XELOX doublet regimen versus EOX triplet regimen as first-line treatment for advanced gastric cancer: An open-labeled, multicenter, randomized, prospective phase III trial (EXELOX)
Trial registration: www.clinicaltrials.gov ( NCT02395640).
Abstract
Background
There is no consensus on whether triplet regimen is better than doublet regimen in the first-line treatment of advanced gastric cancer (AGC). We aimed to compare the efficacy and safety of oxaliplatin plus capecitabine (XELOX) and epirubicin, oxaliplatin, plus capecitabine (EOX) regimens in treating AGC.
Methods
This phase III trial enrolled previously untreated patients with AGC who were randomly assigned to receive the XELOX or EOX regimen. The primary endpoint was non-inferiority in progression-free survival (PFS) for XELOX as compared with EOX on an intention-to-treat basis.
Results
Between April 10, 2015 and August 20, 2020, 448 AGC patients were randomized to receive XELOX (n = 222) or EOX (n = 226). The median PFS (mPFS) was 5.0 months (95% confidence interval [CI] = 4.5-6.0 months) in the XELOX arm and 5.5 months (95% CI = 5.0-6.0 months) in the EOX arm (hazard ratio [HR] = 0.989, 95% CI = 0.812-1.203; Pnon-inferiority = 0.003). There was no significant difference in median overall survival (mOS) (12.0 vs. 12.0 months, P = 0.384) or objective response rate (37.4% vs. 45.1%, P = 0.291) between the two groups. In patients with poorly differentiated adenocarcinoma and liver metastasis, the EOX arm had a significantly longer mOS (P = 0.021) and a trend of longer mPFS (P = 0.073) than the XELOX arm. The rate of grade 3/4 adverse events (AEs) was 42.2% (90/213) in the XELOX arm and 72.5% (156/215) in the EOX arm (P = 0.001). The global health-related quality of life (QoL) score was significantly higher in the XELOX arm than in the EOX arm during chemotherapy.
Conclusions
This non-inferiority trial demonstrated that the doublet regimen was as effective as the triplet regimen and had a better safety profile and QoL as a first-line treatment for AGC patients. However, the triplet regimen might have a survival advantage in patients with poorly differentiated adenocarcinoma and liver metastasis.
Abbreviations
-
- AE
-
- adverse events
-
- CIs
-
- confidence intervals
-
- AGC
-
- advanced gastric cancer
-
- CF
-
- cisplatin and 5-fluorouracil
-
- CS
-
- cisplatin and S-1
-
- CTLA4
-
- cytotoxic T-lymphocyte associated protein 4
-
- DCF
-
- docetaxel, cisplatin and 5-fluorouracil
-
- DCR
-
- disease control rate
-
- DCS
-
- docetaxel, cisplatin, and S-1
-
- ECF
-
- epirubicin, cisplatin, and 5-fluorouracil
-
- ECOG
-
- Eastern Cooperative Oncology Group performance status
-
- ECX
-
- epirubicin, cisplatin, and capecitabine
-
- EOX
-
- epirubicin, oxaliplatin and capecitabine
-
- FGFR
-
- fibroblast growth factor receptor
-
- FLOT4
-
- oxaliplatin, docetaxel, and 5-fluorouracil
-
- FOLFIRI
-
- irinotecan, leucovorin, and 5-fluorouracil
-
- FOLFIRINOX
-
- Irinotecan, oxaliplatin, leucovorin and 5-fluorouracil
-
- FOLFOX
-
- oxaliplatin, leucovorin and 5-fluorouracil
-
- HER2
-
- human epidermal growth factor receptor
-
- HR
-
- hazard ratio
-
- ITT
-
- intention-to-treat
-
- LOCF
-
- last observation carried forward
-
- mDCF
-
- modified DCF
-
- mOS
-
- median overall survival
-
- mPFS
-
- median progression-free survival
-
- mTTP
-
- median time to progression
-
- ORR
-
- objective response rate
-
- OS
-
- overall survival
-
- PD-1
-
- programmed cell death protein-1
-
- PFS
-
- progression-free survival
-
- PP
-
- per-protocol
-
- QoL
-
- quality of life
-
- RECIST
-
- Response Evaluation Criteria in Solid Tumors
-
- SOX
-
- S-1 and oxaliplatin
-
- SP
-
- S-1 and cisplatin
-
- ToGA
-
- trastuzumab for gastric cancer
-
- XELOX
-
- oxaliplatin and capecitabine
1 BACKGROUND
Gastric cancer ranks fourth in cancer incidence and second in mortality worldwide, and more than 40% of patients with gastric cancer are Chinese [1, 2]. Patients with advanced gastric cancer (AGC) under best supportive care have a median survival of 3-4 months, and combination chemotherapy significantly prolongs these patients’ median overall survival (mOS) to 7-11 months and improves their quality of life (QoL) [3, 4]. The cisplatin plus 5-fluorouracil (CF) regimen and the epirubicin, cisplatin, plus 5-fluorouracil (ECF) regimen have been widely used as first-line treatments for AGC. The Trastuzumab for GAstric cancer (ToGA) study demonstrated a significantly longer mOS in the trastuzumab plus chemotherapy arm compared with chemotherapy alone in the first-line treatment of patients with human epidermal growth factor receptor (HER2)-positive