematology investigations | ||||||||||||
| White blood cell count decreased | 0 | 0 | 1 (33.3) | 0 | 5 (27.8) | 2 (11.1) | 42 (48.3) | 6 (6.9) | 16 (64.0) | 5 (20.0) | 64 (47.1) | 13 (9.6) |
| Neutrophil count decreased | 0 | 0 | 1 (33.3) | 0 | 5 (27.8) | 2 (11.1) | 40 (46.0) | 12 (13.8) | 18 (72.0) | 10 (40.0) | 64 (47.1) | 24 (17.6) |
| Anemia | 1 (33.3) | 0 | 1 (33.3) | 0 | 4 (22.2) | 0 | 10 (11.5) | 1 (1.1) | 5 (20.0) | 0 | 21 (15.4) | 1 (0.7) |
| Hepatobiliary investigations | ||||||||||||
| AST increased | 2 (66.7) | 0 | 1 (33.3) | 0 | 11 (61.1) | 1 (5.6) | 64 (73.6) | 4 (4.6) | 19 (76.0) | 1 (4.0) | 97 (71.3) | 6 (4.4) |
| ALT increased | 2 (66.7) | 0 | 0 | 0 | 12 (66.7) | 1 (5.6) | 50 (57.5) | 0 | 20 (80.0) | 1 (4.0) | 84 (61.8) | 2 (1.5) |
| Gamma-glutamyl transferase increased | 0 | 0 | 1 (33.3) | 0 | 1 (5.6) | 0 | 39 (44.8) | 15 (17.2) | 4 (16.0) | 3 (12.0) | 45 (33.1) | 18 (13.2) |
| Blood alkaline phosphatase increased | 0 | 0 | 1 (33.3) | 0 | 1 (5.6) | 0 | 25 (28.7) | 3 (3.4) | 0 | 0 | 27 (19.9) | 3 (2.2) |
| Bilirubin conjugated increased | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 15 (17.2) | 3 (3.4) | 6 (24.0) | 0 | 22 (16.2) | 3 (2.2) |
| Blood bilirubin increased | 0 | 0 | 0 | 0 | 3 (16.7) | 1 (5.6) | 11 (12.6) | 1(1.1) | 2 (8.0) | 0 | 16 (11.8) | 2 (1.5) |
| Peripheral neuropathy | ||||||||||||
| Hypoesthesia | 0 | 0 | 1 (33.3) | 0 | 5 (27.8) | 1 (5.6) | 47 (54.0) | 5 (5.7) | 18 (72.0) | 2 (8.0) | 71 (52.2) | 8 (5.9) |
| Gastrointestinal disorders | ||||||||||||
| Nausea | 2 (66.7) | 0 | 0 | 0 | 3 (16.7) | 0 | 35 (40.2) | 1 (1.1) | 13 (52.0) | 1 (4.0) | 53 (39.0) | 2 (1.5) |
| Vomited | 2 (66.7) | 0 | 0 | 0 | 0 | 0 | 13 (14.9) | 0 | 6 (24.0) | 0 | 21 (15.4) | 0 |
| Constipation | 0 | 0 | 0 | 0 | 1 (5.6) | 0 | 9 (10.3) | 1 (1.1) | 7 (28.0) | 0 | 17 (12.5) | 1 (0.7) |
| Others | ||||||||||||
| Asthenia | 1 (33.3) | 0 | 0 | 0 | 5 (27.8) | 0 | 32 (36.8) | 3 (3.4) | 20 (80.0) | 12 (48.0) | 58 (42.6) | 15 (11.0) |
| Alfa hydroxybutyrate dehydrogenase increased | 0 | 0 | 0 | 0 | 0 | 0 | 36 (41.4) | 0 | 0 | 0 | 36 (26.5) | 0 |
| Weight decreased | 0 | 0 | 0 | 0 | 2 (11.1) | 0 | 27 (31.0) | 1 (1.1) | 4 (16.0) | 0 | 33 (24.3) | 1 (0.7) |
| Blood lactate dehydrogenase increased | 0 | 0 | 0 | 0 | 1 (5.6) | 0 | 32 (36.8) | 1 (1.1) | 0 | 0 | 33 (24.3) | 1 (0.7) |
| Blood glucose increased | 0 | 0 | 0 | 0 | 5 (27.8) | 0 | 18 (20.7) | 0 | 9 (36.0) | 2(8.0) | 32 (23.5) | 2 (1.5) |
| Fever | 0 | 0 | 0 | 0 | 1 (5.6) | 0 | 10 (11.5) | 0 | 13 (52.0) | 0 | 24 (17.6) | 0 |
| Pruritus | 0 | 0 | 0 | 0 | 1 (5.6) | 0 | 9 (10.3) | 0 | 13 (52.0) | 0 | 23 (16.9) | 0 |
| Rash | 1 (33.3) | 0 | 0 | 0 | 1 (5.6) | 0 | 9 (10.3) | 2 (2.3) | 10 (40.0) | 0 | 21 (15.4) | 2 (1.5) |
