ABTL0812 enhances antitumor effect of paclitaxel and reverts chemoresistance in triple-negative breast cancer models
Abbreviations
-
- TNBC
-
- Triple-negative breast cancer
-
- NSCLC
-
- Non-small cell lung cancer
-
- 231PTR
-
- Paclitaxel-resistant MDA-MB-231 cells
-
- MSL
-
- Mesenchymal stem-like
-
- Akt
-
- Protein kinase B
-
- mTOR
-
- Mechanistic target of rapamycin
-
- TRIB3
-
- tribbles homolog 3
-
- LC3
-
- Microtubule-associated protein 1A/1B-light chain 3
-
- PTEN
-
- Phosphatase and tensin homolog
-
- PI3KCA
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
-
- PARP
-
- Poly (ADP-ribose) polymerase
-
- PI3K
-
- Phosphoinositide 3-kinase
-
- IC50
-
- 50% inhibitory concentration
Dear Editor,
Triple-negative breast cancer (TNBC) accounts for 20% of all breast carcinomas and lacks a validated targeted therapy; thus, currently, cytotoxic chemotherapy is the treatment of choice [1]. Compared with other breast cancer types, patients with TNBC are younger, have larger tumors, a higher risk of metastasis, and a higher rate of recurrence [2]. Among all the subtypes described by Lehmann et al. [3], mesenchymal-like and mesenchymal stem-like (MSL) subtypes had the lowest 5-year distant metastasis-free survival rates. Therefore, the aggressiveness and poor prognosis of TNBC call for new and more effective therapies.
ABTL0812 is a novel first-in-class anticancer agent. It was initially selected for preclinical development based on its anti-proliferative effect on different human cancer cell lines and its safety profile in animal models as both single therapy and in combination with chemotherapy [4-8]. A first-in-human phase I clinical trial with ABTL0812 was successfully completed, showing a high safety profile and signs of efficacy in patients with advanced solid tumors who had received ABTL0812 orally after several chemotherapy lines (NCT02201823). Based on these findings, a phase I/IIa clinical trial was performed whereby ABTL0812 was administered as first-line therapy in combination with paclitaxel and carboplatin in patients with advanced/recurrent endometrial and metastatic squamous non-small cell lung cancers (NSCLC). The trial results observed improved efficacy without increasing toxicities, compared to chemotherapy alone (NCT03366480). ABTL0812 is currently in a phase IIb clinical trial as first-line therapy in combination with FOLFIRINOX in patients with metastatic pancreatic cancer (NCT04431258).
ABTL0812 has a dual-action mechanism. It can induce endoplasmic reticular (ER) stress and inhibit the protein kinase B (Akt)/mechanistic target of rapamycin (mTOR) axis via the overexpression of tribbles homolog 3 (TRIB3). These two actions converge to strongly induce cytotoxic autophagy that can cause cancer cell death, while non-tumor cells are spared [4-8]. Deregulation of the phosphoinositide 3-kinase (PI3K)/Akt/mTOR (PAM) signaling pathway is a common oncogenic aberration observed in TNBC that frequently leads to chemoresistance [1]. Based on the anticancer efficacy of ABTL0812 as a single agent or potentiating the effect of chemotherapy and the involvement of the PAM pathway in carcinogenesis and chemoresistance in breast cancer, we hypothesized that ABTL0812 treatment might be effective against TNBC and could revert resistance to paclitaxel.
