Renal cell carcinoma escapes NK cell-mediated immune surveillance through the downregulation of DNAM-1
Abbreviations
-
- NK
-
- Natural killer
-
- RCC
-
- Renal cell carcinoma
-
- ccRCC
-
- clear cell Renal cell carcinoma
-
- DNAM-1
-
- DNAX accessory molecule-1
-
- PVR
-
- Poliovirus receptor
-
- CRISPR/Cas9
-
- Clustered regularly interspaced short palindromic repeats associated protein 9
-
- KIR
-
- Killer cell immunoglobulin-like receptors
-
- MHC
-
- Major histocompatibility complex
-
- PBMC
-
- Peripheral blood mononuclear cell
-
- NCR
-
- Natural Cytotoxicity Receptor
-
- TIGIT
-
- T cell immunoreceptor with Ig and ITIM domains
-
- KO
-
- Knock out
-
- WT
-
- Wild type
-
- TCGA
-
- The cancer genome atlas
-
- PMN
-
- Polymorphonuclear
-
- MDSC
-
- Myeloid-derived suppressor cells
-
- CBLB
-
- Casitas B-Lineage Lymphoma Proto-Oncogene B
-
- Gas6
-
- Growth arrest-specific protein 6
-
- TAM
-
- Tyro3, Axl, and Mer
-
- COX-2
-
- Cyclooxygenase-2
-
- Arg-1
-
- Arginase-1
-
- iNOS
-
- Inducible nitric oxide synthase
-
- t-SNE
-
- t-distributed stochastic neighbor embedding
Dear Editor,
The activating receptor DNAX accessory molecule-1 (DNAM-1) plays an important role in T and natural killer (NK) cell-mediated cytotoxicity via the interaction with its ligands poliovirus receptor (PVR, CD155) and Nectin-2 (CD112). Compared with peripheral blood NK cells, tumor-infiltrating NK cells show reduced expression of DNAM-1 across several solid tumors, including ovarian and breast cancer resulting in impaired NK cell function. Early studies reported an inverse correlation between DNAM-1 expression and its ligands in leukemic blasts and ovarian cancer [1]. It was recently reported that engagement of the PVR results in reduced DNAM-1 expression in murine T cells [2].
In contrast to several other solid tumors, the frequency of tumor-infiltrating T cells is associated with poor prognosis in patients with renal cell carcinoma (RCC) [3]. Instead, high frequencies of NK cells have been associated with improved prognosis in RCC [4]. However, the underlying mechanisms that regulate NK cell activity in RCC are poorly understood. Here we explored the DNAM-1 – PVR axis in RCC and propose a strategy to maintain DNAM-1 expression in NK cells. Analysis of The Cancer Genome Atlas (TCGA) data revealed that high DNAM-1 expression is associated with increased overall survival and progression-free survival in patients with RCC. Conversely, high PVR expression is associated with a worse prognosis (Figure 1A and Supplementary Figure S1A). PVRhigh tumors showed increased neutrophil, M0 and M2 macrophage, and resting memory CD4 T cell signatures and reduced signatures for CD8 T cells, activated NK cells, gamma delta T cells and resting dendritic cells (Supplementary Figure S1B). The detailed methods are described in the Supplementary Materials.
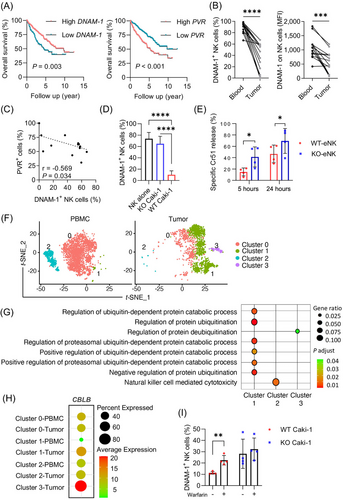
RCC tumors induce DNAM-1 downregulation on NK cells and attenuate their activity via PVR. (A) Overall survival in RCC patients stratified by high and low DNAM-1 or PVR expression. P values were calculated by log-rank test. (B) Frequencies of DNAM-1+ NK cells and mean fluorescence intensity (MFI) of NK cells in blood and tumor of RCC patients (n = 14 patients). (C) Inverse correlation of PVR expression on tumor tissue and frequencies of DNAM-1+ NK cells in RCC tumor tissue (Spearman correlation) (n = 14 patients). (D) Frequencies of DNAM-1+ NK cells after 2-day co-culture of NK cells and WT Caki-1 or PVR-KO Caki-1 cells (n = 7 biological replicates). Data are depicted as mean ± SD. Fold change in the frequency of DNAM-1+ NK cells between co-culture with WT and PVR-KO Caki-1 is 6.5. (E) Specific killing of WT Caki-1 target by tumor-experienced NK cells (n = 4 biological replicates). Data are depicted as mean ± SD. (F) t-distributed stochastic neighbor embedding (t-SNE) of different NK cell clusters origin in PBMC and tumor. (G) GO and KEGG pathway enrichment of different NK clusters. (H) Expression of CBLB on NK cells from different clusters in PBMC and tumor. (I) Frequencies of DNAM-1+ NK after co-culture with Caki-1 in the absence or presence of warfarin (n = 4 biological replicates).