| Decreased appetite | 0 | 0 | 0 | 0 | 0 | 0 | 8 (9.2) | 0 | 13 (52.0) | 0 | 21 (15.4) | 0 |
| Pain | 0 | 0 | 0 | 0 | 0 | 0 | 8 (9.2) | 1 (1.1) | 13 (52.0) | 7 (28.0) | 21 (15.4) | 8 (5.9) |
| Alopecia | 0 | 0 | 0 | 0 | 5 (27.8) | 0 | 13 (14.9) | 0 | 2 (8.0) | 1 (4.0) | 20 (14.7) | 1 (0.7) |
| Pain in limb | 0 | 0 | 0 | 0 | 1 (5.6) | 0 | 10 (11.5) | 3 (3.4) | 9 (36.0) | 2 (8.0) | 20 (14.7) | 5 (3.7) |
| Electrocardiogram QT prolonged | 0 | 0 | 0 | 0 | 0 | 0 | 14 (16.1) | 2 (2.3) | 0 | 0 | 14 (10.3) | 2 (1.5) |
- Note: All values are No. (%). The data cutoff for this analysis was October 30, 2021. This table shows treatment-emergent adverse events occurring in ≥ 10% of patients with HER2-overexpression and HER2-low breast cancer.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
TEAEs resulted in dose interruption in 50/136 (36.8%) of patients and dose reduction in 23/136 (16.9%) of patients; 8/136 (5.9%) of patients discontinued treatment due to an AE (Supplementary Table S1). Patients receiving DV at the 2.5 mg/kg dose had higher rates of any grade TEAEs, grade 3 or higher TEAEs, dose interruption, and dose reduction (Supplementary Table S1). Additional details are available in Supplementary Tables S3-S5. Seven patients died (Supplementary Table S1), and the causes of death were disease progression, cholestatic jaundice, increased blood bilirubin, pneumonitis, respiratory failure, cardiac tamponade and interstitial lung disease (ILD), with none of the deaths being drug-related.
Peripheral neuropathy, which includes symptoms such as hypoesthesia, neurotoxicity, peripheral sensory neuropathy and pain in extremities, led to treatment discontinuation in 4 patients due to hypoesthesia (3/136, 2.2%) and peripheral sensory neuropathy (1/136, 0.7%) (Supplementary Table S3).
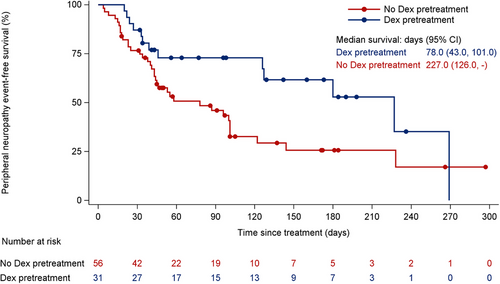
To decrease its incidence, patients in the C001 CANCER and C003 CANCER studies received pretreatment with dexamethasone 10 mg via intravenous drip prior to each DV infusion starting from October 2018. Of the 87 patients receiving the 2.0 mg/kg dose, 31 patients were pretreated with dexamethasone, resulting in a lower incidence of peripheral neuropathy (41.9% vs. 64.3%, P = 0.070) (Supplementary Table S6). No grade 3 or higher cases of peripheral neuropathy occurred in those who received pretreatment compared to patients who did not (0 vs. 8.9%, P = 0.156) (Supplementary Table S6). Dexamethasone also delayed the onset of the first peripheral neuropathy event (227 days with pretreatment vs. 78 days without pretreatment) (Figure 2, Supplementary Table S7). However, the recovery time could not be statistically analyzed due to the short follow-up period after the last dose (4 weeks).