AGC [5]. However, the low HER2 positive rate in AGC patients limited the clinical value of trastuzumab. Recently, new drugs brought more choices for AGC patients. Immune checkpoint inhibitor, programmed cell death protein-1 (PD-1) antibody, was approved by the Food and Drug Administration (FDA) of the United States and the National Medical Products Administration (NMPA) of China in AGC patients as third-line treatment at first, but the survival benefit was limited and only about 20%patients could obtain a chance to receive third-line treatment [2, 6]. Trastuzumab deruxtecan (T-Dxd) showed satisfactory efficacy in HER2-positive patients in the later-line treatment but was applicable in a limited number of patients [7]. PD-1 antibody combined with chemotherapy was approved by the US FDA and China NMPA as the first-line treatment for AGC with increased efficacy, but mOS was prolonged by only 2.2 months in the PD-1 antibody plus chemotherapy arm as compared with the chemotherapy only arm, indicating that chemotherapy remained the basis of the treatment [2, 8].
Next-generation chemotherapy drugs, such as oxaliplatin and capecitabine, have shown good clinical efficacy on gastric cancer. Trials demonstrated that the substitution of oxaliplatin for cisplatin or capecitabine for 5-fluorouracil, oxaliplatin, or capecitabine was at least non-inferior to cisplatin or 5-fluorouracil, or had a trend towards longer median progression-free survival (mPFS) or mOS [9, 10]. Furthermore, a meta-analysis showed that the efficacy of oxaliplatin was better than that of cisplatin [11], and the efficacy of capecitabine was better than that of 5-fluorouracil [12]. The REAL-2 study evaluated several variants of the ECF regimen with a 2 × 2, non-inferiority trial design and confirmed that oxaliplatin and capecitabine were at least non-inferior to cisplatin or 5-fluorouracil, and the epirubicin, oxaliplatin, plus capecitabine (EOX) regimen yielded longer mOS than the ECF regimen [13]. In recent years, there has been a decreasing trend in the application of triplet regimens in palliative treatment, but EOX was still used in clinical practice and trials [14, 15]. Next-generation doublet regimens, such as oxaliplatin and capecitabine (XELOX), showed promising efficacy and tolerability in a phase II study with an objective response rate (ORR) of 44%, median time to progression (mTTP) of 7.2 months, and mOS of 13.3 months in the first-line treatment of AGC [16]. S-1-based doublet regimens, such as SP (S-1 and cisplatin) and SOX (S-1 and oxaliplatin), have also been developed and have become standard regimens for AGC in Asia [17, 18].
Since both triplet and doublet regimens are widely recognized, the question of whether triplet regimens are better than doublet regimens in the first-line treatment of AGC remains controversial. In the V325 study, compared with the CF regimen, the mOS extension in the DCF (docetaxel, cisplatin and 5-fluorouracil) arm was only 0.6 months, and the strong toxicity might make this minimal survival benefit negligible, as the rateof grade 3/4 neutropenia was as high as 82% in the DCF arm [19]. In addition, the modified DCF regimen completed in the Chinese population reduced the dose intensity of chemotherapeutic agents, and the rate of grade 3/4 neutropenia remained as high as 60.5% [20].
It is questionable whether the triplet regimen would continue to have a slight efficacy advantage when next-generation doublet regimen is introduced as a control. Two superior phase III trials compared the next-generation doublet and triplet regimens. The JCOG1013 study yielded negative results in which DCS (docetaxel, cisplatin, and S-1) failed to increase the effects compared with the CS (cisplatin and S-1) regimen [21]. This might be due to the low dose intensity of docetaxel in the DCS regimen. Another phase III trial showed positive results in which FOLFIR (irinotecan, leucovorin, and 5-fluorouracil) yielded a longer median time-to-treatment failure (mTTF) than the ECX (epirubicin, cisplatin, and capecitabine) regimen (5.1 vs. 4.2 months) [22]. However, mTTF is no longer considered a suitable primary endpoint of effects, as it is influenced by the discontinuation of chemotherapy due to toxicity. There is a high frequency of grade 3/4 AEs in both groups (69% vs. 84%) [22]. Therefore, the results and conclusions were questioned [23, 24], and it is still difficult to determine which regimen is better in the first-line treatment of AGC. Moreover, FOLFIRI might not be a suitable choice due to its relatively high toxicity, and it is rarely used in first-line treatment of AGC in clinic.