Abbreviations: RCC, renal cell carcinoma; DNAM-1, DNAX accessory molecule-1; NK, natural killer; PVR, poliovirus receptor; MFI, mean fluorescence intensity; WT, wild type; KO, knockout; eNK, experienced NK; t-SNE, t-distributed stochastic neighbor embedding; PBMC, peripheral blood mononuclear cell; GO, gene ontology; KEGG, Kyoto encyclopedia of genes and genomes; CBLB, Casitas B-Lineage Lymphoma Proto-Oncogene B.
The expression of PVR has shown to be a negative prognostic marker in several cancers, including urothelial carcinoma, hepatocellular carcinoma, and head and neck squamous cell carcinoma. Although PVR is expressed in RCC, its prognostic value has not been well-studied in patients with RCC. Analysis of PVR expression levels in the TCGA RCC cohort (KIRC) revealed that PVR expression correlated with Fuhrman grade, where the highest expression of PVR was observed in Fuhrman grade 4 tumors (data not shown). Compared with peripheral blood, RCC-infiltrating T and NK cells showed reduced DNAM-1 expression (Figure 1B and Supplementary Figure S1C-D). The frequency of intratumoral DNAM-1+ NK and T cells negatively correlated with PVR expression levels in RCC tumors (Figure 1C and Supplementary Figure S1E-F). Collectively, these results suggest that PVRhigh tumors may evade the immune system by downregulating DNAM-1 on NK and T cells, thereby impacting the prognosis of RCC patients.
To investigate if PVR expression by tumor cells influences DNAM-1 expression, peripheral blood mononuclear cells (PBMC) were cultured with RCC cell lines expressing different levels of PVR but at comparable expression of Nectin-2 (Supplementary Figure S2A). Upon co-culture, both the frequency of DNAM-1+ NK and T cells and the expression of DNAM-1 were significantly decreased. Notably, co-culture with Caki-1 cells expressing the highest level of PVR resulted in the strongest down-regulation of DNAM-1 (Supplementary Figure S2B). To confirm that reduced DNAM-1 expression on NK cells depends on PVR expression by tumor cells, PVR was knocked out (KO) with clustered regularly interspaced short palindromic repeats associated protein 9 (CRISPR-Cas9) in Caki-1 cells prior to co-culture with PBMC. Importantly, KO of PVR did not affect the expression of Nectin-2 and major histocompatibility complex (MHC) class I (Supplementary Figure S3A). Although DNAM-1 expression was significantly reduced in both T and NK cells upon culture with wild-type (WT) Caki-1 cells, a stronger reduction in DNAM-1 expression was observed in NK cells (Supplementary Figure S3B). In addition, purified NK cells showed significant downregulation of DNAM-1 after culture with WT but not PVR-KO Caki-1 cells (Figure 1D). Exposure to Caki-1 cell culture supernatant did not influence the expression of DNAM-1 in NK cells suggesting that the reduced expression of DNAM-1 might indeed depend on cell contact (data not shown). Similarly, Chauvin et al. [5] demonstrated that membrane-bound but not soluble PVR reduced DNAM-1 on NK cells.
The expression of T cell immunoreceptor with Ig and ITIM domains (TIGIT) but not CD96 was also reduced upon culture with WT Caki-1 cells, though the effect was not as profound as for DNAM-1 (6.5-fold decrease for DNAM-1; 1.35-fold decrease for TIGIT) (Figure 1D and Supplementary Figure S3C-D). The expression of other NK cell receptors, including CD57, killer cell immunoglobulin-like receptors (KIRs), natural killer group 2 member A (NKG2A), NKG2C NKG2D and the natural cytotoxicity receptors (NCRs) NKp30, NKp44, and NKp46, did not differ between cultures with WT or PVR-KO Caki-1 cells (Supplementary Figure S3C). In comparing the phenotype of DNAM-1+ and DNAM-1− NK cells upon co-culture with WT Caki-1 cells, DNAM-1− NK cells express lower levels of KIRs (KIR3DL1/KIR2DL2/ KIR2DL3), NKG2A, and CD96 (Supplementary Figure S4). In contrast, the expression of KIR2DL1/S1, NKG2C, NKG2D, NKp30, NKp44, NKp46, CD57, and TIGIT did not differ between DNAM-1+and DNAM-1− NK cells. Importantly, the reduced DNAM-1 expression upon exposure to WT but not PVR-KO Caki-1 cells significantly reduced NK cell-mediated cytotoxicity (Figure 1E and Supplementary Figure S5A). These findings demonstrate that RCC tumors downregulate DNAM-1 expression via PVR ligation and attenuate NK activity in vitro.
