Differential risk of 23 site-specific incident cancers and cancer-related mortality among patients with metabolic dysfunction-associated fatty liver disease: a population-based cohort study with 9.7 million Korean subjects
Goh Eun Chung and Su Jong Yu contributed equally to this work as the first authors.
Abstract
Introduction
Although an association between metabolic dysfunction-associated fatty liver disease (MAFLD) and cardiovascular disease or overall mortality has been reported, it is unclear whether there is an association between MAFLD and cancer incidence or mortality. We aimed to investigate the differential risk of all- and site-specific cancer incidence and mortality according to MAFLD subgroups categorized by additional etiologies of liver disease.
Methods
Using the Korean National Health Insurance Service database, we stratified the participants into three groups: (1) single-etiology MAFLD (S-MAFLD) or MAFLD of pure metabolic origin; (2) mixed-etiology MAFLD (M-MAFLD) or MAFLD with additional etiological factor(s) (i.e., concomitant liver diseases and/or heavy alcohol consumption); and (3) non-MAFLD. Hepatic steatosis and fibrosis were defined using the fatty liver index and the BARD score, respectively. Cox proportional hazards regression was performed to estimate the risk of cancer events.
Results
Among the 9,718,182 participants, the prevalence of S-MAFLD and M-MAFLD was 29.2% and 6.7%, respectively. During the median 8.3 years of follow-up, 510,330 (5.3%) individuals were newly diagnosed with cancer, and 122,774 (1.3%) cancer-related deaths occurred among the entire cohort. Compared with the non-MAFLD group, the risk of all-cancer incidence and mortality was slightly higher among patients in the S-MAFLD group (incidence, adjusted hazard ratio [aHR] = 1.03; 95% confidence interval [CI]: 1.02−1.04; mortality, aHR = 1.06; 95% CI: 1.04−1.08) and highest among patients with M-MAFLD group (incidence, aHR = 1.31; 95% CI: 1.29−1.32; mortality, aHR = 1.45; 95% CI: 1.42−1.48, respectively). The M-MAFLD with fibrosis group (BARD score ≥ 2) showed the highest relative risk of all-cancer incidence (aHR = 1.38, 95% CI = 1.36–1.39), followed by the M-MAFLD without fibrosis group (aHR = 1.09, 95% CI = 1.06–1.11). Similar trends were observed for cancer-related mortality.
Conclusions
MAFLD classification, by applying additional etiologies other than pure metabolic origin, can be used to identify a subgroup of patients with poor cancer-related outcomes.
Abbreviations
-
- AUROC
-
- area under the receiver operating characteristic curve
-
- AST
-
- aspartate aminotransferase
-
- ALT
-
- alanine aminotransferase
-
- CI
-
- confidence interval
-
- CCI
-
- Charlson comorbidity index
-
- DBP
-
- diastolic blood pressure
-
- DM
-
- diabetes mellitus
-
- eGFR
-
- estimated glomerular filtration rate
-
- GGT
-
- gamma-glutamyl transferase
-
- HCC
-
- hepatocellular carcinoma
-
- HDL
-
- high-density lipoprotein
-
- IR
-
- incidence rate
-
- HR
-
- hazard ratio
-
- LDL
-
- low-density lipoprotein
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- MAFLD
-
- metabolic dysfunction-associated fatty liver disease
-
- M-MAFLD
-
- mixed etiology MAFLD
-
- PY
-
- person year
-
- TG
-
- triglyceride
-
- SBP
-
- systolic blood pressure
-
- S-MAFLD
-
- single etiology MAFLD
-
- WC
-
- waist circumference
1 BACKGROUND
The term metabolic (dysfunction)-associated fatty liver disease (MAFLD) has been introduced, emphasizing the role of metabolic dysfunction in the clinical outcomes of patients with hepatic steatosis [1, 2]. Recent studies have reported the predictive role of MAFLD in all-cause mortality [3-5] and cardiovascular disease (CVD) outcomes [6-8]. Although CVD is the most common cause of death in the United States (US) [9], cancer may surpass CVD as the leading cause of premature death in many countries [10]. Several studies have reported an association between MAFLD and cancer incidence and mortality. A previous study using the United Kingdom Biobank data reported that MAFLD was associated with a 7% increased risk for overall cancer and a 59% increased risk for liver cancer [8]. In a recent systematic review, MAFLD was associated with an increased risk of overall cancer incidence and mortality [11]. MAFLD has also been associated with an increased risk of a set of cancers, although the effect substantially varied by cancer site [12].
Moreover, MAFLD is an umbrella term whose definition may not accurately reflect the specific underlying causes of MAFLD (i.e., pure metabolic dysfunction and/or concomitant chronic liver disease such as alcoholic liver disease, viral hepatitis or other known liver disease) in individual patients. Additionally, it is believed that a mixed etiology may cause a worse clinical course than a single, pure metabolic etiology in patients with MAFLD [13-15]. From this point of view, a recent study stratified hepatocellular carcinoma (HCC) cases into three groups: single, metabolic etiology MAFLD, mixed etiology MAFLD and non-MAFLD, and reported the differential risk of HCC incidence and mortality by MAFLD subgroups [16].
Although the increased overall cancer incidence and mortality in MAFLD have been investigated [11], there are limited studies regarding the relationship between MAFLD and site-specific cancer-related mortality other than incidence [8, 12]. Therefore, we aimed to investigate the differential risk of overall and site-specific cancers, including their incidence and mortality, based on MAFLD subgroups categorized by additional etiologies other than pure metabolic origin in a nationally representative Korean population.