Therefore, we carried out this comparative study based on the next-generation doublet regimen to observe whether the addition of one drug to the doublet regimen would improve the efficacy and provide the basis for the selection of first-line treatment for AGC. Since XELOX and EOX (i.e., adding epirubicin to XELOX) are used worldwide, this trial intended to compare the efficacy and safety of the next-generation XELOX doublet regimen and EOX triplet regimen in the first-line treatment of AGC to determine whether XELOX is non-inferior to EOX in terms of efficacy and superior in terms of safety.
2 PATIENTS AND METHODS
2.1 Participants
EXELOX (ClinicalTrials.gov identifier: NCT02395640) was an open-labeled, multicenter, randomized, prospective phase III trial conducted at seven sites in China designed to demonstrate the non-inferiority of the XELOX regimen to the EOX regimen as the first-line treatment for AGC patients. AGC included gastric cancer with primary lesion that cannot be radically resected or metastatic lesions. The protocol, attached in the Supplementary file, was approved by the local institutional review board and ethics committee at each participating site. The trial was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki and Chinese law. All patients signed an informed consent form before enrollment in the trial.
Eligible patients had histologically confirmed, unresectable, locally advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. Eligible patients had not received chemotherapy for locally advanced or metastatic disease and had at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1 criteria). Other key inclusion criteria were age of 18 years or older, an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, adequate organ function, and life expectancy of more than 3 months. Key exclusion criteria included HER2-positive patients who were able to afford and willing to receive trastuzumab treatment, and symptomatic brain or leptomeningeal metastases. Full eligibility criteria are provided in the protocol (Supplementary file).
The intention-to-treat (ITT) set included all patients who were enrolled and randomized, irrespective of whether they received the study medications or not. The per protocol (PP) set included patients who met the eligibility criteria, received at least one cycle of chemotherapy, and underwent at least one tumor response evaluation.
2.2 Randomization and treatment
We randomly assigned eligible patients (1:1) to receive either the XELOX (oxaliplatin 130 mg/m2 on day 1 and xeloda 1000 mg/m2 on days 1-14) or EOX regimen (epirubicin 50 mg/m2 on day 1, oxaliplatin 130 mg/m2 on day 1, xeloda 1000 mg/m2 on days 1-14). A computer-generated randomization schedule managed by King Yee Company (Beijing, China) was used. We stratified randomization by ECOG status, extent of disease (locally advanced or metastatic), and clinical trial center. Chemotherapy was repeated every 3 weeks until disease progression, intolerable adverse events (AEs), patient's death, withdrawal of informed consent, or up to eight cycles, followed by xeloda single-agent maintenance. The dose modifications are detailed in the trial protocol (Supplementary file).
2.3 Outcomes
The primary endpoint was PFS (the duration from the date of randomization to any documented tumor progression or death due to any cause). The secondary endpoints included OS (the duration from the date of randomization to the date of death due to any cause), ORR (the proportion of patients whose tumor decreased to a certain size and remained for a certain period of time, including those with complete response and partial response), safety, and QoL. Tumors were assessed radiologically at baseline, and efficacy evaluation was conducted every 6 weeks according to the criteria of RECIST 1.1. The global health/QoL (EORTC QLQ-C30) score was assessed at baseline (7 days before the first dose of study drugs) and every 6 weeks. AEs were classified and graded using the Common Terminology Criteria for Adverse Events (version 4.0).
2.4 Statistical analyses
The primary objective was to determine the non-inferiority of the XELOX regimen to the EOX regimen in terms of PFS. Assuming an mPFS of 7.0 months in the EOX arm, referring to the results of the REAL-2 trial [13], a hazard ratio (HR) < 1.3 was considered non-inferior, with 80% power and a one-sided P = 0.05. Accordingly, an estimated 360 progression events were needed to show non-inferiority, and an enrollment of at least 438 patients, accounting for a 10% dropout rate, was set for this trial. In the two previous phase III trials, before the start of the present trial, the non-inferiority margin in PFS of AGC patients under first-line treatment were 1.25 and 1.4 months, respectively [9, 18]. The non-inferiority margin in PFS in the present study was defined in reference to the trial of SOX non-inferior to SP, in which HR < 1.3 was set [18]. Nevertheless, in a phase III trial showing that the modified DCF (mDCF) triplet regimen was superior to the CF doublet regimen in terms of PFS (mPFS: 7.16 vs. 4.93 months) in Chinese patients in the REAL-2 trial [20], in which the mPFS of patients receiving mDCF (7.16 months) was close to that of patients receiving EOX (7.0 months), the HR of PFS for mDCF versus CF was 0.58, and the reciprocal was 1.72, which led to 1.36, with a 50% retention of 1.72. Therefore, the HR margin of 1.30 in the present study was considered suitable for evaluating non-inferiority.
