Combination of anti-PD-L1 antibody with peptide MEL-dKLA targeting M2 tumor-associated macrophages suppresses breast cancer metastasis
Abbreviations
-
- IFN
-
- interferon
-
- KLA
-
- (KLAKLAK)2
-
- LN
-
- lymph node
-
- TDLN
-
- tumor-draining lymph node
-
- WT
-
- wild-type
-
- MEL
-
- melittin
-
- MEL-dKLA
-
- melittin-GGGGS-d(KLAKLAK)2
-
- TAMs
-
- tumor-associated macrophages
-
- TME
-
- tumor microenvironment
-
- PD-L1
-
- programmed cell death-ligand 1
-
- VEGF
-
- vascular endothelial growth factor
-
- EGF
-
- epidermal growth factor
-
- CSF-1
-
- colony-stimulating factor 1
-
- TIM-3
-
- T-cell immunoglobulin domain and mucin domain 3
-
- LAG-3
-
- lymphocyte activation gene 3
-
- TNF-α
-
- tumor necrosis factor-α
-
- GzmB
-
- granzyme B
-
- IFNG
-
- interferon-gamma
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
Breast cancer is a leading cause of cancer-related deaths worldwide and is the most common cancer among women [1]. The cause of death in patients with breast cancer is not the primary tumor itself but mainly metastasis. The most common sites of breast cancer metastasis include the bones, lungs, brain, lymph nodes, and liver. Importantly, lung metastasis has been a particular concern as it results in high patient morbidity and mortality rates [2].
In the tumor microenvironment (TME), tumor-associated macrophages (TAMs) have been thought to have M2-like phenotypes that lead to tumorigenicity and metastasis by directly increasing epithelial-mesenchymal transition, extracellular matrix remodeling, and angiogenesis [3], and they are key regulators of lung metastasis in breast cancer [4]. Further, M2-like macrophages have been demonstrated to cause dysregulation of the T-cell receptor signaling and subsequently induce CD8+ T-cell unresponsiveness [5].
CD8+ T cells are known to secrete large amounts of interferon-γ (IFN-γ) and the protease granzyme B (GzmB), kill infected or tumorigenic cells, and play a key role as an essential component of tumor-infiltrating lymphocytes and the TME [6]. In breast cancer, high CD8+ T-cell counts are associated with better survival [7].
Melittin (MEL) has been reported to have anticancer effects in various cancers. Recently, it emerged as a promising immunotherapeutic agent that activates cytotoxic T cells and natural killer (NK) cells [8, 9]. In our previous study [10], we synthesized a peptide named MEL-dKLA consisting of melittin, which bound preferentially to M2-like TAMs, a short peptide bridge GGGGS, and pro-apoptotic peptide d(KLAKLAK)2 (dKLA). MEL-dKLA induced mitochondrial membrane disruption when internalized into cells. We further reported that this peptide has M2-like TAM-targeting properties in a lung cancer mouse model and verified its anti-angiogenic effect, demonstrating its anti-metastatic potential [10]. Here, we investigated whether MEL-dKLA and anti-programmed death-ligand 1 (PD-L1) antibody combination therapy would have a synergistic effect on inhibiting breast cancer metastasis through CD8+ T-cell activation and M2-like TAM elimination. The detailed materials and methods can be found in the Supplementary file.
Compared with those in the control group treated with phosphate-buffered saline (PBS), tumor size and weight were significantly decreased in the MEL-dKLA and the combination group (MEL-dKLA and anti-PD-L1 antibody combination therapy) groups and decreased more significantly in the combination group than in the MEL-dKLA group. The combination therapy showed stronger effect on reducing tumor growth than both monotherapies (Figures 1A and 1B). Moreover, it significantly inhibited breast cancer metastasis compared with other groups (Figure 1C).
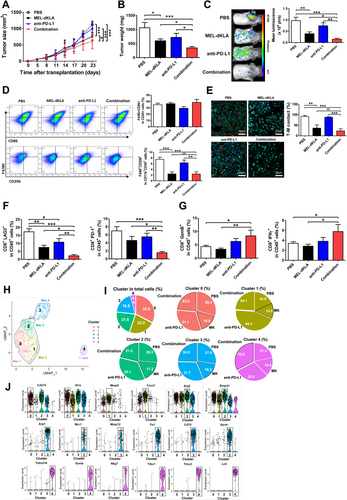
MEL-dKLA alone or in combination with anti-PD-L1 antibody led to a decrease in F4/80+CD206+ M2-like TAMs in tumor tissues. However, the combination therapy did not show stronger effect on the decrease in M2-like TAMs than both monotherapies (Figure 1D). In addition, the ratio of contacted cells between CD8+ T cells and M2 macrophages in tumor tissues was significantly decreased in the MEL-dKLA and combination groups, whereas combination treatment did not significantly enhance the effect compared to MEL-dKLA (Figure 1E). Thus, these results were considered due to MEL-dKLA targeting M2 macrophages. We determined whether the combination therapy could inhibit the exhaustion of CD8+ T cells in the TME and induce their activation. Exhaustion markers lymphocyte activation gene 3 (LAG3) and programmed cell death 1 (PD-1) were significantly reduced in the combination group compared with levels in other groups (Figure 1F). Activation markers GzmB and IFN-γ were significantly increased in the combination group compared to levels in the PBS and MEL-dKLA groups (Figure 1G). Further, the mRNA expression levels of GZMB, IFNG, and tumor necrosis factor-α (TNF-α) in tumor tissues were significantly increased in the combination group compared to those in the PBS group (Supplementary Figure S1). We also confirmed the activity of T cells in response to the combination therapy using in vitro co-culture of CD8+ T cells and TAMs. GzmB and IFN-γ in CD8+ T cells were significantly increased in the combination group compared to levels in other groups, whereas LAG3, cytotoxic T lymphocyte antigen-4 (CTLA4), and T cell immunoglobulin and mucin-domain containing-3 (TIM-3) in CD8+ T cells were significantly decreased in the combination group (Supplementary Figure S2). These results show that the function of T cells in the TME is important not only for macrophage targeting but also for PD-L1 blockade.