2 METHODS
2.1 Data source
The data of this retrospective population-based study were retrieved from the Korean National Health Insurance System (NHIS), which is a national insurer managed by the Korean government and to which approximately 97% of the Korean population is subscribed [17]. The NHIS conducts biennial health examinations for local householders aged ≥40 years or employees of any age. The NHIS database contains health records, including sociodemographic data, anthropometric measurements, laboratory tests and lifestyle behaviors, and claims data based on the International Classification of Diseases, 10th revision (ICD-10). This database has been widely used for previous epidemiologic studies [18, 19]. The study protocol was approved by the Institutional Review Board of Soongsil University (SSU-202007-HR-236-01) and conformed to the ethical guidelines of the Declaration of Helsinki. The requirement for patient informed consent was waived, as this was a retrospective study using deidentified secondary data.
2.2 Study population
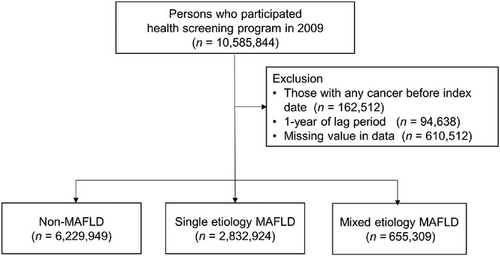
The enrollment process of this cohort is presented in Figure 1. A total of 10,585,844 adults aged 20 years or older who underwent health screening examinations between January 1, 2009, and December 31, 2009, were included. Those with ICD-10 codes for any cancer (Supplementary Table S1) before the index date were excluded (n = 162,512), and we ascertained outcome events after a lag of one year (n = 94,638). After excluding participants with incomplete information (n = 610,512), 9,718,182 remained for analysis.
2.3 Measurement of hepatic steatosis and advanced fibrosis
FLI scores ranged from 0-100, with scores <30 representing a low risk for fatty liver and scores ≥60 representing a high risk [18]. The lower cutoff of FLI score ≥ 30 was used in this study [6, 22].
The presence of advanced liver fibrosis was estimated based on the BARD score for subjects with MAFLD, which was calculated by assigning points for aspartate transaminase/alanine aminotransferase (AST/ALT) ratio ≥ 0.8 (2 points), BMI ≥ 28 kg/m2 (1 point), and diabetes mellitus (DM) (1 point), where a total score of 2-4 points indicated advanced hepatic fibrosis [23].
2.4 Definition of MAFLD subgroups
MAFLD was defined based on the diagnostic criteria proposed by an international expert panel as the presence of metabolic risk factors with hepatic steatosis, including individuals with other concomitant liver diseases and significant alcohol consumption [1]. For the diagnosis of MAFLD, hepatic steatosis had to be present, and at least one of the following criteria had to be met: (1) overweight or obese (BMI ≥ 23 kg/m2), (2) DM, or (3) at least 2 metabolic abnormalities. Metabolic abnormalities consisted of (1) WC ≥ 102 cm for men and ≥ 88 cm for women, (2) blood pressure ≥ 130/85 mmHg or anti-hypertensive drug treatment, (3) fasting triglyceride level ≥ 150 mg/dL or specific drug treatment, (4) high-density lipoprotein (HDL) cholesterol level < 40 mg/dL for men and < 50 mg/dL for women or specific drug treatment, and (5) fasting glucose level 100-125 mg/dL. As homeostasis model assessment of insulin resistance scores and C-reactive protein levels were not available in the NHIS screening program, these criteria for metabolic dysregulation were not used in this study.
The presence of concomitant liver diseases was defined based on the following ICD-10 codes: viral hepatitis (B15-19), cirrhosis (K74), alcoholic liver disease (K70), toxic liver disease (K71), primary biliary cholangitis (K74.3), secondary or unspecified biliary cirrhosis (K74.4-74.5), autoimmune hepatitis (K75.4), Wilson's disease (E83.0), and disorders of iron metabolism (E83.1). One or more diagnoses during hospitalization or two or more diagnoses in outpatient clinics were required for the diagnosis of concomitant liver diseases. Next, the participants were stratified into three groups: (1) single-etiology MAFLD (S-MAFLD) or MAFLD of pure metabolic origin; (2) mixed-etiology MAFLD (M-MAFLD) or MAFLD with additional etiological factor(s) (i.e., concomitant liver diseases and/or heavy alcohol consumption); and (3) non-MAFLD (Figure 1).
2.5 Study outcomes: cancer incidence and mortality
The primary outcomes were cancer incidence and cancer-related mortality. Site-specific cancer included oral cavity and pharyngeal cancer, esophageal cancer, gastric cancer, colorectal cancer, liver cancer, biliary cancer, pancreatic cancer, laryngeal cancer, lung cancer, malignant melanoma, rectal cancer, bladder cancer, cancer of the central nervous system, thyroid cancer, non-Hodgkin lymphoma, multiple myeloma, leukemia, breast cancer, uterine cervical cancer, uterine corpus cancer, ovarian cancer, prostate cancer and testicular cancer. Ascertainment of cancer diagnosis was achieved from the NHIS database using ICD-10 codes (Supplementary Table S1) and rare incurable disease registration codes (V193) [24]. All patients in this category are designated as special medical aid beneficiaries with the expanding benefit of the NHIS. Since 2006, the government has introduced an initiative covering 90% of all medical expenses claimed by these patients. Therefore, the diagnosis of cancer is strictly determined and monitored by a thorough verification with clinical, imaging, and pathological evidence and rigorous reviews by medical experts and health insurance professionals, according to an act established by the Ministry of Health and Welfare [25]. The data for cancer used in this study have been validated in previous studies [26, 27].
Information on mortality and cause of death was available for all subjects in the cohort; the latter was classified according to the Korean Standard Classification of Diseases and Causes of Death provided by the Korean National Statistical Office [28]. The cause of death was classified according to the diagnostic codes of the ICD-10. Participants who had no event were censored at the date of death, the last follow-up, or on December 31, 2018, whichever came first.