In the C001 CANCER study, DLTs occurred in 3/24 (12.5%) of patients (Supplementary Table S8). These included a grade 4 neutrophil count decrease in 2 patients and a grade 3 alanine aminotransferase increase and aspartate aminotransferase increase in 1 patient. The MTD was not determined, and the highest dose of DV obtained during dose escalation was limited to 2.5 mg/kg to ensure safety and optimal PK characteristics.
3.3 Clinical activity
Clinical activity endpoints for HER2-overexpression and HER2-low ABC, as reported by investigators, are provided in Tables 3, 4. The duration of treatment is depicted in Figure 3.
| HER2-overexpression breast cancer | |||||
|---|---|---|---|---|---|
| 0.5-1.0 mg/kg | 1.5 mg/kg | 2.0 mg/kg | 2.5 mg/kg | Total | |
| Outcome | (n = 6) | (n = 18) | (n = 21) | (n = 25) | (n = 70) |
| Confirmed best overall response, n (%) | |||||
| CR | 0 | 0 | 0 | 0 | 0 |
| PR | 0 | 4 (22.2) | 9 (42.9) | 10 (40.0) | 23 (32.9) |
| SD | 3 (50.0) | 12 (66.7) | 11 (52.4) | 13 (52.0) | 39 (55.7) |
| PD | 3 (50.0) | 2 (11.1) | 1 (4.8) | 0 | 6 (8.6) |
| NE | 0 | 0 | 0 | 2 (8.0) | 2 (2.9) |
| Confirmed ORR, n (%) | 0 | 4 (22.2) | 9 (42.9) | 10 (40.0) | 23 (32.9) |
| 95% CI | 0.0%-45.9% | 6.4%-47.6% | 21.8%-66.0% | 21.1%-61.3% | 22.1%-45.1% |
| Confirmed DCR,a n (%) | 3 (50.0) | 16 (88.9) | 19 (90.5) | 22 (88.0) | 60 (85.7) |
| 95% CI | 11.8%-88.2% | 65.3%-98.6% | 69.6%-98.8% | 68.8%-97.5% | 75.3%-92.9% |
| Median DOR, months | NE | 7.2 | 4.4 | 6.4 | 5.7 |
| 95% CI, months | NE-NE | 3.5-15.6 | 4.0-21.4 | 2.7-8.3 | 4.0-7.4 |
| Median PFS, months | 1.7 | 4.0 | 5.7 | 6.3 | 5.5 |
| 95% CI, months | 1.2-6.0 | 2.6-7.6 | 5.3-8.4 | 4.3-8.8 | 4.6-6.5 |
- Note: The data cutoff for this analysis was October 30, 2021. Analysis was performed on the enrolled analysis population, which included patients with HER2-overexpression who provided informed consent and were enrolled in the dose escalation. All patients received ≥ 1 dose of disitamab vedotin.
- Abbreviations: CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; HER2, human epidermal growth factor receptor 2; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
- a The duration of SD was <6 weeks in one patient at 2.0 mg/kg and 2.5 mg/kg, respectively.