We analyzed PFS, OS, and ORR in the ITT set, and analyzed PFS, OS, and safety in the PP set. OS and PFS were estimated using the Kaplan-Meier method. The log-rank test was used to compare the PFS and OS between the two groups. The differences in ORR and AEs between the groups were compared using the χ2 test. The Cox proportional hazards model was used to calculate HRs with 95% confidence intervals (CIs) and Pinteraction values for predefined subgroup analyses. QoL scores were calculated according to the procedures defined in the EORTC QLQ-C30 scoring manual. QoL scores were compared using a two-sample t-test or Wilcoxon rank sum test between arms. We also used the last observation carried forward (LOCF) method and Mean-value method to fill in the missing data. The analysis of the primary endpoint PFS was one-sided, and all other statistical analyses were two-sided. The statistical significance level was set at 0.05. Statistical analyses were all performed using the SAS statistical software (version 9.4; SAS Institute Inc, North Carolina State University, USA and Stata statistical software (version 12.0; StataCorp, College Station, TX, USA).
3 RESULTS
3.1 Patients and treatment
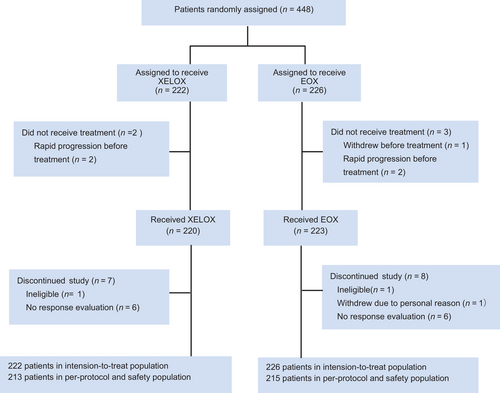
Between April 10, 2015 and August 20, 2020, 448 patients were enrolled (Figure 1) and randomly assigned to the XELOX (n = 222) or EOX arm (n = 226). The baseline characteristics were well balanced between the two arms (Table 1). Two patients in the XELOX arm and 3 in the EOX arm did not receive treatment and were excluded from the PP set. Seven patients in the XELOX arm and 8 in the EOX arm, who were eventually considered ineligible, withdrew due to personal reason, or received only one cycle of treatment without response evaluation, were also excluded from the PP set. One patient was diagnosed with gastric adenocarcinoma at another hospital which did not participate in this trial, but the final histological diagnosis was diffuse large B-cell lymphoma at a participating hospital. For another patient, imaging specialists considered that the lesion of the lung was unmeasurable.
| Characteristic | XELOX [cases (%)] | EOX [cases (%)] |
|---|---|---|
| Total | 222 | 226 |
| Sex | ||
| Male | 154 (69.4) | 148 (65.5) |
| Female | 68 (30.6) | 78 (34.5) |
| Age | ||
| <65 years | 160 (72.1) | 158 (69.9) |
| ≥65 years | 62 (27.9) | 68 (30.1) |
| ECOG PS | ||
| 0 | 22 (9.9) | 23 (10.2) |
| 1 | 198 (89.2) | 199 (88.0) |
| 2 | 2 (0.9) | 4 (1.8) |
| Locally advanced/metastatic | ||
| Locally advanced | 6 (2.7) | 6 (2.6) |
| Metastatic | 216 (97.3) | 220 (97.4) |
| Primary tumor location | ||
| Proximal gastric | 64 (28.8) | 53 (23.4) |
| Gastric body | 68 (30.6) | 77 (34.1) |
| Distal gastric | 60 (27.0) | 60 (26.5) |
| Multicentric | 21 (9.5) | 18 (8.0) |
| Stomach NOS | 9 (4.1) | 18 (8.0) |
| Liver metastasis | ||
| Yes | 78 (35.1) | 94 (41.6) |
| No | 144 (64.9) | 132 (58.4) |
| Lung metastasis | ||
| Yes | 24 (10.8) | 17 (7.5) |
| No | 198 (89.2) | 209 (92.5) |
| Lymphatic metastasis | ||
| Yes | 174 (78.4) | 174 (77.0) |
| No | 48 (21.6) | 52 (23.0) |
| Ovarian metastasis | ||
| Yes | 19 (8.6) | 26 (11.5) |
| No | 203 (91.4) | 200 (88.5) |
| Peritoneal metastasis | ||
| Yes | 58 (26.1) | 55 (24.3) |
| No | 164 (73.9) | 171 (75.7) |
| Number of metastatic sites | ||
| 0 | 6 (2.7) | 6 (2.6) |
| 1 | 89 (40.1) | 90 (39.8) |
| 2 | 94 (42.3) | 98 (43.4) |
| ≥3 | 33 (14.9) | 32 (14.2) |
| Adjuvant chemotherapy | ||