2.6 Covariates
As described previously [29], standardized self-report questionnaires were used to collect data at the time of enrollment. Briefly, age, sex, smoking status (none, former, and current smokers), and alcohol consumption data were used. Participants reported alcohol consumption frequency and amount of alcohol consumed as the number of glasses consumed per occasion. We categorized alcohol consumption as none, mild, and heavy (≥ 30 g for males and ≥ 20 g for females per day). The questionnaire on physical activity consisted of items on the frequency (days per week) of light, moderate, and vigorous physical activity in recent weeks. Regular exercise was defined as vigorous-intensity physical activity ≥ 3 times per week or moderate physical activity ≥ 5 times per week. Income level was dichotomized at the lowest 20%.
We defined comorbidities based on the ICD-10 code for each disease and with a prescription history of relevant medication. Criteria for hypertension were prescription of anti-hypertensive agents in claim codes I10-13 or I15 or systolic/diastolic blood pressure ≥ 140/90 mmHg. The criteria for DM were an E11-14 claim code plus 1 or more prescriptions of an antidiabetic drug per year or a fasting blood glucose level of 126 mg/dL or more. Criteria for dyslipidemia were E78 claim code plus ≥ 1 prescription for a lipid-lowering agent or total cholesterol level ≥ 240 mg/dL. The Charlson comorbidity index (CCI) score was assessed using ICD-10 codes [30].
WC was measured in a horizontal plane at the midpoint between the lower edge of the last palpable rib and the top of the iliac crest. BMI was calculated as a person's weight (in kg) divided by the square of their height (in meters). After overnight fasting, serum glucose and lipid measurements were obtained from each participant. To estimate the glomerular filtration rate, the Modification of Diet in Renal Disease equation was used [31].
2.7 Statistical analyses
Clinical characteristics are presented as the means ± standard deviations for normally distributed continuous variables or otherwise as median (interquartile ranges). Categorical variables were reported as numbers (percent) unless otherwise indicated. Baseline characteristics were compared using independent t-tests or analysis of variance for continuous variables and chi-square tests for categorical variables.
The primary outcome was the incidence rate calculated by dividing the number of incident cancer cases by the total follow-up period and presented per 1,000 person-years. Person-years is a measure of the amount of time that a group of people or an individual has been exposed to a particular risk of an event, such as developing a disease. It is calculated by multiplying the number of people at risk by the time they were observed. Cox proportional hazards regression was performed to estimate the risk of cancer events. First, we conducted a univariate analysis and performed multivariate analysis by considering a range of covariates that may be associated with the primary outcome. The multivariate Cox models were adjusted for age, sex, income, smoking, exercise, CCI score, WC, fasting glucose, total cholesterol, systolic blood pressure, and eGFR. Covariates were selected a priori based on possible associations with MAFLD and outcomes [6, 12]. Tests based on Schoenfeld residuals verified the proportional hazards assumption. Linear trends of cancer events risk across MAFLD categories were assessed by analyzing MAFLD categories as a continuous variable, with non-MAFLD as the comparator. The risk of cancer development and mortality for each site-specific cancer was expressed as a hazard ratio (HR) with a corresponding 95% confidence interval (CI). Stratified analyses were performed according to age (< 65 vs. ≥ 65 years), sex, BMI (< 25 vs. ≥ 25 kg/m2), the presence of diabetes, smoking status and alcohol consumption, and we tested the interactions between subgroups. In addition, we performed competing survival analyses using the Fine–Gray models with adjustment of competing risk of all-cause death in analyses of cancer incidence and with adjustment of other causes of death, including cardiovascular disease, in analyses of cancer-related mortality [32]. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.Rproject.org) software with two-sided tests and a significance level of 0.05.
3 RESULTS
3.1 Clinical characteristics of the study population
Among the total 9,718,182 participants (median age, 46 years; gender proportion, 54.8% male), the prevalence of S-MAFLD and M-MAFLD was 29.2% and 6.7%, respectively. The baseline characteristics of each group are shown in Table 1. Compared with the non-MAFLD group, people in the S-MAFLD and M-MAFLD groups were older, more likely to be male and smokers, and had higher income and alcohol consumption levels (P <0.001 for all). Additionally, subjects with S-MAFLD and M-MAFLD were more likely to have diabetes, hypertension and dyslipidemia than those without MAFLD (P <0.001). Most clinical variables (including BMI, WC, systolic/diastolic blood pressure, fasting glucose, total cholesterol, and HDL cholesterol) were less metabolically favorable in the S-MAFLD and M-MAFLD groups than in the non-MAFLD group (all P <0.001).
