Prognostic impact of circulating tumor cells in patients with soft-tissue sarcomas: a prospective study
Abbreviations
-
- ctDNA
-
- circulating tumor DNA
-
- CTC
-
- circulating tumor cell
-
- ISET
-
- Isolation by Size of Epithelial Tumor cells
-
- PFS
-
- progression-free survival
-
- OS
-
- overall survival
-
- RT-PCR
-
- Reverse Transcriptase polymerase chain reaction
-
- STS
-
- soft-tissue sarcomas
Dear Editor,
Sarcomas represent a heterogenous group of tumors accounting for about 1% of cancer in adults and 15% in children [1]. However, despite adequate locoregional treatment, up to 40% of patients develop metastatic disease. Drugs used in the advanced setting have very limited efficacy, with a response rate of <10% and progression-free survival of <4 months and are mainly used for palliative purposes [1]. It is, therefore, important to monitor individual therapy responses to avoid inefficient therapy and unnecessary toxicity. Such monitoring is currently based on repeated CT imaging which may represent an important burden for patients with advanced disease [2].
Circulating tumor cells (CTCs) have been considered a potentially promising tool to monitor therapy response in cancer patients. The detection of CTCs in the peripheral blood has been associated with prognosis in several tumor types, including breast, colorectal, prostate and non-small cell lung cancer [3]. CTCs count changes during treatment may also be predictive of response to treatment [3].
Data related to sarcoma-derived CTCs are very limited. Indeed, flow cytometry-based technologies for detecting sarcoma CTCs are limited by the lack of a universal marker. In this regard, the isolation by size of tumor cells (ISET) diagnostic technique, which relies on the tumor cellular size, which is larger than leukocytes, may represent an interesting approach for CTC enrichment and detection in patients with sarcoma [4, 5].
The aim of the study was to evaluate the prognostic relevance of CTCs in patients with soft-tissue sarcomas (STS) treated with systemic therapy in the neoadjuvant and advanced setting,
Seventy-six patients with metastatic sarcoma were prospectively enrolled between July 2017 and December 2020 (Supplementary Figure S1). The study design and method are described in the Supplementary Materials. The patients’ characteristics are described in Supplementary Table S1. CTCs were detected at baseline in 56 patients (74%). The average and median number of CTCs were 22 CTC/10 mL (range, 0-123 CTCs/10 mL) and 11 CTC/10 mL (range, 0-123 CTCs/10 mL), respectively. Characterizations of CTCs are presented in Supplementary Figure S2. The number of CTCs at baseline was not significantly correlated with the number of metastatic sites neither with histological subtype nor with histological grade (Supplementary Table S2).
The median follow-up time was 21.9 months. The median progression-free survival was 4.7 months (95% CI 3.7-5.7) (Figure 1A). At the time of statistical analysis, 43 patients had died, and 24 were still alive, while 9 were lost to follow-up. The median overall survival was 14.4 months (95% CI 8.7-20.1) (Figure 1B). CTC count at baseline, irrespective of the cut-off used (CTCs, ≥1 or ≥ median value), was not correlated with PFS and OS (Figure 1 and Supplementary Figure S3).
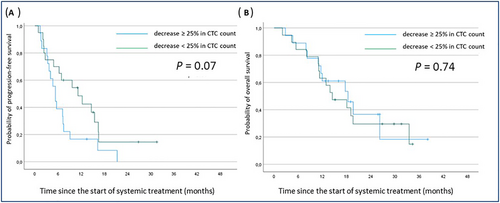
Kaplan Meier curves of progression-free (A) and overall survival (B) according to early CTC count decrease (blue line: decrease > 25% in CTC count at the time of cycle 2 compared to baseline; green line: no change, increase or decrease <25%).
Abbreviations: CTC, circulating tumor cells;
To investigate whether a change in CTC predicts early progression and/or death, we compared changes in levels between treatment onset and cycle 2 (3 to 4 weeks after treatment onset). Patients with no detectable CTCs at any time point (n = 2) and patients with available CTC count at baseline only (n = 27) were excluded from this analysis. As shown in Figure 1, no significant difference was observed in terms of PFS or OS between patients who had decreased ≥ 25% in CTC count at the time of C2 experience and patients with no change, increase or decrease < 25%.
Blood represents the ideal compartment for developing non-invasive approaches to monitor and predict treatment responses in patients with cancer. However, no circulating biomarker is available yet for patients with sarcoma.