| Yes | 37 (16.7) | 30 (13.3) |
| No | 185 (83.3) | 196 (86.7) |
| Prior use of oxaliplatin in adjuvant chemotherapy | ||
| Yes | 30 (13.5) | 26 (11.5) |
| No | 192 (86.5) | 200 (88.5) |
| Prior gastrectomy | ||
| Yes | 52 (23.4) | 43 (19.0) |
| No | 170 (76.6) | 183 (81.0) |
| Pathologic type | ||
| Non-mucinous adenocarcinoma | 180 (81.1) | 180 (79.7) |
| Mucinous adenocarcinoma/SRCC | 42 (18.9) | 45 (19.9) |
| Non-adenocarcinoma | 0 | 1 (0.4) |
| Histologic grade | ||
| Well differentiated | 7 (3.2) | 6 (2.6) |
| Moderately differentiated | 33 (14.9) | 16 (7.2) |
| Poorly differentiated | 153 (68.9) | 156 (69.0) |
| Unknown | 29 (13.0) | 48 (21.2) |
| HER2 | ||
| Positive | 23 (10.4) | 17 (7.5) |
| Negative | 129 (58.1) | 145 (64.2) |
| Unknown | 70 (31.5) | 64 (28.3) |
| MMR/MSI | ||
| pMMR/MSS | 48 (21.6) | 57 (25.2) |
| dMMR/MSI-H | 12 (5.4) | 4 (1.8) |
| Unknown | 162 (73.0) | 165 (73.0) |
- The intention-to-treat set included all patients who were enrolled and randomized, irrespective of whether they received the study medications or not.
- Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status. SRCC, signet ring cell carcinoma. NOS, not otherwise specified. HER2, human epidermal growth factor receptor 2. MMR, mis-match repair. pMMR, proficient mis-match repair. dMMR, deficient mis-match repair.
As a result, 428 randomly assigned patients were included in the PP set, with 213 assigned to the XELOX arm and 215 assigned to the EOX arm. The median number of cycles administered to the two arms was six. The rate of dose delay was 32.9% (70/213) in the XELOX arm and 35.3% (76/215) in the EOX arm. Dose modifications resulting from toxicity were more common with EOX than with XELOX (55.8% vs. 35.2%, P = 0.009). The mean relative dose intensity of oxaliplatin (0.866 vs. 0.807) and xeloda (0.877 vs. 0.802) was higher in the XELOX arm than in the EOX arm. The reasons for stopping intravenous chemotherapy are shown in Supplementary Table S1.
For the patients in the PP set who had disease progression after first-line treatment, 47.5% (94/198) in the XELOX arm received second-line treatment and the information of second-line treatment was unavailable for 21.2% (42/198) patients. In the EOX arm, 41.3% (83/201) received second-line treatment and 18.4% (37/201) were lost to follow-up. The paclitaxel-based regimens were most commonly used for second-line treatment. Besides, in the XELOX arm, 59 patients received best support treatment, 2 received resection of primary lesion, and 1 received radiotherapy of primary lesion; in the EOX arm, 68 patients received best support treatment, 5 received resection of primary lesion, 4 received radiotherapy of primary lesion, and 4 rechallenged oxaliplatin and 5-fluorouracil.
3.2 Efficacy
At the time of data cutoff (February 17, 2021), 198 (89.2%) patients in the XELOX arm and 201 (88.9%) in the EOX arm had disease progressed or died. In the ITT set, mPFS was 5.0 months (95% CI = 4.5-6.0 months) in the XELOX arm and 5.5 months (95% CI = 5.0-6.0 months) in the EOX arm (HR = 0.989, 95% CI = 0.812-1.203; Pnon-inferiority = 0.003; Figure 2A). The upper limit of the 95% CI was lower than the non-inferiority margin of 1.30. The PP set also demonstrated a non-inferiority of PFS in the XELOX arm compared with the EOX arm. The mPFS was 5.0 months (95% CI = 5.0-6.0 months) with XELOX and 5.5 months (95% CI = 5.0-6.0 months) with EOX (HR = 0.983, 95% CI = 0.807-1.198; Pnon-inferiority = 0.003; Figure 2B).