| Non-MAFLD | S-MAFLD | M-MAFLD | |
|---|---|---|---|
| Variables | (n = 6,229,949) | (n = 2,832,924) | (n = 655,309) |
| Age, year, mean ± SD | 45.8 ± 14.3 | 49.6 ± 13.4* | 47.2 ± 12.2#,† |
| Male, n (%) | 2,684,051 (43.1) | 2,043,538 (72.1)* | 593,771 (90.6)#,† |
| Low-income levela, n (%) | 1,027,290 (16.5) | 394,554 (13.9)* | 80,670 (12.3) |
| Smoking, n (%) | |||
| None | 4,250,985 (68.2) | 1,359,717 (48.0)* | 161,577 (24.7)#,† |
| Former | 684,114 (11.0) | 540,484 (19.1) | 161,831 (24.7) |
| Current | 1,294,850 (20.8) | 932,723 (32.9) | 331,901 (50.6) |
| Alcohol consumption, n (%) | |||
| None | 3,556,681 (57.1) | 1,344,784 (47.5)* | 79,301 (12.1)#,† |
| Mild | 2,326,809 (37.3) | 1,488,140 (52.5) | 85,138 (13.0) |
| Heavy | 346,459 (5.6) | - | 490,870 (74.9) |
| Regular exercise, n (%) | 1,088,392 (17.5) | 513,057 (18.1)* | 132,166 (20.2)#,† |
| Diabetes, n (%) | 313,717 (5.0) | 416,436 (14.7)* | 111,851 (17.1)#,† |
| Hypertension, n (%) | 1,112,771 (17.9) | 1,110,688 (39.2)* | 273,444 (41.7)#,† |
| Dyslipidemia, n (%) | 768,892 (12.3) | 807,869 (28.5)* | 179,608 (27.4)#,† |
| Concomitant liver disease, n (%) | 215,703 (3.5) | 108,835 (16.6)† | |
| Liver cirrhosis, n (%) | 12,271 (0.2) | - | 11,608 (1.8)† |
| CCI score, n (%) | |||
| 0 | 4,181,057 (67.1) | 1,737,234 (61.3)* | 354,106 (54.0)#,† |
| 1 | 1,193,238 (19.2) | 587,334 (20.7) | 147,437 (22.5) |
| ≥ 2 | 855,654 (13.7) | 508,356 (17.9) | 153,766 (23.5) |
| BMI, kg/m2, mean ± SD | 22.2 ± 2.4 | 26.5 ± 3.6* | 26.1 ± 2.8#,† |
| WC, cm, mean ± SD | 75.7 ± 7.1 | 88.3 ± 7.7* | 88.3 ± 7.6#,† |
| SBP, mmHg, mean ± SD | 119.3 ± 14.4 | 127.9 ± 14.6* | 129.3 ± 14.6#,† |
| DBP, mmHg, mean ± SD | 74.3 ± 9.6 | 79.8 ± 9.8* | 81.2 ± 10.0#,† |
| Fasting glucose, mg/dL, mean ± SD | 93.5 ± 18.9 | 103.4 ± 29.0* | 106.5 ± 31.9#,† |
| Total cholesterol, mg/dL, mean ± SD | 189.2 ± 38.9 | 207.0 ± 43.0* | 203.2 ± 45.6#,† |
| HDL cholesterol, mg/dL, mean ± SD | 59.0 ± 30.9 | 51.5 ± 35.1* | 53.9 ± 35.7#,† |
| LDL cholesterol, mg/dL, mean ± SD | 112.0 ± 36.5 | 118.1 ± 41.8* | 108.3 ± 43.2#,† |
| eGFR, mL/min/1.732, mean ± SD | 88.7 ± 44.5 | 85.2 ± 46.5* | 88.2 ± 46.6#,† |
| TG, mg/dL, geometric mean (95% CI) | 87.5 (87.5-87.6) | 175.9 (175.8-176.0)* | 184.3 (184.1-184.6)#,† |
| AST, IU/L, geometric mean (95% CI) | 21.5 (21.5-21.5) | 26.5 (26.5-26.5)* | 31.1 (31.1-31.2)#,† |
| ALT, IU/L, geometric mean (95% CI) | 17.6 (17.6-17.6) | 29.3 (29.3-29.4)* | 33.3 (33.2-33.3)#,† |
| GGT, IU/L, geometric mean (95% CI) | 19.3 (19.3-19.3) | 41.8 (41.7-41.8)* | 69.7 (69.6-69.8)#,† |
- Abbreviations: MAFLD, metabolic-associated fatty liver disease; S-MAFLD, single etiology MAFLD; M-MAFLD, mixed etiology MAFLD; CCI, Charlson comorbidity index; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; TG, triglyceride; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; SD, standard deviation; CI, confidence interval.
- a Lower 20% of income
- * P < 0.001 non-MAFLD vs. S-MAFLD
- # P < 0.001 S-MAFLD vs. M-MAFLD
- † P <0.001 non-MAFLD vs. M-MAFLD
3.2 Risk of cancer incidence and mortality by MAFLD subgroup
During the median 8.3 (interquartile range, 8.1-8.6) years of follow-up, 510,330 (5.3%) individuals were newly diagnosed with cancer (Table 2). The incidence rates of overall cancer per 1,000 person-years in the non-MAFLD, S-MAFLD, and M-MAFLD groups were 5.94, 7.32 and 8.55, respectively. In the age- and sex-adjusted model, the risk of all-cancer incidence increased in patients with S-MAFLD and M-MAFLD compared with non-MAFLD patients (HR [95% CI], 1.02 [1.01–1.02] and 1.33 [1.32–1.35]). After further adjustment for income, smoking, exercise, eGFR, CCI score, WC, fasting glucose, total cholesterol and systolic blood pressure, the HR for all-cancer incidence among patients with S-MAFLD and M-MAFLD compared with that among non-MAFLD patients was 1.03 (95% CI = 1.02–1.04) and 1.31 (95% CI = 1.29–1.32), respectively (Table 2).
| Age- and sex-adjusted | Multivariate modela | |||||
|---|---|---|---|---|---|---|
| All-cancer outcomes | No. of subjects (n) | No. of events (development of cancer or cancer-related death) (n) |
Follow-up duration (person-years) |
Incidence rate (per 1,000 person-years) |
HR (95% CI) | HR (95% CI) |
| Incidence | ||||||
| Non-MAFLD | 6,229,949 | 299,203 | 50,353,689 | 5.94 | 1 (reference) | 1 (reference) |
| S-MAFLD | 2,832,924 | 166,545 | 22,739,288 | 7.32 | 1.02 (1.01–1.02) | 1.03 (1.02–1.04) |
| M-MAFLD | 655,309 | 44,582 | 5,213,649 | 8.55 | 1.33 (1.32–1.35) | 1.31 (1.29–1.32) |
| Mortality | ||||||
| Non-MAFLD | 6,229,949 | 67,037 | 51,366,724 | 1.31 | 1 (reference) | 1 (reference) |
| S-MAFLD | 2,832,924 | 42,656 | 23,271,780 | 1.83 | 1.02 (1.01–1.04) | 1.06 (1.04–1.08) |
| M-MAFLD | 655,309 | 13,081 | 5,350,398 | 2.44 | 1.54 (1.51–1.57) | 1.45 (1.42–1.48) |
- Abbreviations: MAFLD, metabolic-associated fatty liver disease; S-MAFLD, single etiology MAFLD; M-MAFLD, mixed etiology MAFLD; HR, hazard ratio; CI, confidence interval.