A recent study showed that ligation of PVR results in the degradation of internalized DNAM-1 mediated by the E3 ubiquitin ligase Casitas B-Lineage Lymphoma Proto-Oncogene B (Cbl-b) in CD8+ T cells [2]. Analysis of the single-cell RNA sequencing dataset from peripheral blood and RCC tumors (Figure 1F) revealed that tumor-infiltrating NK cells (clusters 1 and 3) featured genes that are related to the ubiquitin processes, and peripheral blood NK cells (cluster 2) featured genes that are related to cytotoxicity (Figure 1G). Of note, CBLB was preferentially expressed in tumor-infiltrating NK cells compared with peripheral blood NK cells (Figure 1H), implying Cbl-b-mediated DNAM-1 degradation in NK cells in RCC tumors. The anticoagulant warfarin has been proposed to exert anti-metastatic activity by modulating the growth arrest-specific protein 6 (Gas6)/ Tyro3, Axl, and Mer (TAM)/Cbl-b pathway in NK cells [6]. Consistent with this study, we found that warfarin inhibited PVR-mediated downregulation of DNAM-1 in NK cells cultured with WT Caki-1 cells (Figure 1I), suggesting that DNAM-1 downregulation might be due to ubiquitin-mediated proteolysis. Since CBLB knockout or knockdown has been shown to increase NK cell cytotoxicity against tumor cells, targeting CBLB may be a potential way to enhance NK cell antitumor activities [7, 8].
Various cell populations within the tumor microenvironment can negatively impact NK cell activity. For instance, cancer-associated fibroblasts express PVR, which may influence the expression of DNAM-1 on NK cells [9]. Myeloid-derived suppressor cells (MDSC) display an array of immunosuppressive properties and have been shown to negatively affect overall survival in RCC patients [8]. Furthermore, MDSC can downregulate the expression of NKG2D on NK cells [10]. Herein, we found that the frequency of polymorphonuclear (PMN) MDSC negatively correlated with DNAM-1+ CD56dim NK cells but not total NK cells (Supplementary Figure S5B). In addition, the frequency of DNAM-1+ CD56dim NK cells negatively correlated with the frequency of Lin−CD11b+ myeloid cells, CD11b+ myeloid cells expressing cyclooxygenase-2 (COX-2+), arginase-1 (Arg-1+), and the expression level of inducible nitric oxide (iNOS) on CD11b+ myeloid cells (Supplementary Figure S5C). Collectively, these results suggest additional suppressive factors imposed by RCC are likely responsible for DNAM-1 downregulation in NK cells.
The importance of DNAM-1 in T and NK cell cytotoxicity and tumor surveillance is well-established [1]. However, the high expression of DNAM-1 ligands in tumor cells may also represent an immune escape mechanism. Here, we show that DNAM-1 is downregulated in T but more profound in NK cells upon contact with PVR−positive tumors, resulting in attenuated NK-mediated killing of tumor cells. In support of this finding, analysis of TCGA data revealed that higher PVR expression is associated with a worse prognosis. In conclusion, the downregulation of DNAM-1 on NK cells via ligation of PVR provides a novel mechanism for escaping NK-mediated surveillance by RCC tumors.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Designing research studies: Le Tong, Veronika Kremer, Shi Yong Neo, Ulrika Harmenberg, Eugenia Colón, Ann-Helén Scherman Plogell, Lisa Lei Liu, and Andreas Lundqvist. Conducting experiments: Le Tong, Veronika Kremer, Shi Yong Neo, Arnika Kathleen Wagner, Ziqing Chen, Ying Yang, and Christina Seitz. Acquiring data: Le Tong and Veronika Kremer. Analyzing data: Le Tong, Veronika Kremer, Shi Yong Neo, Yaxuan Liu, Yi Chen, Ziqing Chen, Christina Seitz, Nicholas Patrick Tobin, Maarten Alexander Ligtenberg, Xinsong Chen, and Felix Haglund. Providing reagents and materials: Evren Alici, Xinsong Chen, Barbara Seliger, Ulrika Harmenberg, Eugenia Colón, and Ann-Helén Scherman Plogell. Writing the manuscript: Le Tong, Veronika Kremer, Shi Yong Neo, Lisa Lei Liu, and Andreas Lundqvist.
ACKNOWLEDGMENTS
We thank Anna Malmerfelt, Department of Oncology-Pathology, Karolinska Institutet, for technical histological support. We thank Drs. Björn Önfelt and Valentina Carannante, Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, for intellectual input.
CONFLICT OF INTEREST STATEMENT
No competing interests are related to this work.
FUNDING INFORMATION
This work was supported by grants from The Swedish Cancer Society (#CAN 2018/451 and #21 1524 Pj), The Cancer Research Funds of Radiumhemmet (#161192, #181183, and #211253), The Swedish Society for Medical Research (P17-0134), and The Swedish Society of Medicine (SLS-960960).
CONSENT FOR PUBLICATION
Not applicable
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Regional Ethical Review Board in Stockholm (Ethical approval #2013-570-31). All patients provided informed consent.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