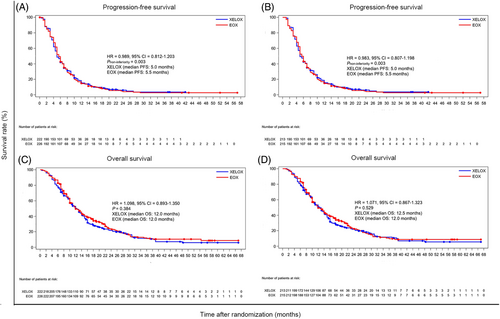
After a median follow-up of 13.4 months (range, 3.0-59.0 months), 184 patients in the XELOX arm and 176 in the EOX arm died. In the ITT set, mOS was 12.0 months (95% CI = 10.4-14.0 months) in the XELOX arm and 12.0 months (95% CI = 11.0-14.0 months) in the EOX arm (HR = 1.098; 95% CI = 0.893-1.350; P = 0.384; Figure 2C). In the PP set, mOS was 12.5 months (95% CI = 10.5-14.0 months) in the XELOX arm and 12.0 months (95% CI = 10.0-14.0 months) in the EOX arm (HR = 1.071; 95% CI = 0.867-1.323; P = 0.529; Figure 2D).
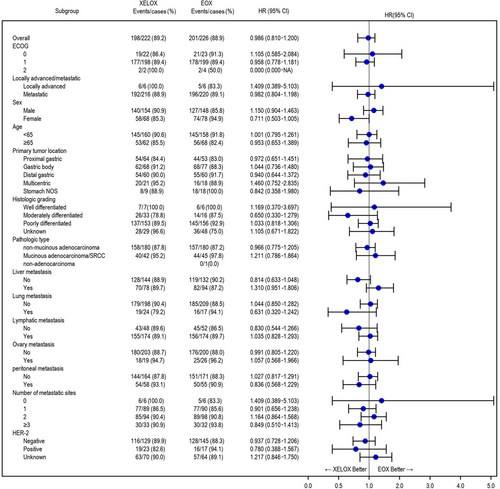
Subgroup analysis was performed to analyze the PFS and OS of the eligible patients according to ECOG status, locally advanced/metastasis, sex, age, primary tumor location, histologic grade, pathologic type, metastasis site, number of metastatic sites, and HER2 status (Figure 3, Supplementary Figures S1-3, Supplementary Table S2). Subgroup analysis showed that the EOX arm had longer OS than the XELOX arm in the HER2 unknown subgroup. This might be due to bias, as the HER2 unknown subgroup included both HER2-positive and -negative patients. However, there was no difference in OS between the two arms in the HER2-positive or -negative subgroup. There were no significant differences in PFS or OS in other subgroup analysis. Further exploratory analysis was performed in patients with poorly differentiated adenocarcinoma and liver metastases. The results showed that, in the ITT and PP sets, the EOX arm had a significantly longer mOS (P = 0.021 and P = 0.020, respectively) and a longer mPFS (P = 0.073, P = 0.057, respectively) than the XELOX arm (Supplementary Figure S4), while the clinicopathological characteristics between the two arms were balanced (Supplementary Table S3).
In the ITT set, the ORR was 39.0% in the XELOX arm and 47.4% in the EOX arm (P = 0.291) (Supplementary Table S4).
3.3 Safety
The most common AEs with a rate of ≥ 5% are summarized in Table 2. The rate of grade 3/4 AEs was 42.2% (90/213) in the XELOX arm and 72.5% (156/215) in the EOX arm (P = 0.001). Grade 3/4 white blood cell decrease, neutrophil decrease, platelet decrease, nausea, and vomiting occurred more often in the EOX arm than in the XELOX arm (all P < 0.05). One treatment-related death (lung infection) was observed in the EOX arm, and none was observed in the XELOX arm.