| IHC 2+/FISH- | IHC1+ | ||
|---|---|---|---|
| 2.0 mg/kg | 2.0 mg/kg | Total | |
| Outcomes | (n = 35) | (n = 31) | (n = 66) |
| Confirmed best overall response, n (%) | |||
| CR | 0 | 1 (3.2) | 1 (1.5) |
| PR | 15 (42.9) | 6 (19.4) | 21 (31.8) |
| SD | 17 (48.6) | 16 (51.6) | 33 (50.0) |
| PD | 3 (8.6) | 7 (22.6) | 10 (15.2) |
| NE | 0 | 1 (3.2) | 1 (1.5) |
| Confirmed ORR, n (%) | 15 (42.9) | 7 (22.6) | 22 (33.3) |
| 95% CI | 26.3%-60.6% | 9.6%-41.1% | 22.2%-46.0% |
| Confirmed DCR, n (%) | 31 (88.6) | 23 (74.2) | 54 (81.8) |
| 95% CI | 73.3%-96.8% | 55.4%-88.1% | 70.4%-90.2% |
| Median DOR, months | 5.6 | 7.3 | 5.6 |
| 95% CI, months | 4.1-8.6 | 2.8-9.5 | 4.3-8.3 |
| Median PFS, months | 6.6 | 4.1 | 5.1 |
| 95% CI, months | 4.1-8.3 | 2.7-5.5 | 4.1-6.6 |
- Note: The data cutoff for this analysis was October 30, 2021. Analysis was performed on the enrolled analysis population that included patients with HER2-low breast cancer who provided informed consent and were enrolled in the dose expansion. All patients received ≥ 1 dose of disitamab vedotin.
- Abbreviations: CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NE, not evaluable; ORR, response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
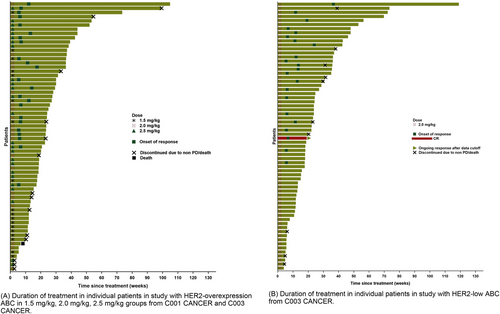
Duration of treatment in individual patients from C001 CANCER and C003 CANCER studies. (A). Duration of treatment in individual patients in study with HER2-overexpression ABC in 1.5 mg/kg, 2.0 mg/kg, 2.5 mg/kg groups from C001 CANCER and C003 CANCER. (B). Duration of treatment in individual patients in study with HER2-low ABC from C003 CANCER. Abbreviations: CR, complete response; HER2, human epidermal growth factor receptor 2; PD, progressive disease.
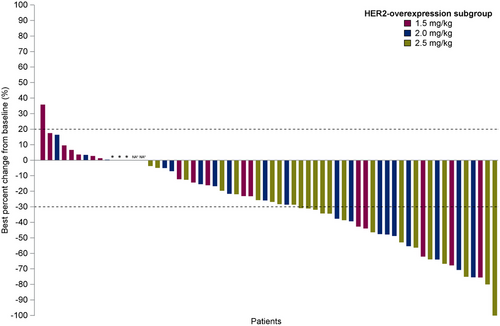
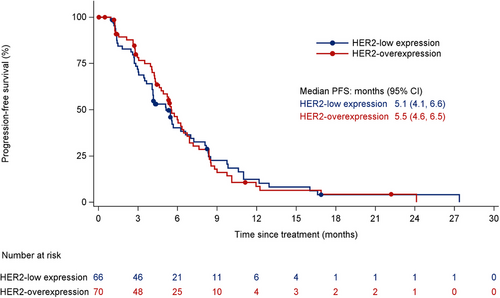
In the HER2-overexpression ABC group, the confirmed ORR was 32.9% (23/70; 95% CI, 22.1%-45.1%) (Table 3 and Figure 4), with doses of 1.5, 2.0, and 2.5 mg/kg showing ORRs of 22.2% (4/18; 95% CI, 6.4%-47.6%), 42.9% (9/21; 95% CI, 21.8%-66.0%), and 40.0% (10/25; 95% CI, 21.1%-61.3%), respectively. The median DOR was 5.7 months (95% CI, 4.0-7.4 months), and the median PFS was 5.5 months (95% CI, 4.6-6.5 months) (Figure 5), with 1.5, 2.0, and 2.5 mg/kg doses showing PFS of 4.0 months (95% CI, 2.6-7.6 months), 5.7 months (95% CI, 5.3-8.4 months), and 6.3 months (95% CI, 4.3-8.8 months), respectively.