The effect of ABTL0812 on cell proliferation was tested in MSL TNBC MDA-MB-231 and paclitaxel-resistant MDA-MB-231 (231PTR) cells (Supplementary Material). The 50% inhibitory concentration (IC50) values determined in these cell lines were 34.0 ± 1.6 × 10–6 and 37.0 ± 3.3 × 10–6 mol/L, respectively. These results are in agreement with previous research. It was described to cause cytotoxicity in different human cancer cell lines with IC50 values ranging 2.0 - 6.0 × 10–5 mol/L [4-8]. No significant differences were found in ABTL0812 cytotoxicity between both TNBC cell models (P = 0.977), indicating that ABTL0812 equally kills cells sensitive or resistant to paclitaxel. TRIB3 and the autophagy marker microtubule-associated protein 1A/1B-light chain 3 II (LC3-II) were also quantified by western blotting (Supplementary Material), detecting an increase in their expression after ABTL0812 treatment in both MSL TNBC cell models (Figure 1A). TRIB3 is a key protein in both actions of ABTL0812 and validated as a pharmacodynamic biomarker in NSCLC [5, 6] and endometrial cancer patients [6, 8]. Hence, ABTL0812 induces autophagy and has anticancer activity in MSL TNBC cells sensitive and resistant to paclitaxel.
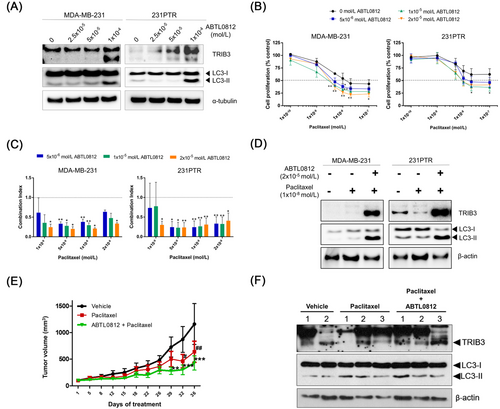
Next, the combination of ABTL0812 and paclitaxel was explored in MSL TNBC sensitive and paclitaxel-resistant cells (Supplementary Material). The combination treatment synergistically inhibited cell proliferation in both cell models, significantly reducing the IC50 value of paclitaxel. The number of living cells was dramatically reduced by the addition of ABTL0812 in 231PTR cells, leading to IC50 values similar to MDA-MB-231 cells; the IC50 of paclitaxel as a single agent decreases from over 1.0 × 10–7 mol/L (maximum concentration tested) to 8.1 ± 1.2 × 10–9 mol/L when given in combination with 1.0 × 10–5 mol/L of ABTL0812 (Figure 1B). Further, all ABTL0812 concentrations (0.5, 1.0, and 2.0 × 10–5 mol/L) tested in combination with paclitaxel (0.1, 0.5, 1.0, and 2.0 × 10–8 mol/L) exhibited synergistic effects in MDA-MB-231 and 231PTR cells (Figure 1C). The molecular action of the combination was studied to determine its effect on TRIB3 expression and autophagy. The co-treatment of paclitaxel with ABTL0812 induced higher expression levels of TRIB3 and LC3-II than the untreated control and the treatment with paclitaxel alone (Figure 1D). Overall, these results demonstrate that ABTL0812 synergizes with paclitaxel in MSL TNBC cell models and, importantly, it re-sensitizes resistant cells.
The reversion of resistance to paclitaxel-induced by ABTL0812 was also tested in vivo in a xenograft animal model (Supplementary Material). The anticancer activity of ABTL0812 in combination with chemotherapy has been previously demonstrated in oncogenic animal models [4-8]. Nude mice were injected with 231PTR cells. Once tumors were established, the mice were treated for 36 days with ABTL0812. As expected, chemotherapy treatment had a mild effect on the growth of tumors derived from paclitaxel-resistant cells. However, the antitumor effect was potentiated when paclitaxel was combined with ABTL0812 (Figure 1E). In addition, the combination treatment was well tolerated, with no significant weight loss and no major clinical signs of toxicity or distress observed. The excised tumors were analyzed by western blotting, detecting an induction of TRIB3 and LC3-II by the combination treatment (Figure 1F). Therefore, these in vivo data further confirm that ABTL0812 reverts the chemoresistance to paclitaxel in an MSL TNBC xenograft model. These data indicate the potentiality of combining ABTL0812 and paclitaxel in MSL TNBC patients to prevent or revert chemoresistance.