- a Adjusted for age, sex, income, smoking, exercise, estimated glomerular filtration rate, Charlson comorbidity index score, waist circumference, glucose, total cholesterol and systolic blood pressure.
During the follow-up period, 122,774 (1.3%) cancer-related deaths occurred (Table 2). The incidence rates of cancer mortality per 1,000 person-years in the non-MAFLD, S-MAFLD, and M-MAFLD groups were 1.31, 1.83, and 2.44, respectively. In the age- and sex-adjusted model, the risk of all-cancer incidence increased in patients with S-MAFLD and M-MAFLD compared with non-MAFLD patients [HR (95% CI), 1.02 (1.01–1.04) and 1.54 (1.51–1.57)]. After adjustment for multiple covariates, the HR for all-cancer mortality among patients with S-MAFLD and M-MAFLD compared with that among non-MAFLD patients was 1.06 (95% CI = 1.04–1.08) and 1.45 (95% CI = 1.42–1.48), respectively (Table 2). In the competing survival analysis, in which all-cause death was considered a competing risk for cancer incidence and death resulting from non-cancer was considered a competing risk for cancer-related mortality, S-MAFLD and M-MAFLD groups showed a higher risk of all-cancer incidence and mortality than non-MAFLD group (Supplementary Table S2).
Next, we performed sensitivity analysis with additional adjustments for alcohol consumption, diabetes and BMI. The associations between MAFLD subgroups and all-cancer incidence or mortality remained significant (Supplementary Table S3).
When stratified by age, sex, obesity, diabetes, smoking status, and alcohol consumption, a significantly higher relative risk for all-cancer incidence and mortality in the M-MAFLD group was observed predominantly among subjects ≥ 65 years of age, non-obese (BMI <25 kg/m2) subjects, those with diabetes, and non or former smokers and non-drinkers (all P for interaction < 0.001) (Supplementary Table S4). Male patients with M-MAFLD showed a higher risk for cancer incidence, whereas mortality risk was higher in female M-MAFLD patients (Supplementary Table S4). To further reduce the possibility of reverse causation, we additionally explored whether these results changed when extending a lag period to 2 years from the enrollment but found little change in our results (Supplementary Table S5).
3.3 Advanced fibrosis based on the BARD score and the risk of cancer incidence and mortality in MAFLD
Among 655,309 subjects with M-MAFLD, 448,911 (68.5%) had advanced fibrosis (defined as a BARD score ≥ 2) versus 1,866,975/2,832,924 (65.9%) with S-MAFLD (P < 0.001). In the multivariate model, the M-MAFLD with fibrosis group showed the highest relative risk of all-cancer incidence (HR = 1.38, 95% CI = 1.36–1.39), followed by the M-MAFLD without fibrosis (BARD < 2, HR = 1.09, 95% CI = 1.06–1.11) and S-MAFLD with fibrosis groups (HR = 1.05, 95% CI = 1.04–1.06) (P for trend < 0.001), whereas the S-MAFLD without fibrosis group was modestly associated with lower cancer risk compared to the non-MAFLD group (HR = 0.98, 95% CI = 0.97–0.99, Table 3). Similar trends were observed for cancer-related mortality. Compared to the group without MAFLD, the M-MAFLD with fibrosis group had a 1.52 (95% CI = 1.48–1.55) times higher cancer mortality risk, followed by the M-MAFLD without fibrosis group (HR = 1.11, 95% CI = 1.06–1.16), and the S-MAFLD with fibrosis group (HR = 1.09, 95% CI = 1.07–1.11, Table 3).
| Age- and sex-adjusted | Multivariate modela | |||||
|---|---|---|---|---|---|---|
| All-cancer outcomes | No. of subjects (n) | No. of events (development of cancer or cancer-related death) (n) | Follow-up duration (person-years) | Incidence rate (per 1,000 person-years) | HR (95% CI) | HR (95% CI) |
| Incidence | ||||||
| Non-MAFLD | 6,229,949 | 299,203 | 50,353,689 | 5.94 | 1 (reference) | 1 (reference) |
| S-MAFLD, BARD < 2 | 965,949 | 40,668 | 7,844,957 | 5.18 | 0.96 (0.95–0.97) | 0.98 (0.97–0.99) |
| S-MAFLD, BARD ≥ 2 | 1,866,975 | 125,877 | 14,894,330 | 8.45 | 1.03 (1.03–1.04) | 1.05 (1.04–1.06) |
| M-MAFLD, BARD < 2 | 206,398 | 9,170 | 1,673,403 | 5.48 | 1.10 (1.08–1.12) | 1.09 (1.06–1.11) |
| M-MAFLD, BARD ≥ 2 | 448,911 | 35,412 | 3,540,245 | 10.00 | 1.41 (1.39–1.42) | 1.38 (1.36–1.39) |
| Mortality | ||||||
| Non-MAFLD | 6,229,949 | 67,037 | 51,366,724 | 1.30 | 1 (reference) | 1 (reference) |
| S-MAFLD, BARD < 2 | 965,949 | 7,895 | 7,981,512 | 0.98 | 0.91 (0.89–0.93) | 0.93 (0.91–0.96) |
| S-MAFLD, BARD ≥ 2 | 1,866,975 | 34,761 | 15,290,268 | 2.27 | 1.05 (1.04–1.07) | 1.09 (1.07–1.11) |
| M-MAFLD, BARD < 2 | 206,398 | 1,921 | 1,703,866 | 1.12 | 1.17 (1.12–1.23) | 1.11 (1.06–1.16) |
| M-MAFLD, BARD ≥ 2 | 448,911 | 11,160 | 3,646,532 | 3.06 | 1.62 (1.59–1.65) | 1.52 (1.48–1.55) |
- Abbreviations: MAFLD, metabolic-associated fatty liver disease; S-MAFLD, single etiology MAFLD; M-MAFLD, mixed etiology MAFLD; HR, hazard ratio; CI, confidence interval.