| Adverse event | All grade [cases (%)] | Grade 3/4 [cases (%)] | ||||
|---|---|---|---|---|---|---|
| XELOX (n = 213) | EOX (n = 215) | P value | XELOX (n = 213) | EOX (n = 215) | P value | |
| Total | 204 (95.8) | 210 (97.7) | 0.891 | 90 (42.2) | 156 (72.5) | 0.001 |
| Hematological | ||||||
| White blood cell decrease | 104 (48.8) | 170 (79.1) | 0.002 | 10 (4.7) | 56 (26.0) | <0.001 |
| Neutrophil count decrease | 105 (49.3) | 168 (78.1) | 0.004 | 28 (13.1) | 104 (48.4) | <0.001 |
| Platelet count decrease | 136 (63.8) | 129 (60.0) | 0.697 | 53 (24.9) | 81 (37.7) | 0.047 |
| Anemia | 119 (55.9) | 147 (68.4) | 0.211 | 13 (6.1) | 23 (10.7) | 0.122 |
| Non-hematological | ||||||
| Alanine aminotranferase increase | 63 (29.6) | 68 (31.6) | 0.765 | 3 (1.4) | 1 (0.5) | 0.623 |
| Aspartate aminotranferase increase | 94 (43.5) | 94 (43.7) | 1.000 | 1 (0.5) | 3 (1.4) | 0.623 |
| Blood bilirubin increase | 44 (20.7) | 31 (14.4) | 0.170 | 4 (1.9) | 4 (1.9) | 1.000 |
| Diarrhea | 22 (10.3) | 21 (9.8) | 0.874 | 3 (1.4) | 3 (1.4) | 1.000 |
| Oral mucositis | 3 (1.4) | 8 (3.7) | 0.221 | 1 (0.5) | 3 (1.4) | 0.623 |
| Anorexia | 86 (40.4) | 109 (50.7) | 0.196 | 1 (0.5) | 4 (1.9) | 0.372 |
| Nausea | 93 (44.1) | 108 (50.2) | 0.442 | 1 (0.5) | 8 (3.7) | 0.037 |
| Vomiting | 66 (31.0) | 87 (40.5) | 0.187 | 4 (1.9) | 17 (7.9) | 0.007 |
| Alopecia | 9 (4.2) | 105 (48.8) | <0.001 | 0 (0) | 0 (0) | 1.000 |
| Fatigue | 72 (33.8) | 71 (33) | 0.923 | 1 (0.5) | 1 (0.5) | 1.000 |
| Peripheral sensory neuropathy | 57 (26.7) | 51 (23.7) | 0.592 | 1 (0.5) | 1 (0.5) | 1.000 |
| Palmar-plantar erythrodysesthesia syndrome | 33 (15.5) | 47 (21.9) | 0.181 | 1 (0.5) | 4 (1.9) | 0.372 |
| Fever | 13 (6.1) | 10 (4.6) | 0.670 | 0 (0) | 0 (0) | 1.000 |
| Febrile neutropenia | 1 (0.5) | 6 (2.8) | 0.122 | 1 (0.5) | 6 (2.8) | 0.122 |
| Constipation | 8 (3.8) | 17 (7.9) | 0.101 | 0 (0) | 0 (0) | 1.000 |
- The per protocol set included patients who met the eligibility criteria, received at least one cycle of chemotherapy, and underwent at least one tumor response evaluation.
- Abbreviations: XELOX, oxaliplatin plus capecitabine; EOX, epirubicin, oxaliplatin, plus capecitabine.
3.4 Quality of life
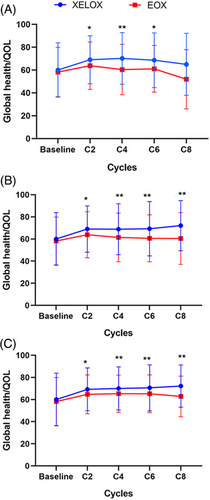
At baseline and at the beginning of cycles two, four, six, and eight, the rates of compliance to the QoL questionnaire were 86.9%, 75.3%, 68.1%, 48.8%, and 15.0% in the XELOX arm, and 83.2%, 81.8%, 74.1%, 45.9%, and 12.1% in the EOX arm, respectively. The QoL scores at baseline were similar between the two arms (P = 0.425). At the beginning of cycles two, four, six, and eight, QoL scores were significantly higher in the XELOX arm than in the EOX arm (Figure 4A), which were confirmed by using the LOCF method (Figure 4B) and Mean-value method (Figure 4C).
4 DISCUSSION
The present study compared the next-generation doublet regimen (XELOX) and triplet regimen (EOX), and obtained positive results that met the primary endpoint of non-inferiority in terms of PFS, with the same mOS (12 months). Furthermore, XELOX showed a good safety profile with a lower rate of grade 3/4 AEs (42.2% vs. 72.5%) and a better QoL compared with EOX.
Except for XELOX, S-1-based doublet regimens, such as SP and SOX, have been widely utilized in Asia for AGC. However, in the FLAG study, SP only achieved an mOS of 8.6 months in European and American populations, which might be due to the low tolerance of Western patients to S-1 [25]. Therefore, XELOX is a potential standard chemotherapy regimen in the first-line treatment of AGC worldwide, as capecitabine is suitable for both Western and Eastern populations.