The strategy of combining paclitaxel with targeted therapies for the treatment of TNBC patients to improve its therapeutic effect is being investigated in clinical trials. The Akt inhibitor ipatasertib (GDC-0068) has reached phase II clinical trials in combination with paclitaxel as a neoadjuvant treatment for early-stage TNBC (NCT02301988) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PI3KCA)/Akt1/phosphatase and tensin homolog (PTEN)-altered advanced TNBC (NCT03337724). AZD2281 (Olaparib), a poly (ADP-ribose) polymerase 1/2 (PARP1/2) inhibitor, in combination with paclitaxel has recently completed a phase I/II study in metastatic TNBC patients (NCT00707707). At preclinical level, other studies using human breast cancer cells have shown the induction of ER stress and the inhibition of the Akt/mTOR axis as a strategy to activate autophagy, causing cell death [9, 10]. For instance, the antiretroviral drug Nelfinavir suppressed Akt-mediated survival, increased ER stress and TRIB3 causing cell death by autophagy, and sensitized drug-resistant cells to chemotherapy [9].
Overall, the levels of evidence that support the combination of ABTL0812 with paclitaxel as a potential therapeutic strategy for TNBC patients are as follows: 1) ABTL0812 causes autophagy-dependent cancer cell death, in part due to the inhibition of the Akt-mTORC axis, which is frequently altered in TNBC and promotes chemoresistance, 2) previous phase II clinical data have shown an increased efficacy by combining ABTL0812 with chemotherapy in endometrial cancer and NSCLC, and 3) preclinical data in MSL TNBC models indicate that ABTL0812 potentiates paclitaxel efficacy and reverts chemoresistance.
In conclusion, we have shown that ABTL0812 has anticancer activity in MSL TNBC models. It synergized with paclitaxel and reverted resistance to chemotherapy. Therefore, these data support further investigation of ABTL0812 in other TNBC subtypes and as a potential therapeutic agent for TNBC patients in combination with paclitaxel.
DECLARATIONS
CONSENT FOR PUBLICATION
Not applicable.
AUTHORS’ CONTRIBUTIONS
Santiago Ruiz-Martínez, Héctor Perez-Montoyo, Marc Yeste-Velasco, Jose Alfón, Carles Domènech, Jose Miguel Lizcano, and Teresa Puig designed the study. Santiago Ruiz-Martínez, Emma Polonio-Alcalá, Sònia Solé-Sánchez, Pau Muñoz-Guardiola, and Elisabet Megías-Roda conducted the experiments, collected, and analyzed the data. Santiago Ruiz-Martínez, Emma Polonio-Alcalá, and Marc Yeste-Velasco wrote the manuscript. All the authors read, reviewed, and approved the final manuscript.
FUNDING
This research was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) (RETOS: RTC-2014-2589-1; EMPLEA: EMP-TU-2015-4576), the Catalan Government (2017SGR00385), and Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF, “A way to make Europe”/“Investing in your future”) (PI19/00372). This work was partially supported by Fundación Ramón Areces and Fundació Oncolliga and RadikalSwim (OncoSwim).
ACKNOWLEDGMENTS
Santiago Ruiz-Martínez acknowledges the postdoctoral grant (POSTDOCUDG-2020-0002) and Emma Polonio-Alcalá the predoctoral grant (2019FI_B01011).
ETHICS APPROVAL
The animal study was approved by the Ethics Committee on Animal Experiments of the Autonomous University of Barcelona (CEEAH) and the Ethics Commission in Animal Experimentation of the Generalitat de Catalunya, Catalonia, Spain.
CONFLICTS OF INTEREST STATEMENT
Sònia Solé-Sánchez, Pau Muñoz-Guardiola, Elisabet Megías-Roda, Héctor Perez-Montoyo, Marc Yeste-Velasco, Jose Alfón, and Carles Domènech are Ability Pharmaceuticals employees; Jose Miguel Lizcano is an advisory board member and Carles Domènech holds shares of the company.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