- a Adjusted for age, sex, income, smoking, exercise, estimated glomerular filtration rate, Charlson comorbidity index score, waist circumference, glucose, total cholesterol and systolic blood pressure.
In the competing survival analysis, the highest relative risk of all-cancer incidence and mortality was consistently observed in the M-MAFLD with fibrosis group (Supplementary Table S6). When we performed sensitivity analysis with additional adjustments for alcohol consumption, diabetes and BMI, the associations between advanced fibrosis and all-cancer incidence or mortality in individuals with MAFLD remained significant (Supplementary Table S7).
3.4 Risk of site-specific cancer incidence and mortality by MAFLD subgroup
Figure 2 and Supplementary Table S8 show site-specific cancer incidence rates according to the MAFLD subgroup. In patients with M-MAFLD, the incidence rate of liver cancer was the highest among all cancers (1.85 per 1,000 person-years). For most types of cancers, the risk of incident cancer significantly increased from subjects without MAFLD to subjects with S-MAFLD and those with M-MAFLD, except for breast cancer (women), ovarian cancer, testicular cancer, melanoma, and hematologic malignancies. Subjects with M-MAFLD were at the highest risk for liver cancer (HR = 3.39, 95% CI = 3.30–3.50), followed by esophageal (HR = 2.15, 95% CI = 1.99–2.33), laryngeal (HR = 1.69, 95% CI = 1.51–1.89) and renal cancer (HR = 1.48, 95% CI = 1.39–1.57) among the MAFLD subgroups. Regarding digestive system cancers, patients with M-MAFLD were more likely to develop biliary (HR = 1.43, 95% CI = 1.35–1.52), oral cavity and pharyngeal (HR = 1.39, 95% CI = 1.28–1.51), colorectal (HR = 1.33, 95% CI = 1.29–1.36), gastric (HR = 1.24, 95% CI = 1.21–1.27), and pancreatic cancer (HR = 1.23, 95% CI = 1.16–1.30) among the MAFLD subgroups. Women with M-MAFLD had a significantly higher risk for cervical cancer (HR, 1.26; 95% CI, 1.06–1.49) than those with S-MAFLD or without MAFLD. Similar trends were observed for prostate cancer in men. There were no significant differences in the risk of hematologic malignancies, including leukemia, non-Hodgkin lymphoma and multiple myeloma, among the MAFLD subgroups.
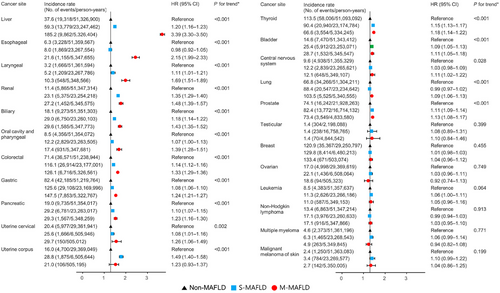
Association between MAFLD subtypes and site-specific cancer incidence. Incidence rates were presented per 100,000 person-years. The hazard ratio and its 95% confidence interval were derived from the Cox regression model. Adjusted for age, sex, income, smoking, exercise, estimated glomerular filtration rate, Charlson comorbidity index score, waist circumference, glucose, total cholesterol and systolic blood pressure.
Abbreviations: MAFLD, metabolic-associated fatty liver disease; S-MAFLD, single etiology MAFLD; M-MAFLD, mixed etiology MAFLD; HR, hazard ratio; CI, confidence interval.
*P for trend was estimated across MAFLD categories (modeled continuously), compared to non-MAFLD; for details, see Methods.
Regarding site-specific cancer mortality, the incidence rate of liver cancer-related mortality was the highest among all cancers in the M-MAFLD group (0.78 per 1,000 person-years). The highest risk in M-MAFLD remained consistent for the liver (HR = 3.70, 95% CI = 3.54–3.88), esophageal (HR, 2.32; 95% CI, 2.04–2.63), oral cavity and pharyngeal (HR = 1.57, 95% CI = 1.32–1.88), biliary (HR = 1.39, 95% CI = 1.26–1.52), colorectal (HR = 1.25, 95% CI = 1.16–1.34), and pancreatic cancer (HR = 1.17, 95% CI = 1.08–1.26) among the MAFLD subgroups (Figure 3 and Supplementary Table S9). Women with M-MAFLD had a significantly higher mortality risk for cervical cancer (HR = 1.17, 95% CI = 1.05–1.22), and men with M-MAFLD showed similar trends regarding prostate cancer-related mortality (HR = 1.17, 95% CI = 1.00–1.35)
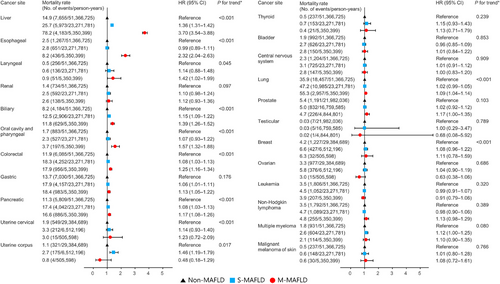
Association between MAFLD subtypes and site-specific cancer mortality. Mortality rates were presented per 100,000 person-years. The hazard ratio and its 95% confidence interval were derived from the Cox regression model. Adjusted for age, sex, income, smoking, exercise, estimated glomerular filtration rate, Charlson comorbidity index score, waist circumference, glucose, total cholesterol and systolic blood pressure.