It is noteworthy that the ORR in the EOX arm was slightly increased, but not significantly higher than that in the XELOX arm in the present study, suggesting that the triplet regimen may still have advantages in conversion therapy of local AGC. However, the majority of patients in the present study had no chance of receiving conversion therapy, and the slightly higher ORR could not be translated into the prolongation of PFS and OS. Recently, a phase II trial showed that the triplet regimen FOLFIRINOX (irinotecan, oxaliplatin, leucovorin and 5-fluorouracil) yielded high ORR and long mOS [26]. The effects need to be confirmed in randomized controlled trials, and high toxicity (79% of patients experienced grade 3/4 neutropenia) also limits its clinical utility. However, these results suggest that a higher ORR produced by a high-intensity regimen might translate into a longer OS in certain local AGC patients with a poor prognosis. Therefore, we tried to identify which subset might benefit from triplet regimen in the present study. First, poor differentiation is a widely recognized factor for poor prognosis. Second, the programmed cell death protein 1 (PD-1) antibody nivolumab combined with XELOX or FOLFOX (oxaliplatin, leucovorin and 5-fluorouracil) chemotherapy has become the standard first-line treatment in AGC [8], and usually liver metastasis is resistance to PD-1 antibody. Therefore, we tried to explore whether triplet regimen has clinical advantages in these two subgroups and lay the foundation for future investigation of triplet regimen combined with PD-1 antibody for patients with poor prognostic factors. Our analysis found no significant survival benefit from the triplet regimen in the two subgroups. Nevertheless, we found that in patients with poorly differentiated adenocarcinoma and liver metastasis, the EOX arm had a longer mOS (P = 0.021) and a longer mPFS (P = 0.073) than the XELOX arm, suggesting that the triplet regimen might have an advantage in selected patient subpopulations. Therefore, finding subsets suited for high-intensity regimens and biomarkers that could help in regimen selection is crucial.
The Checkmate-649 trial explored the potential of PD-1 antibody (nivolumab) plus chemotherapy (XELOX/FOLFOX) as the first-line treatment for AGC [8]. However, the survival benefit from the combined treatment was still limited with a prolonged mOS of only 2.2 months compared with chemotherapy alone in the all randomized-population. Therefore, the major research focuses in the future will include the exploration of biomarkers for the selection of subpopulations who would benefit from PD-1 antibody combined with chemotherapy and the development of new generation of immune checkpoint inhibitors, such as PD-1/cytotoxic T-lymphocyte associated protein 4 (CTLA4) bi-specific antibody, and new generation of molecular targeted drugs, such as Clauding 18.2 antibody [14] and fibroblast growth factor receptor (FGFR) antibody [27, 28]. Incorporating new generation of immune checkpoint inhibitors and molecular targeted drugs into first-line treatment of AGC might further increase the efficacy. As the present study showed that triplet regimen EOX might have a survival advantage in patients with poorly differentiated adenocarcinoma and liver metastasis, the value of PD-1 antibody combined with triplet regimen in AGC patients with poor prognosis deserves further investigation and needs to be confirmed in clinical trials.
The present study had several limitations. First, HER2 status was unknown in approximately 30% of patients in both arms. Second, the present study did not compare the efficacy of next-generation doublet regimen with that of taxane-contained triplet regimens, such as FLOT4 (oxaliplatin, docetaxel, and 5-fluorouracil) or DCF. Third, some patients were lost to follow-up, especially in later cycles, and the information of QoL was lacked.
In conclusions, this non-inferiority trial demonstrated that the XELOX doublet regimen is as effective as the EOX triplet regimen in the first-line treatment for AGC patients, with a better safety profile and QoL. EOX regimen might have a survival advantage in patients with poorly differentiated adenocarcinoma and liver metastasis. This trial provides solid evidence for guideline updates and guidance for future clinical trial design.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This trial was approved by ethics committee at each participating site and the trial obtained patients’ consent to participate.
CONSENT FOR PUBLICATION
All authors have consent for publication.
CONFLICT OF INTERESTS
The authors declare no potential conflicts of interest.
ACKNOWLEDGEMENTS
We would like to extend our thanks to Dr. XiChun Hu's advice on data analysis and Dr. Jin Li's advice on research design.
AUTHOR CONTRIBUTIONS
Conception and design: WG, XDZ
Funding support: WG, XDZ
Provision of study materials or patients: WG, XDZ, MH, YW, ZC, YH, XWZ, XL, CW, WZ, JY, JW, LY, YQ, XZ, WL, ZZ, LQ, HZ
Collection and assembly of data: WG, XDZ, MH, WF, QG, XS, SW, CZ, QL, XW, QX, QW, JJ, JZ
Data analysis and interpretation: MH, WF, JL, JZ, ZG
Manuscript writing: WF, WG, JZ
Final approval of the manuscript: All authors
Accountable for all aspects of the work: All authors
PRIOR PRESENTATION
Part of the results has been accepted and presented in the poster discussion section in 2021 ASCO meeting.
Open Research
DATA AVAILABILITY STATEMENT
The data are available from corresponding authors upon reasonable request.