We also analyzed single-cell transcriptomes to determine the changes in related genes in TAMs and T cells from breast tumor tissues treated with the MEL-dKLA and anti-PD-L1 antibody combination therapy. Cells were divided into five clusters using uniform manifold approximation and projection, and clusters 0-3 highly expressed macrophage-related genes chemokine (C-X-C motif) ligand 2 (Cxcl2), C-C motif chemokine ligand 3 (Ccl3), cluster of differentiation 274 (Cd274), C-X-C motif chemokine receptor 2 (Cxcr2), haem oxygenase 1 (Hmox1), and interleukin 1 receptor antagonist (Il1rn). However, cluster 4 distinctly expressed T-cell receptor-related genes T cell receptor beta constant 1 (Trbc1), Trbc2, lymphocyte-specific protein tyrosine kinase (Lck), and linker for activation of T cells (Lat). Therefore, these results suggest that the immune cells in the TME of the breast cancer mouse model were mostly composed of macrophages (Figure 1H, Supplementary Figure S3). In particular, cluster 3 was mainly composed of M2-like TAMs with the high expression of genes associated with M2 macrophages, such as arginase 1 (Arg1), mannose receptor C-type 1 (Mrc1), matrix metallopeptidase 12 (Mmp12), and fibronectin 1 (Fn1). It was decreased in the MEL-dKLA group (16.2%) compared to that in the PBS group (27.5%). Moreover, in cluster 4, effector T cells with highly expressed genes such as TNF receptor superfamily member 18 (Tnfrsf18), Gzmb, and natural killer cell granule protein 7 (Nkg7) were increased in the combination group (32.8%) compared to levels in the PBS group (21.3%; Figures 1I and 1J). These results suggest that MEL-dKLA and anti-PD-L1 antibody combination therapy had stronger effect by inducing effector T cell activity and suppressing M2-like TAMs than both monotherapies.
Here, we used a pro-apoptotic peptide (MEL-dKLA) to target M2-like TAMs in breast cancer metastasis mouse models. As MEL was previously reported to have significant antitumor effects with a high binding affinity to M2-like TAMs [10], we sought to improve the antitumor activity of MEL-dKLA for an enhanced targeting effect. We verified the inhibitory effect of MEL-dKLA on invasive breast cancer metastasis based on its M2-targeting property in an orthotopic implantation model. Notably, among immunosuppressive cells in the TME, M2 macrophages suppress the recruitment of immune cells, such as CD4 and CD8 effector T cells and NK cells, and the immune response of these cells by overexpressing PD-L1 and CTLA4 [5, 9]. Therefore, strategies targeting M2 macrophages might be needed to improve the efficacy of anti-PD-L1 immunotherapy.
In this study, we verified that MEL-dKLA has enhanced anti-metastatic effect by inducing CD8+ T-cell activation and eliminating M2-like TAMs through combination therapy with PD-L1 blockade in breast cancer metastasis. Thus, we suggest that MEL-dKLA could be a novel therapeutic agent for breast cancer metastasis inhibition by targeting M2-like TAMs and can be used in combination with anti-PD-L1 antibody to induce CD8+ T-cell activation.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All animal studies were approved by the University of Kyung Hee Institutional Animal Care and Use of Committee (KHUASP(SE)-17-087).
CONSENT FOR PUBLICATION
Not applicable
CONFLICT OF INTEREST
No conflicts of interest to disclose.
DATA AVAILABILITY STATEMENT
The data used and/or analyzed for this study will be available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
The authors would like to thank Jungmo Kim for technical help with single-cell sequencing at the Institute for Basic Science, Daejeon, Republic of Korea. We would like to thank Editage (www.editage.co.kr) for English language editing. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; NRF-2020R1A2B5B03002164).
AUTHORS’ CONTRIBUTIONS
Study concept and design: HB. Methodology and technical support: CL, HL, HJ, SK, IC, YSH, HJ, SP, and IHH. Analysis and interpretation of data: CL, HL, HC, DSH, and IHH. Writing, review, and/or revision of the manuscript: CL, HL, IHH, and HB Study supervision: IHH and HB. Approval of the manuscript: All authors