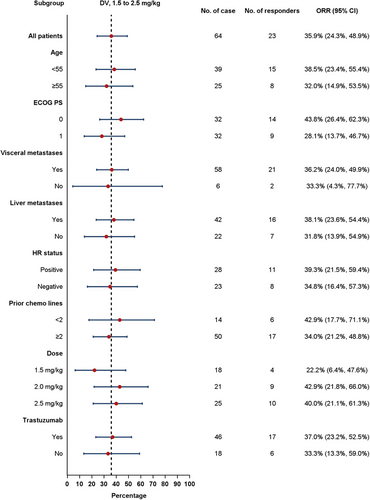
Subgroup analysis of confirmed ORR in HER2-overexpression ABC was conducted based on factors such as age, ECOG PS, visceral metastases, liver metastases, HR status, dose of DV (1.5-2.5mg/kg), prior chemotherapy lines, and trastuzumab prior treatment. No significant differences in confirmed ORR results were observed across these subgroups (Figure 6).
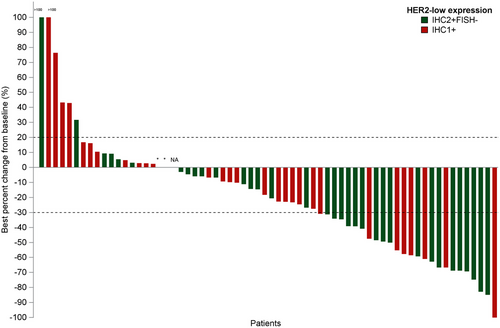
In the HER2-low ABC group, all patients received DV at a 2.0 mg/kg dose, resulting in a confirmed ORR of 33.3% (22/66; 95% CI, 22.2%-46.0%) (Table 4 and Figure 7), a median DOR of 5.6 months (95% CI, 4.3-8.3 months), and a median PFS of 5.1 months (95% CI, 4.1-6.6 months) (Figure 5). Among HER2-low patients, those with HER2 IHC 2+/FISH- had an ORR of 42.9% (15/35; 95% CI, 26.3%-60.6%) and a median PFS of 6.6 months (95% CI, 4.1-8.3 months), while patients with HER2 IHC 1+ exhibited an ORR of 22.6% (7/31; 95% CI, 9.6%-41.1%) and a median PFS of 4.1 months (95% CI, 2.7-5.5 months).
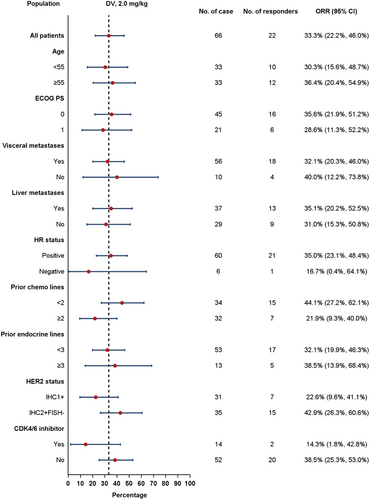
The confirmed ORR in HER2-low ABC was further analyzed based on various factors, such as age, ECOG PS, visceral metastases, liver metastases, HR status, prior chemotherapy lines, prior endocrine therapy, HER2 status, and prior cyclin-dependent kinase 4/6 (CDK4/6) inhibitor treatment. Subgroup analysis revealed no notable differences in confirmed ORR results across these subgroups (Figure 8).
3.4 PK
Blood samples from 65 patients in the C001 CANCER and C003 CANCER studies were analyzed to measure antibody-drug conjugate (ADC), total antibody (TAb), and free MMAE. Figure 9 illustrates the average serum concentration profiles following the first dose within the dose range of 0.5-2.5 mg/kg. TAb and ADC reached their Cmax at the end of the infusion, with a median Tmax of 0.92-1.50 h, while free MMAE had a median Tmax of 24.02-48.02 h. The mean t1/2 values ranged from 12.86 to 30.75 h for TAb and from 17.38 to 32.71 h for ADC (Supplementary Table S9). Multiple-dose administration of DV showed Ra(AUC) ratios of 0.93-1.14, 1.11-1.69, and 0.96-1.57 for TAb, ADC, and free MMAE, respectively (Supplementary Table S10).