To our knowledge, we report here the first prospective study investigating the enumeration of sarcoma CTCs, including patients treated with approved therapies (Supplementary Table S1). Only a few studies have performed CTC detection in sarcoma patients, with most of them only including a very low number of patients (mean number of cases, 44; range 1-225) (Supplementary Table S3). Most of these studies used reverse transcriptase polymerase chain reaction (RT-PCR)-based methods to detect CTCs (Supplementary Table S3). However, this approach is reliable only for translocation-related sarcomas such as Ewing's sarcoma, representing <30% of all sarcomas.1 More recently, several studies investigated the capacity of flow cytometry-based technologies for detecting sarcoma CTCs (Supplementary Table S3). However, this approach is limited by the high heterogeneity of sarcoma and the lack of a universal marker. We have used the ISET diagnostic technique here, which is an antibody-independent whole blood filtration method relying on the tumor cellular size, which is larger than leukocytes4. Chinen et al. [5] already demonstrated the relevance of ISET to isolate, identify, and characterize sarcoma CTCs in a study including 11 patients with different histological subtypes. One advantage of the ISET method is that it allows the study of CTC morphological features. In a study including 808 patients (3 of them had sarcoma), Hofman et al. [6] demonstrated that cytopathologic analysis of CTCs detected by ISET was a reliable procedure associated with high specificity and sensitivity in addition to its simplicity, rapidity and low cost.
A limitation of our study was that the molecular features of the CTC isolated with ISET were not investigated. This would have required extensive molecular experiments that were out of this study's scope. However, the evaluation of CTC was performed by experts in the field of sarcoma pathology by using rigorous cytological criteria as previously described [6]. It is also important to note that mechanical influences such as surgical or interventional procedures may also influence CTC count by enhancing the release of viable tumor cells into the circulation [7]. Further studies investigating the role of CTC in patients with advanced STS should also capture this potential source of bias.
The mean and median numbers of CTC detected at baseline in our study (22 and 11, respectively) were similar to that observed in the study of Chinen et al. [5] (21 and 5), who used the same methodology. Contrary to that observed in several other epithelial tumors such as breast, colorectal, prostate or lung cancer [3-6], the baseline level of CTC was not associated with overall survival, suggesting a lack of intrinsic prognostic value. Martin Broto et al. [8] showed in a study including 35 patients with advanced sarcoma that a decrease in CTC counts after treatment with the experimental drug olaratumab was higher in patients with controlled disease (Supplementary Table S3). Such correlation with treatment outcome was not found in our study.
Another limitation of our study is related to the heterogeneity of the study population, which included several histological subtypes. For this reason, we cannot exclude that CTC count may have a prognostic value in a specific STS histology. Moreover, although filtration-based techniques are simple to use, they can only process small volumes of diluted blood at a time and are prone to membrane clogging. However, a comparison between the ISET and other methods based on positive selection of tumor cells, such as CellSearch, showed that ISET had a higher sensitivity in several tumor types, including prostate, lung and renal cancer patients [9].
Overall, our results suggest that monitoring CTC count during treatment might not be useful in predicting patient outcomes. Further studies are therefore needed to identify innovative methods for therapeutic response monitoring in patients with advanced sarcoma. In this regard, monitoring circulating tumor DNA (ctDNA) may represent a promising approach. Indeed, monitoring ctDNA was previously shown to be useful in evaluating treatment responses and predicting disease progression or relapse in patients with leiomyosarcoma [10].
DECLARATIONS
AUTHORSHIP
Concept and design: Antoine Italiano; Acquisition, analysis, or interpretation of data: Blanchi Julie, Laroche-Clary Audrey, Bonhomme Benjamin, Le Loarer François, Italiano Antoine; Drafting of the manuscript: Italiano Antoine, Blanchi Julie; Critical revision of the manuscript for important intellectual content: all authors; Statistical analysis: Blanchi Julie, Italiano Antoine; Administrative, technical, or material support: Italiano Antoine; Validation of the submission: all authors; Supervision: Italiano Antoine.
ETHICS IN APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the local Research Ethics Committee of Bergonié Insitute (Bordeaux, France) according to good clinical practices and applicable laws and regulations. All methods were performed in accordance with the relevant guidelines and regulations. All patients provided written informed consent.
CONSENT FOR PUBLICATION
The article does not contain any person's data.
CONFLICT OF INTEREST STATEMENT
AI: Research grant (AstraZeneca, Bayer, BMS, Merck, MSD, Pharmamar). The other authors declare that they have no competing interests.
FUNDING
This research was funded by the Fondation Bergonié.
ACKNOWLEDGMENTS
None.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are not publicly available due to the clinical and confidential nature of the material but can be made available from the corresponding author upon reasonable request.