Abbreviations: MAFLD, metabolic-associated fatty liver disease; S-MAFLD, single etiology MAFLD; M-MAFLD, mixed etiology MAFLD; HR, hazard ratio; CI, confidence interval.
*P for trend was estimated across MAFLD categories (modeled continuously), compared to non-MAFLD; for details, see Methods.
When subjects with MAFLD were divided according to BARD scores, fibrosis in M-MAFLD was associated with higher estimates for the risk of site-specific cancer incidence (Supplementary Figure S1) and mortality (Supplementary Figure S2) for most types of cancers.
4 DISCUSSION
In this large, nationally representative, population-based cohort analysis, we demonstrated the differential risk of cancer development and mortality by MAFLD subgroups categorized by additional etiologies other than pure metabolic origin using a database of health insurance claims in Korea. Subjects with M-MAFLD had an approximately 1.3-fold increased risk of cancer incidence and a 1.5-fold higher risk of cancer mortality than those without MAFLD (Table 3), whereas those with S-MAFLD showed only modestly increased risks. Furthermore, advanced fibrosis, defined by the BARD score in MAFLD, was associated with higher risks for overall cancer incidence and mortality. Our findings suggest the importance of careful assessment of cancer risk in subjects with MAFLD, and the classification of MAFLD used in this study might help risk stratification of MAFLD patients according to the relative effect estimates of factors defining etiology and hepatic fibrosis.
Cancer is the second most frequent cause of death among patients with non-alcoholic fatty liver disease (NAFLD) [33], and a positive association between NAFLD and incident cancers, including HCC, colorectal cancer in males, and breast cancer in females, have been reported [34]. In addition to fatty liver, metabolic syndrome, obesity and diabetes are associated with an increased risk of several cancers, such as liver, colorectal and kidney cancers [35, 36]. Although there is not enough data on MAFLD regarding the risk of cancer, a recent study based on UK Biobank data reported a significant association between MAFLD and the incidence of 10 cancers, including uterine corpus, gallbladder, liver, kidney, thyroid, esophagus, pancreas, bladder, breast, and colorectal and anus cancers [12]. Broadly in line with previous results, MAFLD-associated specific cancer risk was greatest for liver cancer (HR = 1.63), followed by uterine corpus (HR = 1.47), kidney (HR = 1.37), esophagus (HR = 1.25), larynx (HR = 1.24) and biliary cancer (HR = 1.22) in our study (data not shown).
When we evaluated the effect of etiological factors defining MAFLD on cancer outcome, the risks of overall cancer incidence and mortality were the greatest in M-MAFLD, while S-MAFLD showed only modestly increased risks compared to non-MAFLD. These trends have been similarly observed in various digestive system cancers, including liver, oral cavity and pharyngeal, esophageal, biliary, pancreatic, and colorectal cancers, suggesting that the presence of different etiological factors in MAFLD, such as at-risk alcohol consumption or chronic viral hepatitis, can be a risk factor for cancer, not only in the liver but also in extra-hepatic digestive organs. As organs of the digestive system share a similar embryologic background [37], and alcohol or chronic viral hepatitis infection is associated with an increased risk of various extra-hepatic cancers [38, 39], it is plausible that M-MAFLD is associated with the highest relative risks of cancer development and mortality for various digestive system cancers among MAFLD subtypes.
The M-MAFLD group in our study was defined due to mixed etiology other than pure metabolic MAFLD, which was similar to the individuals who met the definition of MAFLD but not NAFLD (non-NAFLD MAFLD group) in another study by Nguyen et al., in which the authors classified the study population according to the criteria of NAFLD and MAFLD [5]. In contrast to our results, although the non-NAFLD MAFLD group had higher cancer-related mortality than the control group, the difference was not statistically significant. However, the reference group in that study was set as subjects with NAFLD but not MAFLD, contrary to the present study, leading to different results between studies. In addition, the small number of events in the previous study may contribute to nonsignificant results.
The mechanisms for the association of MAFLD with increased cancer risk have not been fully elucidated, but several hypotheses exist. First, insulin resistance, as the key determinant in the pathology of MAFLD, is involved in the dysregulation of insulin-like growth factors and might contribute to malignant transformation at various sites, including the liver, pancreas, colon and breast [40-42], by promoting cell proliferation and angiogenesis and inhibiting apoptosis [43, 44]. Second, overweight/obesity, another main factor in MAFLD, may result in a state of chronic systemic low-grade inflammation attributed to a proinflammatory environment, which may affect cancer risk by increasing the formation of reactive oxygen species, increasing cell cycle rates, and decreasing tumor suppressor function [45]. Third, DM and hyperglycemia, which are determinant factors of MAFLD, are known as potential risk factors for cancer [46, 47]. Since high blood glucose levels increase mitochondrial glucose oxidation, they promote DNA damage through oxidative stress. However, MAFLD involves a wide spectrum, not only metabolic dysfunction, and could be a multisystem disease. Further studies are needed to reveal the potential mechanisms of the development of MAFLD-associated cancers.