Mean serum concentration profiles of ADC, TAb and MMAE after the first dose (Semi-Log Scale Plot).
Abbreviations: ADC, antibody-drug conjugate; MMAE, microtubule inhibitor monomethyl auristatin E; TAb: Total antibody.
4 DISCUSSION
ADCs have garnered interest for their potential in treating various cancers [7], including breast cancer [8, 22-26]. DV, a novel humanized anti-HER2 antibody conjugated with MMAE, has shown effectiveness against advanced gastric cancer [27] and urothelial carcinomas [28] with varying HER2 expression. In this study, we evaluated the safety and efficacy of DV in patients with either HER2-overexpression or HER2-low ABC and observed promising preliminary activity alongside a manageable safety profile.
The PK results indicated that ADC and free MMAE had t1/2 values of approximately 30 and 60 h, respectively, at doses of 1.5-2.5 mg/kg. The PKs for both unconjugated MMAE and ADC were consistent between single- and multiple-dose administrations. The Ra(AUC) for ADC and free MMAE at doses of 2.0 and 2.5 mg/kg were no more than 1.57, suggesting no substantial PK accumulation over 2 weeks. These PK profiles aligned with previously published data [28].
DV maintained a consistent safety profile with no newly emerged TEAEs [16, 17, 27, 28]. The incidence of TEAEs was lower in the 2.0 mg/kg dose compared to the 2.5 mg/kg dose(Supplementary Table S1). For reference, the platelet count decreased incidence with trastuzumab emtansine (T-DM1) was 30.0%, and the ≥ grade 3 TEAE incidence was 14.0% as reported in the EMILIA trial [23], while the incidence of platelet count decreased with DV in this study was much lower than T-DM-1. Based on previous studies, ILD was a rare but shared AEs in several anti-HER2 drugs. The incidence of ILD with DV was 0.7% (1/136) (Supplementary Table S2), while the incidence of ILD with other anti-HER2 ADCs in a similar population was comparatively higher [25, 29]. In addition, DV also showed less cardiotoxicity compared to other HER2-targeted therapies like trastuzumab [30, 31] and pertuzumab [32, 33], which had a risk of cardiomyopathy, particularly left ventricular dysfunction. For these AEs, DV seems to have more advantage over other anti-HER2 drugs.
Peripheral neuropathy, a known off-target effect of MMAE, mainly presents as hypoesthesia and occurs in a dose-dependent manner [34]. Pretreatment with anti-inflammatory agents, such as dexamethasone, which decreases cyclooxygenase-2 activity and inhibits prostaglandin synthesis, might decrease the risk of chemotherapy-induced neurotoxicity [35, 36]. In the C001 CANCER and C003 CANCER studies, dexamethasone pretreatment tended to decrease the incidence and delay the onset of peripheral neuropathy, suggesting that DV-associated neurotoxicity might be manageable. Therefore, corticosteroids could be a viable option to manage DV-induced neurotoxicity, warranting further investigation.
DV's antitumor mechanism targets HER2 on tumor cells and delivers MMAE with cytotoxic microtubule inhibition. Additionally, the bystander killing effect may lead to increased HER2 selectivity for its antitumor effect. Therefore, it is beneficial in targeting tumors with varying levels of HER2 expression. That explains its efficacy in both HER2-overexpression and HER2-low breast cancer.
