Gene expression profiling improves prognostication by nomogram in patients with soft-tissue sarcomas
Abbreviations
-
- c-index
-
- concordance index
-
- CI
-
- confidence interval
-
- CINSARC
-
- complexity index in sarcomas
-
- HR
-
- hazard ratio
-
- MFS
-
- metastatic relapse-free survival
-
- OS
-
- overall survival
-
- Pr-OS
-
- predicted overall survival
-
- STS
-
- soft-tissue sarcoma
-
- UPS
-
- undifferentiated pleomorphic sarcomas
Dear Editor,
Surgery remains the cornerstone of treatment for soft-tissue sarcomas (STS) [1]. However, despite adequate locoregional treatment, 30%-40% of the patients eventually develop metastases [2, 3]. Accurate risk assessment is crucial to tailor therapeutic strategies and to identify high-risk patients who may benefit from perioperative chemotherapy. Hence, several efforts have been made to develop prognostic nomograms that enable individual prognosis prediction; representing an important decision-making aid for oncologists and surgeons involved in sarcoma care [4-6]. The most recent nomogram, named SARCULATOR, has been adapted to retroperitoneal sarcoma and extremities STS, possibly treated with perioperative chemotherapy, to predict overall survival (OS) and metastasis-free survival (MFS) based on histological type, grade, tumor size and patient's age with good performances on international validation cohorts [4-6]. However, patients treated for STS with similar clinical and pathological features may have distinct outcomes, suggesting the existence of underlying prognostic molecular features. Chibon et al. developed a prognostic gene expression signature named Complexity INdex in SARComas (CINSARC), which, for instance, could discriminate intermediate grade II patients with opposing prognosis [7]. Here, our objective was to investigate whether the CINSARC signature could improve the prediction of prognosis achieved by the SARCULATOR nomogram in STS patients.
We included 227 adult patients who underwent curative surgery for newly-diagnosed, locally-advanced, histologically-proven STS from the extremities or the trunk wall between 1991 and 2006 in 14 centers of the French Sarcoma Group (Supplementary Table S1). Study design and method are described in details in the Supplementary Material (Supplementary Methods). Gene expression profiling data (ATG-Sarc database, http://atg-sarc.sarcomabcb.org/) were available for all patients and all histological diagnoses were validated by an expert pathologist member of the French Sarcoma Group. CINSARC was established by profiling RNA extracted from frozen tissue material of the initial tumor collected before any treatment, as previously described [8]. The 10-year predicted probability of OS (Pr-OS) was computed for each participant using the SARCULATOR free application [5]. Patients were divided into 3 categories of Pr-OS: low (≤51%), intermediate (>51% and ≤66%), and high (>66%) as previously described [9, 10]. The corresponding new categorical variable was named SARCULATOR.
All patients were in complete remission after initial treatments and none of them required continued therapy afterwards. The latest follow-up was obtained in August 2020. At the time of analysis, 81/227 (35.7%) patients had metastatic relapse and 122/227 (53.7%) patients died.
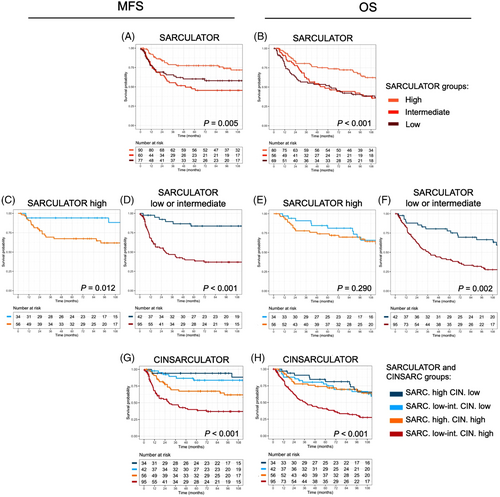
We first investigated the association of risk stratification according to SARCULATOR with MFS and OS. The 5-year MFS probabilities in the high, intermediate and low Pr-OS SARCULATOR groups were 0.77 (95%CI: 0.69-0.87), 0.47 (95%CI: 0.36-0.63) and 0.60 (95%CI: 0.49-0.74), respectively (Supplementary Table S2, Figure 1A). The 5-year OS probabilities in the high, intermediate and low Pr-OS SARCULATOR groups were 0.78 (95%CI: 0.70-0.87), 0.50 (95%CI: 0.39-0.65) and 0.54 (95%CI: 0.43-0.67), respectively (Supplementary Table S2, Figure 1B). The SARCULATOR was significantly correlated with MFS and OS, but heterogeneous outcomes were encountered within the same SARCULATOR group, especially for the intermediate group. The other variables significantly associated with the two survival outcomes in univariate analysis were CINSARC for MFS, and age, tumor depth and CINSARC for OS (Supplementary Table S2 and S3).
Multivariate Cox modeling is displayed in Supplementary Table S4. First, no correlations were observed between the SARCULATOR Pr-OS and CINSARC, and between the SARCULATOR groups and CINSARC (Supplementary Figure S1). However, there were significant correlations between the patients’ age and the SARCULATOR groups, which was explained by the fact that the SARCULATOR requires age to compute Pr-OS. Overall, both CINSARC and SARCULATOR remained independent predictors of MFS and OS.