In this study, the presence of hepatic fibrosis in individuals with MAFLD was associated with higher risks for overall and various site-specific cancer incidence and mortality. Consistent with our results, patients with bridging fibrosis showed an increased risk of extra-hepatic cancers, and those with NAFLD cirrhosis showed an increased risk of liver-related events [48]. Although the mechanisms involved in the association between hepatic fibrosis and the risk of extra-hepatic cancer are still unestablished, the dysregulation of endothelial cell structure, upregulated tissue inhibitors of matrix metalloproteinases, alterations in the extracellular matrix, and angiogenesis induce excessive wound healing responses during hepatic fibrosis [49]. These proinflammatory conditions may underlie the link between high-grade fibrosis and extra-hepatic malignancy [50]. In addition, patients with hepatic steatosis are more likely to have chronic inflammation and insulin resistance, creating a microenvironment suitable for cancer development [51, 52]. Increased proinflammatory cytokine or altered adipokine production may promote the development of cancer through proliferative and anti-apoptotic effects [53].
In the stratified analysis, the highest risk of cancer incidence and mortality in the M-MAFLD group was observed predominantly in non-obese subjects, nonsmokers and alcohol abstainers in this study. To reduce the effect of reverse causality, we excluded people with pre-existing cancer and introduced a lag period of 1 year. In a sensitivity analysis, we extended the lag period to 2 years but found little change in the results. However, reverse causality cannot be ruled out, considering the long latency period of some cancers [54], and our results should be validated in further studies.
Our study presents the substantial strengths of large sample size, a population-based design, and categorization of MAFLD considering additional etiologies. This study provides new insights for understanding cancer-related clinical outcomes of MAFLD, which can be used to develop cancer screening strategies for patients with MAFLD. However, there were also some limitations in this work. First, our study cannot establish a causal relationship because of its population-based observational design. Second, although histological or radiological modalities are preferred methods for detecting hepatic steatosis and fibrosis, we used the FLI and BARD as surrogate markers of fatty liver and hepatic fibrosis. The FLI cannot accurately quantify hepatic steatosis and differentiate simple steatosis from steatohepatitis [55]. However, due to the advantages of easy access, using the FLI is practical for screening the general population in epidemiologic studies [56, 57]. It was also validated in the Korean population with acceptable sensitivity and specificity [58, 59]. Moreover, the median (interquartile range) value of FLI in individuals with a relevant ICD10 code for steatosis (K76.0) was 30.5 (12.8-56.2), while that in those without K76.0 was 19.1 (7.5-41.4) in this study. The area under the receiver operating characteristic curve (AUROC) of BARD for discriminating advanced liver fibrosis in patients with NAFLD ranged 0.77–0.81 [60], and the BARD score yielded a high negative predictive value to rule out advanced fibrosis (90.9%) in Korean NAFLD [61]. Recently, Wu et al. reported the diagnostic performance of BARD in MAFLD with an AUROC of 0.609 [62]. Because the two variables for calculating the BARD score (BMI and diabetes) were also included in the diagnostic criteria of MAFLD, this may lead to a limited performance in detecting advanced fibrosis in MAFLD patients. Nonetheless, its high negative predictive value makes the BARD score an alternative tool for ruling out advanced fibrosis in epidemiologic studies, and only the BARD score was available for evaluating advanced liver fibrosis in the Korean NHIS database [63, 64]. Third, there might be residual confounding factors such as family history or occupational exposures related to cancer development since the NHIS does not collect these data. Finally, because we used FLI rather than ICD codes to define hepatic steatosis, there is potential for misclassification. However, the use of ICD codes relies on the accuracy of clinical documentation and administrative coding, which can be influenced by country-specific codes and coding rules. Since patients with hepatic steatosis often fail to receive medical attention due to its mild severity, patients selected using diagnostic codes would not include a large number of patients with fatty liver disease [62]. Further replicative research is warranted to validate and elucidate the underlying mechanisms for our results.
In conclusion, the M-MAFLD group showed the greatest risk of cancer incidence and mortality among the MAFLD subgroups. Classifying MAFLD subgroups by additional etiologies other than pure metabolic origin and the presence of fibrosis could identify high-risk groups for cancer and contribute to cancer prevention.
DECLARATIONS
AUTHORS CONTRIBUTION
The corresponding authors (Eun Ju Cho and Kyungdo Han) had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Eun Ju Cho, Kyungdo Han
Provision of study materials or patients: Goh Eun Chung, Su Jong Yu, Jeong-Ju Yoo, Yuri Cho
Collection and assembly of data: Kyuna Lee, Yuri Cho, Su Jong Yu, Jeong-Ju Yoo, Dong Wook Shin
Data analysis and interpretation: Yuri Cho, Su Jong Yu, Kyu-na Lee, Dong Wook Shin, Yoon Jun Kim, Jung-Hwan Yoon
Manuscript writing: Goh Eun Chung, Su Jong Yu, Kyungdo Han, Eun Ju Cho
Final approval of manuscript: All authors.
ACKNOWLEDGMENT
This study was performed using a database from the Korean National Health Insurance System.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
This work was supported in part by grants from the Seoul National University Hospital (04-2022-3140 and 30-2022-0340) and the Liver Research Foundation of Korea (Bio Future Strategies Research Project).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This was a retrospective study with clinical data collection, and the Institutional Review Board of Soongsil University (ID: SSU-202007-HR-236-01) approved waiving the participation consent.
CONSENT FOR PUBLICATION
All the authors consented to publish this work.
Open Research
DATA AVAILABILITY STATEMENT
The dataset supporting the conclusions of this article is available on the homepage of the National Health Insurance Sharing Service (NHIS) [http://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do]. To gain access to the data, a completed application form, a research proposal, and the applicant's approval document from the institutional review board should be submitted to and reviewed by the inquiry committee of research support in NHIS. Currently, the use of NHIS data is allowed only for Korean researchers.