DV had an ORR of 42.9% and a median PFS of 5.7 months at a 2.0 mg/kg dose in HER2-overexpression patients. The ORRs of other anti-HER2 agents, such as margetuximab [37], T-DM1 [38], neratinib [39] and tucatinib [40], were relatively lower than those of DV in similar populations. The median PFS was comparable to that of T-DM1 [38], margetuximab [37], and lapatinib [41]. Moreover, DV's safety profile is distinct, with a low incidence of ILD compared to other ADCs, such as T-DXd [24] and ARX788 [42]. In HER2-low breast cancer, DV had a 33.3% confirmed ORR, similar to T-DXd and SYD985 observed in phase I studies [43]. Therefore, this study demonstrated a promising efficacy with a manageable safety profile of DV in HER2-overexpression and HER2-low ABC, which means DV can be used as a treatment option for ABC. However, caution is advised when making cross-trial comparisons because there were differences in patient demographics and previous antitumor treatments.
This study treated patients with HER2-overexpression and HER2-low ABC, finding that DV had promising antitumor activity at doses of 1.5 mg/kg and higher. Although the efficacy was similar at 2.0 mg/kg and 2.5 mg/kg for HER2-overexpression ABC, the higher dose caused more TEAEs. After considering the PK profiles, risks and benefits, the RP2D was set at 2.0 mg/kg Q2W.
In China, chemotherapy remains the standard care for patients with HER2-low breast cancer who have failed prior therapies, including endocrine therapy [6]. Mono-chemotherapy was typically associated with only 2.8 [44]-4.2 [45] months of PFS for those previously treated with anthracyclines and taxanes. For HER2-overexpression metastatic breast cancer (MBC) patients who failed third-line treatment, no standard care is currently recommended [6, 46]. Thus, DV may be a promising option for treating HER2-overexpression and HER2-low breast cancer, given this clinical need. Based on our present results, two key registered studies are being conducted to further verify the safety and efficacy of DV in patients with HER2-overexpression or HER2-low breast cancer: Ongoing clinical studies for HER2- overexpression and HER2-low ABC include RC48-C006 (NCT03500380), a phase II/III study evaluating DV and lapatinib plus capecitabine in HER2-positive ABC and HER2-positive ABC with liver metastasis, and RC48-C012 (NCT04400695), a phase III study assessing DV and the physician's choice of treatment in HER2-low expression ABC.
This study had some limitations. The analysis was constrained by the relatively small sample of HER2-low breast cancer cases, particularly among HR+ patients who experienced progression while on CDK4/6 inhibitors. In addition, our study was limited by the diverse nature of the study population. Furthermore, we did not obtain updated HER2 status immediately prior to treatment, as most patients' statuses were determined using archived tumor samples, and the HER2 status and response to treatment were initially evaluated at individual study sites rather than at a centralized location.
5 CONCLUSIONS
This phase I/Ib study demonstrates the promising efficacy of DV in HER2-overexpression and HER2-low ABC and shows that DV 2.0 mg/kg Q2W had favorable efficacy and safety and dexamethasone pretreatment tends to mitigate the risk of peripheral neuropathy. These results support further investigation into the efficacy and safety of DV monotherapy or combination therapy for ABC with HER2-overexpression and HER2-low.
AUTHOR CONTRIBUTIONS
Binghe Xu was the leading principal investigator of this study. Binghe Xu and Jiayu Wang made substantial contributions to the study conception and design. Binghe Xu, Jiayu Wang, Yunjiang Liu, Qingyuan Zhang, Wei Li, Jifeng Feng, Xiaojia Wang, Jianmin Fang and Yiqun Han were involved in patient recruitment and data acquisition. Binghe Xu, Jiayu Wang, Yunjiang Liu, Qingyuan Zhang and Wei Li did the literature search, research formal analysis, and methodology. Binghe Xu, Jiayu Wang also contributed to manuscript drafting and Writing - review. Binghe Xu was responsible for supervision review. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
ACKNOWLEDGMENT
We sincerely appreciate the patients who participated in our study and their families and health providers. We also thank the investigators and staff who contributed to this study at the medical centers. This study was funded by RemeGen Co., Ltd. This work was also supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-014).
CONFLICT OF INTEREST STATEMENT
Jianmin Fang is a shareholder of RemeGen, Ltd. All the other authors declare no conflict of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (Approval No. 15-112/1039 and 16-180/1259), and patients consented under an IRB-approved protocol prior to any study procedures.
Open Research
DATA AVAILABILITY STATEMENTS
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.