We then assessed whether CINSARC was able to robustly discriminate subgroups of patients within each SARCULATOR category (Supplementary Table S2). We found that for all SARCULATOR groups, MFS differed significantly according to the CINSARC status. In the SARCULATOR low groups, the 5-year MFS probabilities were 0.90 (95%CI: 0.78-1) in patients with CINSARC low risk versus 0.46 (95%CI: 0.33–0.64) in patients with CINSARC high risk. In the SARCULATOR intermediate group, the 5-year MFS probabilities were 0.77 (95%CI: 0.59-1) in patients with CINSARC low risk versus 0.33 (95%CI: 0.20–0.52) in patients with CINSARC high risk. In the SARCULATOR high group, the 5-year MFS probabilities were 0.94 (95%CI: 0.87-1) in patients with CINSARC low risk versus 0.67 (95 CI: 0.56–0.81) in patients with CINSARC high risk. Oppositely, OS differed significantly according to the CINSARC status in the SARCULATOR intermediate group but not in the high and low groups. Indeed, in the SARCULATOR intermediate group, the 5-year OS probabilities were 0.84 (95%CI: 0.69-1.00) in patients with CINSARC low risk versus 0.35 (95%CI: 0.22-0.54) in patients with CINSARC high risk.
Since the Kaplan-Meier curves for the intermediate and low SARCULATOR groups were almost superimposed and not significantly different whatever the outcome (OS or MFS), we merged these two categories for the subsequent analyses in order to facilitate the combination into a single CINSARCULATOR variable of 4 levels instead of 6 levels (i.e., CINSARC high risk – SARCULATOR low-intermediate, CINSARC high risk – SARCULATOR high, CINSARC low risk – SARCULATOR low-intermediate and CINSARC low risk – SARCULATOR high). Kaplan-Meier curves for OS and MFS depending on the CINSARC status and the SARCULATOR high group, or low-intermediate group are shown in Figure 1C-F.
When the SARCULATOR and CINSARC status were then combined (Figure 1G-H), the Harrell concordance index (c-index) for MFS improved to 0.71 (95%CI: 0.68-0.73) compared with CINSARC alone (c-index = 0.65 [95% CI: 0.63-0.67], P < 0.001) or SARCULATOR alone (c-index = 0.60 [95%CI: 0.57-0.62], P < 0.001), respectively. Combining SARCULATOR and CINSARC status also improved death prediction, with a c-index of 0.65 (95%CI: 0.62-0.67) compared with 0.59 (95%CI: 0.57-0.61, P = 0.018) for CINSARC alone and 0.60 (95%CI: 0.58-0.62, P < 0.001) for SARCULATOR alone.
Finally, we investigated whether the combination of SARCULATOR and CINSARC would also help in predicting survivals in the main histotypes of the cohort, namely undifferentiated pleomorphic sarcoma (UPS, n = 62) and leiomyosarcoma (n = 56). Kaplan-Meier curves for MFS and OS for both histotypes depending on the CINSARCULATOR categories are shown in Supplementary Figure S2.
Regarding UPS, the highest c-index for MFS was reached with the CINSARCULATOR (0.65, 95%CI: 0.62–0.72), followed by CINSARC and SARCULATOR alone (Supplementary Table S6). Similarly, the highest c-index for OS was reached with the CINSARCULATOR (0.67, 95%CI: 0.59–0.71), followed by SARCULATOR and CINSARC alone (Supplementary Table S6).
Regarding leiomyosarcoma, again, the highest c-index for MFS was found with the CINSARCULATOR (0.68, 95%CI: 0.63–0.73), followed by CINSARC and SARCULATOR alone (Supplementary Table S6). Combining CINSARC and SARCULATOR significantly improved the c-index for MFS compared with CINSARC alone (P = 0.035) and SARCULATOR alone (P = 0.023). Finally, the highest c-index for OS was obtained with the CINSARCULATOR (0.64, 95%CI: 0.59–0.69) followed by SARCULATOR and CINSARC alone (Supplementary Table S6).
In conclusion, we confirm the significant prognostic value of CINSARC and SARCULATOR for OS and MFS in patients with newly-diagnosed and locally-advanced STS. Combining CINSARC and SARCULATOR into a hybrid and synergistic new CINSARCULATOR variable showed promising value in the whole cohort and in the main histological types. This study paves the way to new prognostic nomograms for STS patients incorporating clinical, histologic and molecular features, in order to tailor perioperative chemotherapy and to adapt the rhythm of follow-up.
DECLARATIONS
AUTHORSHIP
Concept and design: AI; Acquisition, analysis, or interpretation of data: AC, MSC, AM, YL, CL, MT, KB, FLL, AI; Drafting of the manuscript: AC, AI; Critical revision of the manuscript for important intellectual content: AC, MSC, AM, YL, CL, MT, KB, FLL, AI; Statistical analysis: AC, AI; Administrative, technical, or material support: YL, CL, FLL, AI; Validation: AC, MSC, AM, YL, CL, MT, KB, FLL, AI; Supervision: AI.
ETHICS IN APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the local Research Ethics Committee of Bergonié Insitute (Bordeaux, France) according to good clinical practices and applicable laws and regulations. All methods were performed in accordance with the relevant guidelines and regulations. The need for written informed consent was waived because of its retrospective nature.
CONSENT FOR PUBLICATION
The article does not contain any individual person's data.
CONFLICT OF INTEREST STATEMENT
FLL: Blueprint Medicines and PharmaMar. The other authors declare that they have no competing interests.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are not publicly available due to the clinical and confidential nature of the material but can be made available from the corresponding author on reasonable request.
FUNDING
This research did not receive funding.
ACKNOWLEDGEMENTS
None.




